Abstract
Natural glucocorticoids (Gc) produced during stress have profound effects on the immune system. It is well known that Gc induce apoptosis in precursor T and B cells, markedly altering lymphopoiesis. However, it has been noted that marrow myeloid cells expanded both in proportion and absolute numbers in the mouse after Gc exposure. Mice were implanted with a corticosterone (CS) tablet that increased serum Gc and caused atrophied thymuses, both classic signs of activation of the stress axis. Blood neutrophil counts were elevated (4.8×), whereas lymphocyte counts declined. Flow cytometric analysis of the marrow revealed that the phenotypic distribution of the various major classes of cells was shifted by Gc exposure. As expected, marrow lymphocyte numbers declined >40% after 3 days of exposure to Gc. Conversely, in the myeloid compartment, both monocytes and granulocytes increased in number by >40%. Further, all granulocyte developmental stages showed large increases in both total number and percentage of cells. To investigate the functional capacity of mature granulocytes from Gc-treated mice, an improved granulocyte isolation method was developed. Gc exposure had little effect on the ability of granulocytes to produce superoxide or undergo chemotaxis or phagocytose bacteria. These results indicate that Gc treatment shifts bone marrow composition and provides evidence that granulocytes and their progenitors are selectively preserved under stressful conditions without losing function.
Keywords: granulopoiesis, lymphopoiesis
Stress resulting from nutritional deficiency, burns, trauma, psychological distress, or other events can trigger profound physiological responses, leading to synthesis of glucocorticoids (Gc) and causing large increases in serum Gc (1). Chronic elevation of Gc can last days or weeks and can result in dramatic alterations to the immune system. The focus of this article will be the impact of Gc-mediated stress on the development of murine bone marrow granulocytes.
Gcs have long been known to have potent antiinflammatory activity. Although the antiinflammatory activity of Gc, especially at pharmacological levels, may help reduce inflammatory reactions and thus avert tissue damage, prolonged Gc exposure can lead to a variety of unwanted side effects, including immunosuppression (2, 3). Indeed, both exogenous (4) and endogenous (5) Gc have immunosuppressive effects, including inhibition of MHC class II expression (6), disruption of Th1/Th2 balance (7), and increased susceptibility to infection during wound healing (8, 9).
Gcs are well known for their ability to initiate apoptosis in precursor lymphoid cells in both the thymus and bone marrow, reducing lymphopoiesis. Endogenous Gc caused by zinc deficiency resulted in lymphopenia, reduced splenic lymphocyte number, thymic atrophy due to accelerated apoptosis, and reductions of lymphocyte numbers in the bone marrow (10). In related studies, exposure to corticosterone (CS) alone led to apoptotic losses of 30–70% in marrow pre-B cells in mice (11, 12). In contrast, myeloid cells appeared to be resistant to Gc-induced apoptosis, with granulocytes and monocytes increasing in number in the mouse bone marrow (11) and neutrophil counts increasing significantly in the circulation (13). Moreover, Gc significantly increased the half-life of neutrophils (14, 15). The mechanisms by which Gc exerted its effects on granulocytes and their precursors in the marrow were not known. Nevertheless, the significant reprogramming of the immune system subsequent to the introduction of stress deserved further exploration.
In this article, the effects of chronic elevation of Gc in mice on bone marrow granulocyte development were investigated. Delivery of stress levels of CS to mice resulted in shifts in bone marrow composition, including reduction in lymphocyte number and increases in myeloid cells. Granulocytes increased both as a percentage of the nucleated marrow cells and in total number, including all stages of granulocyte development. CS had little or no effect on the gross function of mature granulocytes from the marrow. These data indicate exposure of mice to stress levels of CS has different effects on granulocytic cells and their development than lymphoid-like cells in the marrow. It suggests that the first line of immune defense is preferentially preserved.
Results
CS Exposure Results in Elevated Serum CS, Thymic Atrophy, and Neutrophilia.
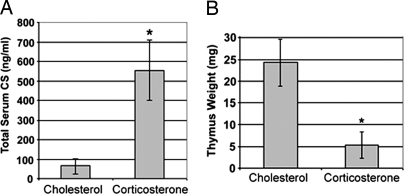
The delivery of stress levels of CS to mice was accomplished by s.c. implantation of CS tablets, as described (11, 12). On day three of CS exposure, mice were killed, and serum CS was determined. Mice with CS implants (20 mg of CS, 20 mg of cholesterol) had significantly higher serum CS levels and severely reduced thymus weights (78.3% reduced) compared with control mice with cholesterol (40 mg) implants (Fig. 1).
Fig. 1.
CS administration to mice results in systemic immune alterations. Mice implanted with CS tablets for 3 days were monitored for serum CS concentration (A) and thymic atrophy (B). Data shown are mean +/− standard deviation for eight (A) and 13 mice (B). *, P < 0.01.
Blood was collected from each group, and white blood cell (WBC) counts were performed. Total WBC numbers were not statistically different between control and CS-treated mice. CS-treated mice, however, had a 4.8-fold increase in neutrophil numbers in the blood (Table 1) and a concomitant decrease in lymphocyte numbers, from 83% to 29% of total WBC. Monocytes did not significantly increase in percentage (Table 1). Thus, elevated CS increased neutrophil numbers in the blood.
Table 1.
CS increased neutrophil counts in mouse blood
| Treatment, day 3† | Percent of WBC in mouse blood* |
||
|---|---|---|---|
| Neutrophils‡ | Lymphocytes | Monocytes | |
| Chol | 14.0 ± 7.0%§ | 83.0 ± 8.7% | 2.0 ± 1.7% |
| CS | 67.4 ± 19%¶ | 29.4 ± 16.3%¶ | 3.5 ± 2.1% |
*Mouse blood was harvested 3 days after tablet implant.
†Mice were implanted subcutaneously with cholesterol (Chol) or CS tablets.
‡Cell types were identified by morphological assessment.
§Data shown as percent ± standard deviation, n = 5.
¶Difference between controls and CS-treated mice was significant (P < 0.01).
Effect of CS on Bone Marrow Classes: Increase in Myeloid Cells.
It was of particular interest to discern how these shifts in WBC composition were manifested in the marrow of mice subjected to CS. Although total nucleated bone marrow cell numbers did not change after 3 days of exposure to CS, there were large shifts in the percentage and overall number of the various classes of cells in major compartments of the bone marrow. To analyze these cell classes, a flow cytometric system allowing discrimination of at least five distinct cell populations was used (10–12, 16) [supporting information (SI) Fig. 5].
Using this protocol, the effects of CS exposure on immune cells and their precursors in the mouse bone marrow were investigated. After 3 days of CS treatment, lymphoid cells of the mouse bone marrow declined sharply compared with control mice (44% decrease, Fig. 2), as has been demonstrated in our previous studies (11). Similarly, erythroid cells also showed a sharp decrease in proportion (37%, Fig. 2) in CS-treated mice.
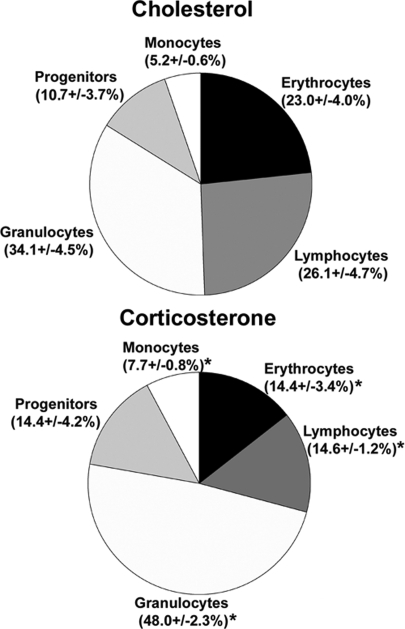
Fig. 2.
Overall changes in bone marrow cellular distribution in control mice (Upper) and CS-treated mice (Lower) are shown as percentage of total bone marrow nucleated cell numbers (n = 8 per group). Data were obtained by phenotypic labeling of bone marrow cells with anti-Ly-6C and -CD31. Lymphoid cells (Ly-6C−/CD31+); erythroid cells (Ly-6C−/CD31−); monocytes (CD31+/Ly-6Chi); granulocytes (CD31−/lo/Ly-6Cmed); mixed progenitors (CD31+/Ly-6Cmed). One representative experiment of four similar experiments is shown. *, P < 0.01.
The key question was to ascertain the status of the development of granulocytes and monocytes after CS exposure. Increases in the proportion of granulocyte and monocyte subpopulations of mouse bone marrow were observed after 3 days of CS exposure (Fig. 2). Granulocytes increased in proportion of marrow from 34.1% to 48.0%, whereas monocytes increased from 5.2% to 7.7% (Fig. 2). Mixed progenitors, which contain a percentage of myeloid progenitors, increased from 10.7% to 14.4% (Fig. 2). Because the overall number of bone marrow nucleated cells did not change upon CS exposure, these changes also represent increases in total numbers of cells for these populations. The data clearly show that in the presence of CS at stress levels, myeloid cells increase in absolute number in the mouse bone marrow whereas lymphoid cells decline sharply.
Development of Flow Cytometric System for Granulocyte Developmental Stages.
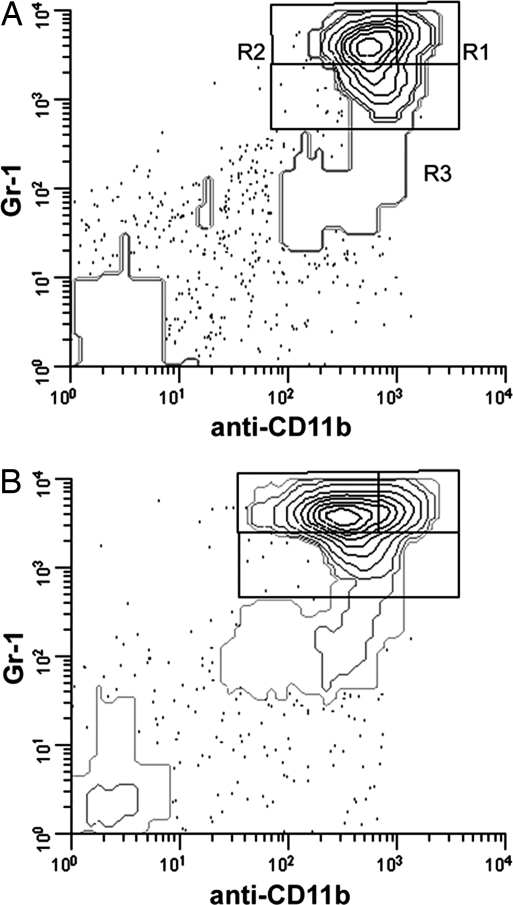
Because overall myeloid cell numbers increased in the bone marrow of CS-treated mice, and because neutrophils but not monocytes increased in number in blood, the effects of CS on bone marrow granulocyte development were further investigated. For this reason, an alternative flow cytometric marker system was developed. Neutrophilic granulocytes develop from hematopoietic stem cells in the bone marrow through a series of successive steps. To delineate immature (promyelocyte/myelocyte), intermediate (metamyelocyte and band cells), and mature (segmented neutrophils) stages of granulocyte development, a common granulocyte marker system consisting of anti-Ly-6G/Ly-6C (Gr-1) and anti-CD11b was used. In this case, Gr-1hi/CD11bhi populations represented segmented neutrophils (R1 in Fig. 3A), Gr-1hi/CD11bmed populations represented metamyelocytes, and band cells (R2) and Gr-1med/CD11bmed/hi represented promyelocytes and myelocytes (R3). Monocytes are also positive for both Gr-1 and CD11b; thus, to exclude monocytic cells, a third antibody was included: anti-Ly-6C (clone ER-MP20). Cells labeled ER-MP20hi are monocytic, whereas ER-MP20med cells are mostly granulocytic. Thus, bone marrow cells were gated on ER-MP20med and analyzed as shown in Fig. 3. The identity of cells in regions in Fig. 3 was verified by sorting followed by cytocentrifugation and histological staining (SI Fig. 6). Thus, the flow cytometric marker systems described above allowed delineation of the various cell compartments of whole bone marrow and the various stages of granulocyte development.
Fig. 3.
CS treatment of mice results in increases in all stages of granulocyte development. Bone marrow cells from mice implanted with cholesterol (A) or CS (B) were analyzed by flow cytometry by using the Gr-1/anti-CD11b/-Ly-6C system, with cells gated on Ly-6Cmed to eliminate contaminating monocytes. Regions corresponding to segmented neutrophils (R1), metamyelocytes and band cells (R2) and promyelocytes and myelocytes (R3) were analyzed. Representative data shown are from a control mouse (A) and a mouse with CS tablet implant (B) and are representative of typical myeloid changes in tablet implant experiments.
Impact of CS on Granulocyte Development.
CS-treated mice showed increases in the three developmental stages delineated by Gr-1/CD11b/ER-MP20 labeling compared with control mice. Segmented neutrophils increased from 3.9% of bone marrow cells to >7.8% in CS-treated mice. Similarly, band and metamyelocytic cells increased from 22.3% to 31.2% of marrow cells, whereas immature granulocytes increased from 7.8% to 11.3% (Table 2). These changes also represent increases in total numbers, because nucleated marrow cell number did not change after CS exposure. In conclusion, all stages of granulocyte development survived and increased in total number upon exposure to CS.
Table 2.
CS expanded granulocyte subpopulations in marrow of CS-treated mice
| Treatment, day 3† | Granulocyte subpopulation* |
||
|---|---|---|---|
| Mature, %‡ | Intermediate, % | Immature, % | |
| Chol | 3.9 ± 1.6 | 22.3 ± 3.9 | 7.8 ± 1.6 |
| CS | 7.8 ± 1.6§ | 31.2 ± 1.7§ | 11.1 ± 1.5§ |
*Data represent percent of total bone marrow cells ± standard deviation (n = 8).
†Mice were implanted subcutaneously with cholesterol (Chol) or CS tablets.
‡Maturational stage determined by flow cytometry (Fig. 2).
§Difference between control- and CS-treated mice was significant (P < 0.01).
Method for Preparation of Mature Bone Marrow Granulocytes.
To determine the effects on bone marrow granulocyte function of mice exposed to CS, an improved isolation technique was developed to separate high-purity mature granulocytes from the marrow. Percoll gradient centrifugation allowed enrichment of mature granulocytes to 70–85% purity. After immunomagnetic depletion of contaminants, cells were 91% and 93% mature granulocytes in controls and experimental samples, respectively (n = 17 for each). Of these granulocytic cells, ≈85% were either band cells or segmented neutrophils. Of special note, ≈3-fold more mature granulocytes were isolated from the bone marrow of CS-treated mice than from controls.
Mature Granulocytes Isolated from Bone Marrow of CS-Treated Mice Are Not Impaired in Superoxide Production.
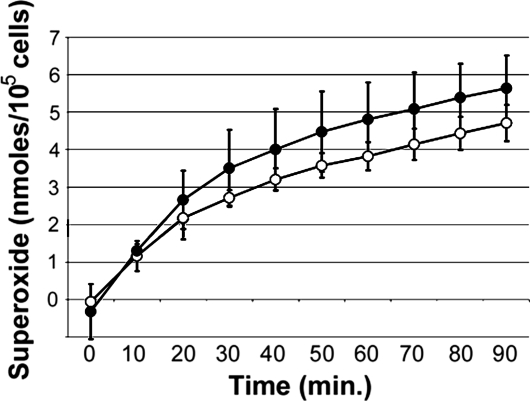
The ability of mature bone marrow granulocytes from CS-treated or control mice to produce superoxide in response to phorbol myristate acetate (PMA) was determined. Granulocytes produced steadily increasing amounts of superoxide up to 90 min after PMA addition (Fig. 4), with no significant differences observed in cells from the marrow of control or CS-treated mice. Similar results were obtained with cells stimulated with 2 μM PMA (data not shown). These findings suggested exposure to CS did not affect superoxide production by granulocytes residing in the marrow. In addition, the ability of marrow granulocytes to undergo chemotaxis in response to murine CXCL 1 chemokines MIP-2 or KC was not affected by CS exposure (SI Fig. 7).
Fig. 4.
Mature bone marrow granulocytes from CS-treated mice were able to produce superoxide at levels comparable to or higher than granulocytes from control mice. The production of superoxide by PMA-stimulated mature granulocytes was monitored over time. Background correction was achieved by using parallel samples in the absence of PMA. Specificity of superoxide production was determined by the addition of SOD to parallel samples. Granulocytes from control (open circles) and CS-treated (filled circles) mice were analyzed, n = 4 per group. Data shown are a representative of two experiments using 6 μM PMA.
Mature Granulocytes Isolated from Bone Marrow of CS-Treated Mice Are Not Impaired in Phagocytic Function.
The ability of marrow granulocytes to engulf Escherichia coli was investigated. Phagocytosis, as determined by visual inspection and measured by percentage of mature granulocytes positive for internalized fluorescein-conjugated E. coli, increased with the ratio of E. coli to granulocyte for both controls and CS-treated samples (Table 3). At a 10:1 E. coli:granulocyte ratio, 54% of granulocytes contained internalized bacteria, whereas at a 100:1 ratio, 87% had internalized bacteria. No statistically significant difference was observed in the percentage of granulocytes with internalized bacteria between control and CS-treated mice. Additionally, the number of E. coli particles per cell were counted. The number of particles per cell increased as the ratio of E. coli to granulocytes increased, with more than three particles per cell at the lowest E. coli to granulocyte ratio (10:1) and up to 10 particles per cell at the highest ratio (100:1). Only at the highest ratio of E. coli to granulocytes (100:1) was a significant difference observed in phagocytosis, with cells from CS-treated mice showing a decrease in the number of E. coli per cell (6.4) compared with control cells (10.3). Samples incubated at 4°C served as negative controls and showed <2% of neutrophils internalized bacteria. These results indicated that marrow granulocytes from CS-treated mice were able to phagocytose bacteria to a degree similar to that of cells from control mice. The only significant difference observed was a decreased number of E. coli particles per cell in granulocytes from CS-treated mice when very high ratios of E. coli to granulocyte were used.
Table 3.
Granulocytes from CS-treated mice were competent in carrying out phagocytosis
| Treatment* | Ratio† | Percent with E. coli, %‡ | E. coli per cell§ |
|---|---|---|---|
| Cholesterol | 10:1 | 54.4 ± 7.3 | 3.3 ± 0.90 |
| Corticosterone | 10:1 | 63.2 ± 10.1 | 3.6 ± 0.49 |
| Cholesterol | 25:1 | 66.3 ± 21.9 | 5.9 ± 1.6 |
| Corticosterone | 25:1 | 67.0 ± 6.7 | 6.9 ± 1.6 |
| Cholesterol | 100:1 | 87.2 ± 3.5 | 10.3 ± 2.9 |
| Corticosterone | 100:1 | 85.9 ± 3.6 | 6.4 ± 1.0¶ |
*Mice were treated with CS by tablet implant. Marrow cells were harvested at day 3 after implant. n = 4 per group, one of two experiments.
†Ratio of E. coli to granulocytes in assay.
‡Percent of granulocytes with ingested E. coli.
§Data obtained by counting individual E. coli-fluorescein particles in each mature granulocyte.
¶Difference from control was significant (P < 0.05).
Discussion
This study focuses on cells of the granulocytic lineages in murine bone marrow and clearly demonstrates they not only survive exposure to CS in vivo but also increase in absolute numbers. As might be expected, this also resulted in increases in peripheral neutrophils (≈480%). Analysis of maturation stages of granulocyte development was possible due to improved flow cytometric methods which used anti-Ly-6C to eliminate monocytes from Gr-1+/CD11b+ myeloid cells, leaving mostly granulocytes. When followed by a combination of Percoll gradient centrifugation and immunomagnetic depletion, this allowed for the isolation of highly pure mature granulocytes. Using this methodology, we found that mature marrow granulocytes (band cells, segmented cells) were able to adequately carry out phagocytosis, produce superoxide, and respond to chemotactic factors subsequent to the Gc exposure. This is in marked contrast to many previous studies where exposure of peripheral neutrophils to pharmacological steroids was shown to alter their function (17).
To carry out these studies, CS was delivered to mice continuously via tablet implant to mimic chronic stress events. At 3 days postimplant, mice had CS levels that averaged >500 ng/ml serum, which is comparable with levels observed in natural stress models including nutritional deficiencies, social stress, and burns (18–22). Thymic atrophy and reductions in precursor B cells in the marrow, which are hallmarks of the stress response, were also observed in mice with CS implants as expected (11, 12), making the model a useful system for the study of stress-induced Gc exposure.
To assess the effects of CS on developing granulocytes in the marrow, a three-color flow cytometric system consisting of antibodies Gr-1, anti-CD11b, and anti-Ly-6C was used. Gr-1 and anti-CD11b have commonly been used to label murine bone marrow myeloid cells. Anti-Ly-6C was used as well, allowing the separation of monocytes (anti-Ly-6Chigh) from granulocytes (anti-Ly-6Cmed). Using this system, three developmental stages of bone marrow granulocytes could be readily discerned: immature (promyelocytes, myelocytes), intermediate (metamyelocytes, band cells), and mature (segmented cells), verified by histological staining (SI Fig. 6) and by cell cycle analysis that showed mature cells were not dividing (SI Table 4). Exposure of mice to CS for 3 days resulted in accumulation of all three major stages of granulocyte development in the bone marrow (increases of 100%, 40%, and 42% in mature, intermediate, and immature granulocytes, respectively; Table 2). This is in keeping with the observations that the addition of small amounts of Gc to bone marrow cultures promoted an accumulation of myeloid cells and gradual loss of lymphoid cells (23), with similar accumulations, including immature granulocytes, observed in vivo in mice responding to social stress (21). Thus, the findings here expand on these earlier studies.
It has been shown that increased egress of granulocytes from bone marrow accounts for a portion of the increased blood neutrophil count in response to Gc (24). In our study, elevated granulocyte numbers in the bone marrow translated to an elevation in blood granulocytes, from 14% of WBC in controls to 67.4% after CS treatment. Under normal conditions, granulocytes in blood have a short lifespan (25). Gcs have been shown to delay the spontaneous apoptosis of senescent neutrophils both in vivo and in vitro (15). The increased half-life of neutrophils after Gc exposure would also contribute to the observed increase in blood neutrophils (Table 1). Taken together, the data show significant alterations of granulocytes in the marrow and peripheral blood subsequent to exposure to CS.
It has long been known that Gc therapy leads to increased susceptibility to infections (26, 27), as does increased social stress (28). Indeed, elevated Gc leads to rapid apoptotic loss of lymphoid cells both peripherally and in the bone marrow (12, 21, 29, 30). Numerous studies have been undertaken to assess the effects of Gc on granulocyte function. Most these studies focused on pharmacological Gc and their effects on peripheral blood or peritoneal neutrophils from different organisms. In these studies, inhibition of neutrophil chemotaxis (31, 32), phagocytosis (33), and superoxide production (34, 35) by Gcs such as fluticasone and dexamethasone was demonstrated both in vitro and in vivo. In contrast, data showing that synthetic Gc has no effect, or even enhances neutrophil function, have also been reported (36, 37). Reasons for the conflicting results are most likely due to the different models being studied and the variation in drug type, dose, and exposure time. It has been shown that synthetic and natural Gc vary in their potency and effects on gene expression and apoptosis (38). Our results differ from many of these studies in that CS, a natural Gc, was administered in vivo to mice continuously for a period of 3 days, with Gc levels that were consistent with natural models of stress. Another difference between this study and previous studies was the use of mature bone marrow granulocytes for functional analysis instead of blood or peritoneal neutrophils (39, 40). Our studies indicated that chemotaxis, phagocytosis, and superoxide production were unaffected or slightly enhanced by in vivo CS treatment.
The evolutionary reason behind Gc-mediated granulocyte expansion by natural steroids remains a mystery. The most obvious explanation is that stress situations promote strengthened innate immune defense. Neutrophils are the first line of defense against microbial pathogens and of critical importance in human immunity. As such, Gcs may serve the purpose of bolstering innate immunity to help protect against as many infections as possible.
Materials and Methods
Mice and Gc Implants.
Female CAF1 mice (4–5 weeks old) were purchased from The Jackson Laboratory or Harlan–Sprague–Dawley. Mice were maintained according to guidelines approved by the University Laboratory Animal Research committee at Michigan State University in a facility maintained at 25°C with 12-h light and dark cycles. For implantation of tablets, age- and weight-matched mice (8–10 weeks of age) were anesthetized with ether and maintained with 30% isoflurane (Halocarbon Products). Tablets consisted of 20 mg of CS (92%; Sigma–Aldrich)/20 mg of cholesterol (Sigma–Aldrich) or 40 mg of cholesterol only and were implanted s.c. Incisions were sealed with wound clips (11). Mice were monitored daily for survival and infections.
Harvesting and Processing of Tissues.
Mice were anesthetized with isoflurane and blood collected by cardiac puncture. Whole blood was used for total blood counts, including differential counts and serum for CS analysis. Thymuses were removed and weighed. Bone marrow was harvested from femurs and tibiae into Harvest Buffer [HBSS (Invitrogen), 10 mM Hepes, 4 mM sodium bicarbonate, 4% heat-inactivated FBS (Atlanta Biologicals, pH 7.2], as described in ref. 41. After red blood cell lysis, bone marrow cells were resuspended in Label Buffer (HBSS, 10 mM Hepes, 4 mM sodium bicarbonate, 2% heat-inactivated FBS, 0.15% sodium azide, pH 7.2) for flow cytometry analysis. Alternatively, cells were resuspended in Harvest Buffer for functional assays.
Determination of Serum CS.
Serum CS was determined by using a Corticosterone (125I) Double Antibody RIA kit (MP Biomedicals), following the manufacturer's recommendations.
Flow Cytometry.
All antibodies were from BD Biosciences, except where indicated. Phenotypic labeling of distinct bone marrow cell classes is described in SI Text. Phenotypic labeling of three granulocyte maturational stages, immature (promyelocytes, myelocytes), intermediate (metamyelocytes, band cells), and mature (segmented neutrophils) was performed by using antibodies anti-Ly-6G and Ly-6C (Gr-1; biotinylated), anti-CD11b (PE-conjugated) and anti-Ly-6C (clone ER-MP20; FITC-conjugated; Bachem). Mature neutrophils were Gr-1hi/CD11bhi, intermediate cells were Gr-1hi/CD11bmed, and immature granulocytes were Gr-1med/CD11bmed/hi. To eliminate monocyte contamination, cells were gated on anti-Ly-6Cmed (16).
For labeling, bone marrow cells (1 × 106) in Label Buffer were incubated with antibody for 25 min on ice. Biotinylated antibodies were detected by addition of streptavidin-PE-Cy5 conjugate (BD Biosciences).
Isotype control antibodies used were FITC-conjugated rat IgG2a, biotinylated rat IgG2b, and PE-conjugated rat IgG2b
After labeling, cells were incubated with 1.5% formaldehyde in PBS (pH 7.2–7.4) overnight on ice. For cell cycle analysis, instrumentation and software used, see SI Text.
Isolation of Mature Bone Marrow Granulocytes.
Bone marrow cells were loaded onto Percoll gradients (GE Healthcare Biosciences), comprised of 50% (vol/vol), 55% (vol/vol), and 62.5% (vol/vol) Percoll layers in HBSS and centrifuged at 300 × g for 20 min at room temperature. Cells at the 55%/62.5% interface were collected. For high purity, cells were subjected to immunomagnetic depletion of contaminants by using anti-CD5, anti-CD45R/B220, Ter119, and anti-CD117/c-kit antibodies for 25 min on ice. Cells were then mixed with sheep anti-rat IgG-conjugated Dynabeads for 30 min with gentle mixing, followed by application to a Magnetic Particle Concentrator (Invitrogen) to remove the contaminating cells.
Determination of Superoxide Production.
Superoxide production by bone marrow granulocytes was determined by monitoring reduction of ferricytochrome c (42). Briefly, 1.5 × 105 high-purity bone marrow granulocytes were mixed with cytochrome c (50 μM; Sigma–Aldrich) and PMA (Sigma–Aldrich; 2 or 6 μM). Negative controls omitted PMA. Samples with and without superoxide dismutase (400 units/ml) were included to determine superoxide-specific reduction of cytochrome c. Reactions were incubated at 37°C.
Analysis of Phagocytosis.
Phagocytosis of E. coli by bone marrow granulocytes was analyzed by visualization of internalized E. coli by using a fluorescent microscope. Fluorescein-labeled E. coli (Invitrogen) was opsonized with fresh normal mouse serum for 1 h at 37°C. Reactions consisted of 1 × 105 bone marrow granulocytes, 10% fresh autologous mouse serum, and fluorescein-conjugated E. coli (ratio of E. coli to granulocytes was 10:1, 25:1 or 100:1) in 500 μl of HBSS with Mg2+ and Ca2+. After 1-h incubation at 37°C with shaking, washing with cold Harvest Buffer, and resuspension stop buffer containing sodium fluoride, cells were centrifuged onto slides, fixed in 10% buffered formalin and mounted by using Prolong Antifade Gold reagent (Invitrogen) containing 0.5 μg/ml DAPI. The extent of phagocytosis was determined by counting at least 250 cells per slide and counting the number of E. coli particles per cell. Associated and ingested E. coli were discriminated by comparing phagocytosis in samples incubated at 4°C.
Statistical Analysis.
Student's t test was used for comparison between control and CS-treated groups.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Olatundun Williams for technical assistance and Joe Frentzel for helpful discussions and other assistance. This work was supported by National Institutes of Health Grant DK 52289-29.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712003105/DC1.
References
- 1.Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan JF, Dobbs C, Brown D, Zwilling B. Psychoneuroimmunology: stress effects on pathogenesis and immunity during infection. Clin Microbiol Rev. 1994;7:200–212. doi: 10.1128/cmr.7.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwilling BS, Brown D, Pearl D. Induction of major histocompatibility complex class II glycoproteins by interferon-gamma: attenuation of the effects of restraint stress. J Neuroimmunol. 1992;37:115–122. doi: 10.1016/0165-5728(92)90162-e. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SK, Marshall GD., Jr Dexamethasone promotes type 2 cytokine production primarily through inhibition of type 1 cytokines. J Interferon Cytokine Res. 2001;21:147–155. doi: 10.1089/107999001750133159. [DOI] [PubMed] [Google Scholar]
- 8.Rojas IG, Padgett DA, Sheridan JF, Marucha PT. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- 9.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 10.King LE, Osati-Ashtiani F, Fraker PJ. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology. 1995;85:69–73. [PMC free article] [PubMed] [Google Scholar]
- 11.Laakko T, Fraker P. Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology. 2002;105:111–119. doi: 10.1046/j.1365-2567.2002.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology. 1993;80:587–592. [PMC free article] [PubMed] [Google Scholar]
- 13.Athens JW, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop CR, et al. Leukokinetic studies. 13. A nonsteady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J Clin Invest. 1968;47:249–260. doi: 10.1172/JCI105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- 16.de Bruijn MF, et al. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- 17.Goulding NJ, Euzger HS, Butt SK, Perretti M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm Res. 1998;47(Suppl 3):S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- 18.Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol. 2005;163:110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.DePasquale-Jardieu P, Fraker PJ. The role of corticosterone in the loss in immune function in the zinc-deficient A/J mouse. J Nutr. 1979;109:1847–1855. doi: 10.1093/jn/109.11.1847. [DOI] [PubMed] [Google Scholar]
- 20.Kagan RJ, Bratescu A, Jonasson O, Matsuda T, Teodorescu M. The relationship between the percentage of circulating B cells, corticosteroid levels, and other immunologic parameters in thermally injured patients. J Trauma. 1989;29:208–213. doi: 10.1097/00005373-198902000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Alleyne GA, Young VH. Adrenocortical function in children with severe protein-calorie malnutrition. Clin Sci. 1967;33:189–200. [PubMed] [Google Scholar]
- 23.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, et al. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–2313. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 25.Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies. II. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (DFP). J Clin Invest. 1960;39:1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oehling AG, et al. Suppression of the immune system by oral glucocorticoid therapy in bronchial asthma. Allergy. 1997;52:144–154. doi: 10.1111/j.1398-9995.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 27.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 28.Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Neurol Clin. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Garvy BA, Telford WG, King LE, Fraker PJ. Glucocorticoids and irradiation-induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology. 1993;79:270–277. [PMC free article] [PubMed] [Google Scholar]
- 30.Amsterdam A, Sasson R. The anti-inflammatory action of glucocorticoids is mediated by cell type specific regulation of apoptosis. Mol Cell Endocrinol. 2002;189:1–9. doi: 10.1016/s0303-7207(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 31.Llewellyn-Jones CG, Hill SL, Stockley RA. Effect of fluticasone propionate on neutrophil chemotaxis, superoxide generation, and extracellular proteolytic activity in vitro. Thorax. 1994;49:207–212. doi: 10.1136/thx.49.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirasawa N, et al. Induction of neutrophil infiltration by rat chemotactic cytokine (CINC) and its inhibition by dexamethasone in rats. Inflammation. 1992;16:187–196. doi: 10.1007/BF00918958. [DOI] [PubMed] [Google Scholar]
- 33.Petroni KC, Shen L, Guyre PM. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J Immunol. 1988;140:3467–3472. [PubMed] [Google Scholar]
- 34.Fukushima K, Ando M, Ito K, Suga M, Araki S. Stimulus- and cumulative dose-dependent inhibition of O2- production by polymorphonuclear leukocytes of patients receiving corticosteroids. J Clin Lab Immunol. 1990;33:117–123. [PubMed] [Google Scholar]
- 35.Tsuji C, Shioya S. In vivo effect of methylprednisolone on lipopolysaccharide-induced superoxide production by pulmonary and circulating blood neutrophils in rats. Circ Shock. 1994;42:128–134. [PubMed] [Google Scholar]
- 36.Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250:598–605. [PubMed] [Google Scholar]
- 37.Clark RA, Gallin JI, Fauci AS. Effects of in vivo prednisone on in vitro eosinophil and neutrophil adherence and chemotaxis. Blood. 1979;53:633–641. [PubMed] [Google Scholar]
- 38.Hofmann TG, Hehner SP, Bacher S, Droge W, Schmitz ML. Various glucocorticoids differ in their ability to induce gene expression, apoptosis and to repress NF-kappaB-dependent transcription. FEBS Lett. 1998;441:441–446. doi: 10.1016/s0014-5793(98)01609-3. [DOI] [PubMed] [Google Scholar]
- 39.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukocyte Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 40.Chervenick PA, Boggs DR, Marsh JC, Cartwright GE, Wintrobe MM. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968;215:353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- 41.King LE, Fraker PJ. Zinc deficiency in mice alters myelopoiesis and hematopoiesis. J Nutr. 2002;132:3301–3307. doi: 10.1093/jn/132.11.3301. [DOI] [PubMed] [Google Scholar]
- 42.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.