Abstract
Drugs with poor oral bioavailability usually are administered by hypodermic injection, which causes pain, poor patient compliance, the need for trained personnel, and risk of infectious disease transmission. Transdermal (TD) delivery provides an excellent alternative, but the barrier of skin's outer stratum corneum (SC) prevents delivery of most drugs. Micrometer-scale microneedles (MNs) have been used to pierce animal and human cadaver skin and thereby enable TD delivery of small molecules, proteins, DNA, and vaccines for systemic action. Here, we present a clinical study of MN-enhanced delivery of a medication to humans. Naltrexone (NTX) is a potent mu-opioid receptor antagonist used to treat opiate and alcohol dependence. This hydrophilic and skin-impermeant molecule was delivered from a TD patch to healthy human subjects with and without pretreatment of the skin with MNs. Whereas delivery from a standard NTX TD patch over a 72-h period yielded undetectable drug plasma levels, pretreatment of skin with MNs achieved steady-state plasma concentrations within 2 h of patch application and were maintained for at least 48 h. The MNs and NTX patch were well tolerated with mild systemic and application site side effects. The MN arrays were painless upon administration and not damaged during skin insertion, and no MNs were broken off into the skin. This human proof-of-concept study demonstrates systemic administration of a hydrophilic medication by MN-enhanced TD delivery. These findings set the stage for future human studies of skin-impermeant medications and biopharmaceuticals for clinical applications.
Keywords: naltrexone, stratum corneum, drug delivery, microfabrication
Transdermal (TD) drug delivery has proven to be of great therapeutic utility (1, 2). Patient acceptance of the technology is evident given the commercial success of products intended for chronic pain management, angina and congestive heart failure, and hormone replacement therapies. A TD patch can provide continuous drug administration, minimizing peaks and troughs in plasma levels throughout the day. TD systems can take the place of more risky and invasive injection-based drug delivery, thus improving regimen compliance. Moreover, they are more efficient, use less medication, and are less variable compared with some oral medications that undergo presystemic metabolism.
Even greater utilization of TD delivery for systemic drug administration is inhibited by several key factors. First, the stratum corneum (SC) outer layer of the skin is a very effective barrier at preventing entry of xenobiotics, infectious agents, and other substances into the body. This barrier prevents therapeutic delivery of most drugs other than those with high potency (dose in milligrams or less), low molecular mass (<1,000 Da), and optimal octanol-water partition coefficient (3).
Design of TD product formulations has attempted to overcome some of these limitations and enhance drug permeation (2). Chemical formulation methods may include the use of solvents, surfactants, and other chemical additives to diffuse and partition drug into the skin or to act as a carrier. Chemical enhancers may also disrupt SC structure (4). Prodrug and codrug medicinal chemistry, whereby physicochemical properties are modified through chemical synthesis with additional nontherapeutic or therapeutic substrates, has also been attempted (5, 6).
Physical approaches apply energy to enhance permeation, causing disruption of the SC, generally on a temporary basis (7). Successful methods include iontophoresis for polar molecules, electroporation and sonophoresis, the use of ultrasound, and even microdermabrasion or laser ablation.
Microneedles (MNs) represent a unique technological approach to enhance drug permeation across the SC (8). MN-based delivery involves micrometer-scale solid or hollow needles that painlessly pierce the SC. For example, an array of stainless steel, solid MNs produces a grid of holes, or micropores, through which medications delivered via a standard patch, may be delivered to the skin for local or systemic drug absorption (9). Modern microfabrication techniques have been used to make MNs by methods suitable for inexpensive mass production (10). In addition to solid, stainless-steel arrays, a number of other designs have been created, including hollow needles through which a drug solution may be transported, MNs that have been coated with a drug for dissolution upon application, and polymer MNs that dissolve upon application (11–13).
MNs have been used to pierce the skin and thereby enable TD delivery of small molecules, proteins, DNA, and vaccines for systemic action, as shown in numerous in vitro and animal studies (9, 13–15). Although there have been a number of studies in animals, there is very little scientific literature describing the use of MNs on humans. Kaushik et al. (16) reported that blinded healthy volunteers could not distinguish a MN array from a smooth surface relative to pain sensation. The same subjects could clearly distinguish a MN array from a 26-gauge hypodermic needle. Mikszta et al. (15) compared microarrays to ECG electrode pads for their ability to disrupt human skin, cause pain, or induce inflammation. MNs of 200 μm in length were successful in breaching the SC as measured by transepithelial water loss. Pain perception was negligible and a minor skin irritation lasting to 48-h postapplication was noted in several subjects. Sivamani et al. (17), in a test of localized drug deposition and effect, found greater skin blood flow after injection of 1 μl of methyl nicotinate with MNs as compared with topical methyl nicotinate alone.
To date, there are no published human reports describing MN- enhanced TD delivery of a medication to the systemic circulation to our knowledge. Our article builds on previous preclinical publications and describes a study in which MN arrays were administered to humans in conjunction with TD systemic delivery of a therapeutic molecule. We selected naltrexone (NTX), which is used to block opioid effects in detoxified patients and treat alcohol dependence, as a model drug because it would benefit from TD administration and is difficult to deliver across intact skin (5, 6, 18). NTX is a small, hydrophilic molecule that when administered orally undergoes significant first-pass metabolism with a highly variable 5–40% systemic bioavailability (19). A recently marketed 30-day NTX depot injection provides therapeutic levels for 1 month but with a 10- to 15-fold peak-to-trough variation and in an administration system most patients find objectionable (20). Relatively constant NTX blood levels, and perhaps lower side effects, could be achieved if a TD product was developed that provided a relatively constant delivery rate (21).
The following study was conducted to determine, in an initial human proof-of-concept study, whether water-soluble NTX hydrochloride could be successfully administered systemically from a TD patch after pretreatment of the SC with MNs. As reported by Vereby et al. (22), a 2 ng/ml plasma concentration in humans resulted in an 85.6% narcotic blockade in response to a 25-mg i.v. injection of heroin. A study performed in healthy volunteers who were given a single oral dose of 50 mg NTX showed maximum plasma concentration values ranging from 20 to 25 ng/ml, and after 24 h those levels had dropped to ≈3 ng/ml (23, 24). Plasma levels of NTX are in the 2–4 ng/ml range for days 7–28 of the monthly depot injection regime (20) and could be a target concentration range for this study.
Given the preclinical data on MNs and the known pharmacokinetics of NTX administered by other routes, our hypotheses are that (i) a modest TD NTX dose of ≈10 mg per day will produce steady-state plasma levels of 2 ng/ml when the skin is pretreated with MNs, (ii) control subjects whose skin is not pretreated with MNs will demonstrate no significant systemic absorption of NTX, and (iii) the MNs and NTX TD system will be well tolerated by healthy subjects.
Results
NTX Pharmacokinetics in Humans After TD Administration.
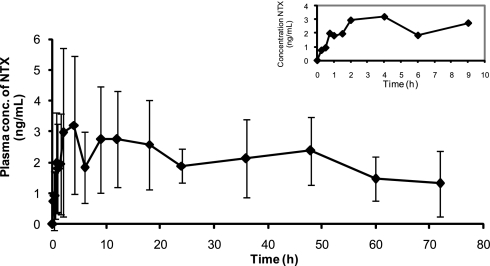
The principal question we wanted to address was whether pretreatment of skin with MNs, and subsequent placement of a TD patch, would permit rapid attainment of pharmacologic, clinically relevant and sustained plasma levels of a hydrophilic molecule not normally absorbed across intact skin. To address this question, our data show that MN pretreatment of the skin permitted rapid systemic exposure to NTX. Measurable plasma levels were demonstrable within 15 min after patch placement in three MN-treated subjects and within 30 min for the remaining three subjects. The concentration–time curve (Fig. 1) shows a rapid rise or burst of absorption within the first several hours of application. Maximum concentrations occurred within a range of 1.5 to 18 h.
Fig. 1.
Mean (SD) NTX plasma concentrations for 72 h of patch application. (Inset) Early sampling points.
Additional questions regarding our technique relate to the length of time the micropores remain open. Would the pore function for several days, permitting relatively constant rates of drug delivery for systemic absorption, or rapidly undergo healing changes that would retard drug absorption? We demonstrate steady-state or a relatively constant plasma concentration of NTX was achieved within hours, to at most 1 day after patch placement, which indicates rapid transit, localized diffusion, equilibrium of NTX through dermal layers, and absorption into capillary beds (Table 1). Approximately zero-order delivery appeared to be achieved for 48 h with a steady-state plasma concentration of ≈2.5 ng/ml, consistent with levels associated with pharmacologic activity. The maximum concentration obtained was somewhat variable and ranged from 1.6 to 8.1 ng/ml. Subjects with the lower concentration profiles tended to have lower peak-to-trough differences over the 72-h administration period. Similarly, the time to maximum concentration had a wide range, from as little as 1–2 h in two subjects, to as long as 18 h in two other subjects.
Table 1.
NTX and NTXOL exposure after MN-enhanced TD delivery
| Parameters | NTX | NTXOL |
|---|---|---|
| Css, ng/ml | 2.5 (1.0) | 0.6 (0.5) |
| Tlag, h | 1.8 (1.1) | 1.4 (1.4) |
| Cmax, ng/ml | 4.5 (2.4) | 1.9 (1.3) |
| Tmax,h | 8.8 (7.6) | 37.5 (31.3) |
| AUC0-t, ng·h/ml | 142.9 (43.9) | 39.7 (25.9) |
| Clast, ng/ml | 1.8 (1.0) | 0.4 (0.6) |
Results are expressed as means ± SD (in parentheses) for six MN-treated subjects. Css = concentration at steady-state condition; Tlag = time to reach steady-state condition; Cmax = maximum concentration achieved; Tmax = time to achieve maximum concentration; AUC0-t = area under the concentration-time curve from time 0 to 72 h; Clast = concentration at time of patch removal after 72 h of application.
Skin micropores appeared to have remained open for at least 48 h as plasma levels appeared to be relatively constant for the first 48 h of administration. Two subjects had a profile suggesting drug permeation up to 72 h. Average plasma levels appeared to be consistent for at least 48 h, with a modest average decline of NTX concentration by ≈50% at 72 h. The 72-h time-point average concentration of 1.8 ng/ml was ≈25% of the maximum concentration and 50% of the steady-state concentration. Pharmacologically active plasma concentrations were still evident at the last time point, 72 h after patch placement. The apparent plasma clearance of NTX indicates an approximate half-life of 4.4 h, suggesting that 95% of NTX would be eliminated within 24 h of patch removal. In contrast to the MN-treated subjects, the control subjects had undetectable (<1 ng/ml) NTX plasma levels, indicating minimal transfer of drug across the skin. Average peak levels in MN-treated subjects were 4.5 times larger than the assay detection limit.
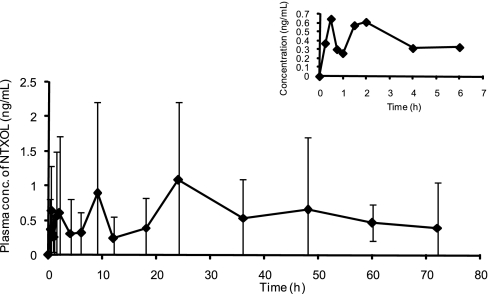
Drug delivery systems that avoid presystemic drug clearance have been demonstrated to remarkably change the metabolite profile of certain medications. Similarly, we wanted to understand whether the TD system would significantly alter, and perhaps even reverse the ratio of the parent drug NTX to the metabolite. Naltrexol (NTXOL; primary metabolite of NTX) plasma concentrations were significantly less than the parent drug NTX (Fig. 2). This finding is consistent with avoiding presystemic first-pass metabolism of NTX. In contrast, NTXOL plasma concentrations are significantly higher than the parent drug NTX after oral administration (24). The reversal of parent-to-metabolite ratio is a desirable outcome, because the NTXOL metabolite is associated with adverse effects.
Fig. 2.
Mean (SD) NTXOL plasma concentrations for 72 h of patch application. (Inset) Early sampling points.
A steady-state concentration of 0.6 ng/ml NTXOL was obtained throughout the 72-h patch placement. The time to reach steady state was comparable to NTX, indicating a slight delay in presentation to the hepatic system for metabolism. Maximum NTXOL concentrations were also modest and were not obtained until an average of 45 h after patch placement. Thus, the MN-facilitated TD delivery of NTX resulted in generation of a much lower quantity of the metabolite (NTXOL) and at a much later time, i.e., 45 h after TD administration using MN versus 1 h after administration of a tablet (19). The study by King et al. (21) suggests our result could lower the side-effect profile of NTX, and improve compliance and retention in treatment, because withdrawal from oral drug treatment is associated with subjects who generate high levels of NTXOL.
Effect of MNs and NTX Patch on Human Skin.
Limited previous studies have been reported on local tolerance of MNs themselves in humans. In our study we wanted to determine the tolerability of the MN arrays combined with a drug formulation and delivery system in humans. Our article demonstrates the tolerability of the combined technologies in humans. MN arrays and patches were easy to administer. Administration of MNs simply required removing their protective liner and pressing the MN against the skin by hand. Application time for MNs and TD patches took 1–2 min, which is quite short, given the nature of the prototype MN and patch systems. MN arrays were examined for physical damage after their application to each subject. No MN array had bent or broken needles, and no needles were broken off into the subjects' skin.
The MN-treated subjects tolerated the MN and patch application system well. Subjects reported no pain when MNs were applied to their skin. The sensation of placement was described as simply of pressure applied at the site. Two subjects reported mild systemic side effects associated with NTX, such as nausea and lethargy, which are believed to be drug-specific and not directly associated with the MN delivery route. There was no clinically significant change in vital signs or liver function tests as a result of TD NTX administration. Control subjects also tolerated the patch system and reported no systemic-related side effects.
Four of the six MN-treated subjects did have skin changes observed when the patch and occlusive dressing were removed after 72 h of application, such as localized irritation and erythema outside of the patch placement site but within the dressing area for two of the subjects. Upon removal of the patch, two of the four subjects demonstrated contact dermatitis that exactly outlined the dimensions of the MN arrays insertion grid. Inside the raised areas were very small crusts that may represent the insertion points of the MNs and the subsequent micropores. The affected subjects were prescribed diphenhydramine capsules (antihistamine) and topical hydrocortisone cream as treatment. Subjects were seen in the clinic 4–6 days later and had responded to treatment. Re-examination of the skin demonstrated the contact dermatitis was greatly diminished and within 1–2 weeks had disappeared. However, the crusts continued healing throughout the 2-week observation period, with a faint outline of the MN array insertion points still evident. Only one control subject demonstrated any finding on skin examination. The subject complained of itchiness and irritation, which was under the occlusive dressing and the patch. The findings disappeared upon removal of the patch.
To better understand the possible causes of skin irritation seen in this study, we carried out an additional study in 10 subjects to assess the effect of just MN insertion followed by occlusion of the skin. No NTX or patch formulation was used. Immediately after insertion, erythema was typically seen at the punctate sites where each MN penetrated into the skin (data not shown). The degree of erythema varied from barely visible to moderate, highly localized, submillimeter spots of redness. Within a few hours, erythema disappeared in most cases, such that it was not possible to distinguish between MN-treated skin and adjacent skin. The more dramatic effects of contact dermatitis observed in the two patients administered NTX were not seen in any of the subjects treated with MNs alone. We therefore conclude that MNs themselves cause little to no skin irritation that is highly transient and that the NTX and/or formulation excipients were responsible for the skin irritation observed in some subjects in this study. Further optimization of patch formulation could reduce or eliminate this irritation.
To better understand the lifetime of TD transport pathways created in the skin by MNs, we carried out a supplemental study in which skin electrical resistance was measured as a function of time after piercing the skin with MNs in 10 human subjects. Skin electrical resistance has been shown to correlate well with skin permeability to various molecules (25). Average skin resistance dropped from 397 ± 183 kΩ before treatment to 11.7 ± 5.5 kΩ after MN insertion and removal. After covering with an occlusive dressing, skin resistance steadily increased but remained significantly less than the resistance of an adjacent control site of untreated skin for 30 h (Student's t test, P < 0.05). These measurements are consistent with the measurements of NTX pharmacokinetics, which both show that brief treatment of skin with MNs creates long-lived permeability. There are quantitative differences between the measurements, however, because the electrical resistance measurement indicated a permeability lifetime of 30 h, whereas the NTX delivery measurement indicated a lifetime up to 72 h. This difference may exist because the electrical measurement assessed the barrier properties of skin's SC, whereas the NTX measurement assessed the kinetics of drug delivery into the bloodstream, which is influenced not only by the SC barrier, but can also be influenced by diffusion, pooling, and possible binding in the skin en route to the bloodstream, which can delay the kinetics of drug clearance.
Discussion
Delivery of NTX from the TD Patch.
This study demonstrates MN pretreatment of the skin and subsequent TD delivery of a drug to humans. Previous research to demonstrate TD transport has been conducted on human cadaver skin and small animals. Studies in humans have focused on the aesthetic nature of avoiding pain upon administration of the MNs or local action in the skin itself. Thus, this work is a significant advancement by combining the MN technology enabling skin permeation with a drug formulation and delivery system for administration of a drug of clinical significance. This report provides the scientific basis and justification for future studies to test MN technology with skin-impermeant medications of different chemistry, such as peptides and proteins, married with a TD drug delivery system.
This proof-of-concept study supports the hypothesis that in vivo insertion of MNs into the skin before placement of a standard TD patch drug delivery system results in pharmacologically active and clinically relevant plasma levels of a skin-impermeant medication. The MN-facilitated TD delivery was very efficient by providing pharmacologically active steady-state plasma levels of 2.5 ng/ml at a very modest estimated dose of 12.6 mg per day. The absorbed TD daily dose is roughly one-quarter of the daily dose administered as an oral tablet to achieve similar plasma levels. This observed increase in efficiency is ascribed to the avoidance of gastrointestinal and hepatic first-pass metabolism.
Moreover, as a result of avoiding first-pass metabolism, the ratio of plasma NTX to NTXOL at steady state was dramatically different from oral delivery. TD delivery using MNs produced a ratio of 4:1, such that most of the drug remained in the parent NTX form. In contrast, the NTX/NTXOL ratio after oral delivery is 1:5, with NTXOL being formed rapidly after gastrointestinal absorption. Moreover, the NTX depot injection also does not achieve the desired metabolic ratio of NTX/NTXOL. The Cmax ratio of NTX to NTXOL is 3:4 on the second day after injection (24). Altering this ratio through constant rate TD delivery could result in an improved side-effect profile because at least one report (25) suggests the commonly observed side effects (nausea, lethargy, dizziness) seen after acute oral administration are associated in subjects with more rapid metabolism and greater relative formation of NTXOL.
Variability in pharmacokinetic parameters across subjects was small after TD delivery using MNs. For comparison, bioavailability of the oral NTX tablet has an 8-fold variation, from 5% to 40%, and similar variability in pharmacokinetic parameters. Rate and extent of NTX exposure was similar across the subjects, with standard deviations being approximately half of the mean, which is a characteristic desirable for drugs and delivery systems. This result is encouraging, given the relatively early proof-of-concept prototype design used in this pilot study.
Of great interest is the observation that most subjects appeared to have an initial burst of NTX into the systemic circulation. The result suggests that there is a loading-dose phenomenon, in which rapid absorption to therapeutic levels takes place initially and then is moderated over the course of the next few days. Subsequent to the burst, steady-state concentrations were achieved in a matter of hours, which is unusually fast for a TD delivery system. For example, conventional clonidine and fentanyl TD delivery systems do not achieve steady-state concentrations for 1–2 days (Catapres TTS and Duragesic Transdermal System product labels; www.fda.gov). The rapid and consistent achievement of steady-state drug concentrations observed in our study provides a pharmacokinetic profile that is ideal for many medication classes.
The observation of a burst of systemically available medication could potentially be explained by the combination of two effects. The first effects concern the relatively hydrophilic nature of NTX. Without the use of MN, or other enhancement techniques, drugs that can cross the skin are very hydrophobic and therefore form a large depot in the hydrophobic environment of the SC (1). The filling of this depot delays drug delivery into the circulation. The use of MNs created hydrophilic micropores across the SC, which bypassed the SC depot and permitted the use of a hydrophilic drug (i.e., NTX) that would not form a depot. This expedited NTX delivery to the circulation.
The second effect is that micropores close over time. Our electrical resistance measurements indicated that skin conductivity steadily decreased with time after MN insertion. NTX plasma levels also were reduced over time, suggesting a slow return of skin barrier properties caused by initiation of the healing process after microinjury to the epidermis. Elastic rebound of the skin back to its original conformation, release of cytokines upon skin piercing, closure of the pore by interstitial fluid proteins, reepitheliazation initially through cell migration and subsequent regeneration, and forming a crust over the pore all may contribute to this resealing process (26, 27).
Tolerability of MNs and NTX Patch.
In general, the subjects tolerated the MN insertion and application of the NTX gel patch. Most subjects had mild erythema underneath the occlusive dressing that was added to further secure the prototype patches for several days and protect them from water. Several subjects also had skin changes under the patch system, in contact with the gel and at the MN insertion sites. These effects were not seen when applying MNs without an NTX patch. Observation of contact dermatitis could be related to the medication, NTX, because opiate structures are known to cause histamine release after local or systemic administration (9). Another possible explanation for local skin irritation is the use of benzyl alcohol as an antimicrobial preservative. Another form of asepsis for the drug product could eliminate this potential irritant.
An outline of the MN insertion site grids, along with punctate crusts over the insertion sites, was observable to varying degrees in most subjects. The observation did not appear to cause distress or discomfort in any subject. Disappearance of the outline and crusts over the next 1–2 weeks likely represented a continued healing process of the skin.
Implications for MN-Assisted TD Patch Delivery.
This proof-of-concept study in humans demonstrated successful TD delivery of NTX, a small hydrophilic molecule. It is likely that other small hydrophilic molecules would be amenable to delivery using methods similar to those in this article. A significant new test would be to demonstrate human delivery of pharmaceuticals with significantly larger molecular weight, such as peptides and proteins, as shown in animals (9, 13–15). Additional challenges may be faced with larger molecules relative to formulation, the function of the skin barrier that may inhibit delivery such as binding of protein drugs to skin constituents, and exposure to proteases. Moreover, preclinical data suggest that TD vaccination via MN-pretreated skin is feasible.
This pilot study has a number of limitations that must be taken into context relative to the results. The systems used are relatively early-stage prototypes as far as TD delivery systems are usually designed. The patch and gel used standard components assembled to approximate pharmaceutical products. However, these data easily translate into preparation of 200-needle MN patches and NTX gel patches to deliver the desired dose in a more practical integrated delivery system. Application of MNs with this device was an imprecise manual administration process. Additional engineering is required to standardize the force necessary to insert MNs to the appropriate depth. Results could be impacted by other factors such as choice of subjects and condition of their skin, anatomical site of MN insertion and patch application, and other well known variables to consider for TD drug administration.
In conclusion, this study reports the systemic delivery of a skin-impermeant medication via MN-facilitated TD delivery in humans. This study supports the significant body of preclinical research in animal and human cadaver skin, and in limited in vivo studies, that MN-enhanced TD delivery is feasible, well tolerated, and pain-free. Methods to enhance standardization of MN insertion and patch formulation are necessary for this application. The results open the possibility of further studies to examine the effect of known variables of TD delivery. Moreover, this study opens the possibility of further research of NTX using a zero-order delivery system in the treatment of various substance abuse disorders.
Materials and Methods
Fabrication and Assembly of Solid MNs.
Using methods described in detail previously, solid MN adhesive patches were fabricated for insertion into the skin (16). Briefly, fixed MN geometries were cut into 75-μm-thick stainless-steel sheets (Trinity Brand Industries; McMaster-Carr) using an infrared laser (Resonetics Maestro) and were then manually bent perpendicular to the plane of their metal substrate. For better insertion and adhesion of patches to the skin, MN arrays were assembled into adhesive patches as described. The adhesive served to hold the MNs firmly against the skin by compensating for the mechanical mismatch between the flexible skin tissue and the rigid MN substrate. The MN patches were assembled in a laminar flow hood for cleanliness and then ethylene oxide-sterilized (AN 74j; Andersen Sterilizers) before use.
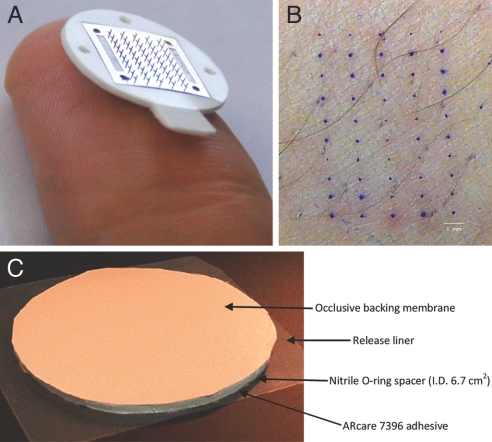
MN arrays were fabricated to produce patches containing 50 MNs arranged in 5 × 10 arrays of MNs (Fig. 3A). Each MN measured 620 μm in length, 160 μm in width at the base, and <1 μm in radius of curvature at the tip. To validate that these MNs pierced into the skin to increase skin permeability, individual arrays were inserted into the forearms of 10 human subjects. After removing the MN patches, the skin was stained with a dye that selectively stains sites of skin barrier perforation. As shown in Fig. 3B, all 50 MNs on the patch inserted into the skin and pierced the skin's SC barrier, as indicated by the 5 × 10 array of dyed spots corresponding exactly to the geometry of the MN array. These 10 subjects were also asked immediately after MN insertion and removal to score the pain on a 0–100 visual analog pain scale. The average pain score was 6 ± 5 for the MN patch and 24 ± 16 for a 5-mm-deep hypodermic needle insertion that served as a positive control. Comparing the ratio of these scores indicates that the subjects felt that insertion of the 50-MN array caused just one-fourth of the pain caused by the hypodermic needle.
Fig. 3.
MN patch for TD delivery. (A) Image of a 50-MN patch resting on the tip of a human thumb. (B) Image of human skin after insertion of a 50-MN patch and staining with gentian violet, a dye that selective stained sites of skin perforation. (C) NTX TD patch and covering.
Dose Estimation for Design of TD Patch.
A method was needed to estimate dosing rate and patch size (area) for the initial human study. An initial estimate was derived by simply using the relationship of K0 = Cl × Css, where K0 = the dosing rate in mg per day, Cl is the total body clearance of the medication in liters/min, and Css is the target plasma concentration at steady state. Assuming a target of 2 ng/ml concentration, and a total body clearance for NTX of 3.5 liters/min, a total daily dose of ≈10 mg was estimated (24).
In preliminary in vitro studies of NTX HCl on human skin treated with MNs, we were able to achieve a steady-state flux of 14.7 ± 4.9 μg/cm2 per h at a patch formula NTX concentration of 160 mg/ml. To make an in vivo correlation for patch size we used the equation: A = Cl × Css /Js, where A is the area of the applied patch and Js is the NTX flux constant. Thus, the patch surface area, at a NTX formulation concentration of 160 mg/ml, and providing 2 ng/ml plasma levels at steady state, was estimated at ≈28 cm2. The daily dose estimate from four commercially accessible 6.7-cm2 patches (26.8 cm2 total) was estimated at 9.5 mg, very close to the previous estimation method.
Preparation of NTX TD Patches.
A 16% NTX hydrochloride (HCl) gel was formulated, prepared, and tested according to current good manufacturing practices as outlined by the Food and Drug Administration. The gel formulation composition (% wt/wt) was NTX HCl [16% U.S. Pharmacopeia (USP)], sterile water for injection (20.25% USP), propylene glycol (60.75% USP), 2% Aerosol (hydroxyethyl cellulose), and 1% benzyl alcohol. The 16% NTX formulation was a clear, colorless gel and had a pH of 4.96 ± 0.03 and a viscosity of 1.69 ± 0.44 × 104 cP.
The TD occlusive protective covering patches of NTX HCl (6.7 cm2) were fabricated by using commercially accessible components by sandwiching a rubber-ringed barrier to create a reservoir between a drug-impermeable backing membrane (Scotchpak 1109 SPAK 1.34 MIL heat-sealable polyester film; 3M) and an Arcare 7396 adhesive (Adhesives Research) around the edge of the rubber spacer (Fig. 3C). The impermeable backing laminate was adhered to the rubber retaining ringed barrier with 3M double-sided tape. Finally, Arcare 7396 was placed on the bottom of the rubber-ringed barrier to maintain intimate contact with the skin and prevent evaporation of the gel formulations. The protective patch was placed on a release liner composed of Scotchpak 9742. The circular NTX patch area slightly exceeded the surface area of MN-treated skin to ensure adequate coverage of the micropores and reduce formulation pooling in square TD patches.
Clinical Study Procedures.
This study was approved by the University of Kentucky Institutional Review Board and carried out in compliance with the ethical and scientific principles governing clinical research as set out in the World Medical Association Declaration of Helsinki. Nine male and female healthy volunteers were medically examined and interviewed to determine appropriateness for study. Inclusion/exclusion criteria to confirm general wellness were compared with data collected through a physical examination, blood sample (chemistry, cell counts, hepatitis), urine sample (urinalysis, pregnancy test if applicable, and drug of abuse screen), and drug and medical history. Subjects could not have a history of opiate use/abuse as indicated by verbal report and a negative urine drug screen or hepatitis. A standardized examination of the patch placement skin site was conducted to confirm normal skin. Subjects with inflammatory diseases of the skin, who had recent sunburn, or other conditions that may cause changes in skin physiology, or who used skin exfoliant dermatologic products or antibacterials were excluded. Subjects were not taking any medications at the time of study with the exception of stable use of oral contraceptives. Subjects were nonsmokers, did not use tobacco products, and agreed to not consume alcoholic beverages during the study.
The six MN-treated and three control subjects were admitted to the inpatient facility of the General Clinical Research Center of the University of Kentucky Hospital. Subject's good health was confirmed by interview and brief examination, and urine drug of abuse screens were repeated. Demographic characteristics of the six MN-treated subjects were average (SD) age of 25.3 (3.2) years, weight of 74 (13.7) kg, and height of 175.9 (7.8) cm, and they were split evenly between males and females. The three control subjects were 23.3 (2.1) years of age, 65.8 (11) kg, 173.9 (7.6) cm, and female.
The morning of NTX gel-patch administration, the subjects had an indwelling i.v. catheter placed in an antecubital or forearm vein contralateral to the arm that received patch placement. Before patch administration, a single blood sample was obtained as baseline, along with vital signs, and a repeat of the standardized skin examination of the test site to document baseline conditions. Six healthy subjects were treated with two 50 MN arrays (100 MN insertions per single patch application site) on the hairless (nonshaved) upper arm before each patch application (four patch sites and 400 MN insertions total per patient). The same procedure, except omission of MN insertion, was used for control subjects. MN insertion simply means placing the MN array over the skin and gently pressing down for a few seconds. The NTX-gel and patch covering were placed over the same area of the skin. An occlusive dressing was placed over the skin site and NTX patches to hold them in place for the study duration.
After patch administration serial blood samples and vital signs were obtained at 15, 30, 45, and 60 min and at 1.5, 2, 4, 6, 9, 12, 18, 24, 36, 48, 60, and 72 h after administration. Subjects were queried, using a standardized instrument, regarding any pain associated with administration and untoward symptoms from the product. An examination of the skin was conducted when the patch was removed and at 4–6 days after administration to detect any irritation, inflammation, discharge, etc., using the standardized skin examination format. A blood chemistry test was repeated to evaluate any gross effects on liver function.
NTX and NTXOL Plasma Assay.
The assay was similar to that published by Valiveti et al. (28). The extraction efficiency was 86.0 ± 6.8% for NTX and 78.0 ± 19.6% for NTXOL. The limits of quantification for NTX and NTXOL were 1.0 and 0.5 ng/ml, respectively, with r2 > 0.95 for both NTX and NTXOL.
Assessment of Skin Irritation and Resealing Kinetics.
An additional study was conducted to measure skin irritation and resealing kinetics. Ten human volunteers (seven males, 23–51 years of age) were recruited from the Georgia Institute of Technology community. The protocol was approved by the Institutional Review Board at Georgia Institute of Technology and informed consent was obtained from the subjects.
A MN patch (Fig. 3A) was inserted into the volar forearm of the subjects and then immediately removed. Treatment sites were occluded by using an occlusive tape (3M Blenderm surgical tape; 3M Healthcare). The occluded sites were further covered with a waterproof dressing (Nexcare absolute waterproof premium adhesive pad; 3M Healthcare) and Saran wrap (SC Johnson), which was secured with waterproof tape (Nexcare absolute waterproof first aid tape; 3M Healthcare).
Skin electrical resistance was measured hourly with an impedance meter (EIM-105 Prep-Check electrode impedance meter; General Devices) modified with a 200-kΩ resistor (Ack Electronics) in parallel. Ag/AgCl dry electrodes (Thought Technology T-3404; Stens) were used as the measurement electrodes for the MN treatment sites. A large electrode with a highly conductive gel (Superior Silver Electrode with PermaGel; Tyco Healthcare Uni-Patch) was used as the reference electrode to keep the impedance contribution of the reference site at a negligibly low value. Resistance measurements were made by connecting lead wires to the reference and measurement electrodes, respectively. Immediately after MN insertion and periodically during the study, skin irritation was assessed by visually examining the MN-treated sites for erythema, edema, and other adverse skin reactions and comparing them with adjacent untreated skin sites.
Data Analysis.
The pharmacokinetic analysis of NTX and NTXOL plasma-concentration versus time profiles after MN treatment and gel patch application was carried out by fitting the data to a noncompartmental model with extravascular input (WinNonlin Professional, version 4.0; Pharsight). The data generated after TD application were analyzed by a noncompartmental method using WinNonlin. The steady-state plasma concentration of NTX after the application of patches was calculated by using the equation Css = AUC0-t/time. Clast was the final plasma concentration measured from the 72-h sampling time point. The flux constant for human skin was calculated as Js = Css × Cl/A. The average daily dose delivered was calculated as K0 = Css × Cl. Statistical analysis of the subject and pharmacokinetic data obtained after the TD application of the patches was performed by one-way ANOVA using SigmaStat. Clinical data were analyzed with SAS.
ACKNOWLEDGMENTS.
We thank the University of Kentucky General Clinical Research Center and the Center for Pharmaceutical Science and Technology at the University of Kentucky College of Pharmacy for support. This research was supported by National Institutes of Health Grants M01RR02602, R01DA13425, R01EB00260, U01AI074579, and R01EB006369 and the University of Kentucky Research Foundation.
Footnotes
Conflict of interest statement: D.P.W., S.L.B., D.A.H., and J.G. have no conflicts to disclose. H.S.G. and M.R.P. are inventors on microneedle-based patents that have been licensed to companies. In addition, M.R.P. is a consultant and advisor to companies working on microneedles. S.L.B. and A.L.S. are inventors on patents related to naltrexone and transdermal delivery.
This article is a PNAS Direct Submission.
References
- 1.Guy RH, Hadgraft J, editors. Transdermal Drug Delivery. New York: Dekker; 2003. [Google Scholar]
- 2.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 3.Hampton T. Breaking barriers in transdermal drug delivery. J Am Med Assoc. 2005;293:2083. doi: 10.1001/jama.293.17.2083. [DOI] [PubMed] [Google Scholar]
- 4.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Vaddi HK, et al. Human skin permeation of branched-chain 3–O-alkyl ester and carbonate prodrugs of naltrexone. Pharm Res. 2005;22:758–765. doi: 10.1007/s11095-005-2592-9. [DOI] [PubMed] [Google Scholar]
- 6.Kiptoo PK, Hamad MO, Crooks PA, Stinchcomb AL. Enhancement of transdermal delivery of 6-beta-naltrexol via a codrug linked to hydroxybupropion. J Control Release. 2006;113:137–145. doi: 10.1016/j.jconrel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Cross SE, Roberts MS. Physical enhancement of transdermal drug application: Is delivery technology keeping up with pharmaceutical development? Curr Drug Deliv. 2004;1:81–92. doi: 10.2174/1567201043480045. [DOI] [PubMed] [Google Scholar]
- 8.Prausnitz MR. Microneedles for transdermal delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 9.McAllister DV, Wang PM, Davis SP. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed ML, Lye W-K. Microsystems for drug and gene delivery. Proc IEEE. 2004;92:56–75. [Google Scholar]
- 11.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Yoshimitsu J, Shiroyama K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14:255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- 13.Gardeniers JGE, Luttge R, Berenschot JW. Silicon micromachined hollow microneedles for transdermal liquid transport. J MEMS. 2003;6:855–862. [Google Scholar]
- 14.Cormier M, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Mikstza JA, et al. Improved gene immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 16.Kaushik S, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 17.Sivamani RK, et al. Clinical microneedle injection of methyl nicotinate: Stratum corneum penetration. Skin Res Technol. 2005;11:152–156. doi: 10.1111/j.1600-0846.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 18.Anton R, O'Malley SS, Ciraulo RA. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE Study. J Am Med Assoc. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 19.American Hospital Formulary Service. Naltrexone Monograph 2102. Bethesda: American Society of Health System Pharmacists; 2004. [Google Scholar]
- 20.Turncliffe RZ, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45:1259–1267. doi: 10.1177/0091270005280199. [DOI] [PubMed] [Google Scholar]
- 21.King AC, Volpicelli JR, Gunduz M. Naltrexone biotransformation and incidence of side effects: A preliminary study. Alcoholism Clin Exp Res. 1997;21:906–909. [PubMed] [Google Scholar]
- 22.Verebey K, Volavka J, Mule SJ, Resnick RB. Clin Pharmacol Ther. 1976;20:315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- 23.Wall ME, Brine DR, Perez-Reyes M. Naltrexone disposition in man after subcutaneous administration. Drug Metab Dispos. 1981;9:369–375. [PubMed] [Google Scholar]
- 24.Licko V. Research Monograph 28. Rockville, MD: National Institute on Drug Abuse; 1980. [Google Scholar]
- 25.Karande P, Jain A, Mitragotri S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. J Control Release. 2006;110:307–313. doi: 10.1016/j.jconrel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112:357–361. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum; 1996. pp. 3–35. [Google Scholar]
- 28.Valiveti S, Nalluri BN, Hammell DC, Paudel KS, Stinchcomb AL. Development and validation of a liquid chromatography-mass spectrometry method for the quantitation of naltrexone and 6beta-naltrexol in guinea pig plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:259–267. doi: 10.1016/j.jchromb.2004.08.016. [DOI] [PubMed] [Google Scholar]