Abstract
Nonsense mutations inactivate gene function and are the underlying cause of a large percentage of the individual cases of many genetic disorders. PTC124 is an orally bioavailable compound that promotes readthrough of premature translation termination codons, suggesting that it may have the potential to treat genetic diseases caused by nonsense mutations. Using a mouse model for cystic fibrosis (CF), we show that s.c. injection or oral administration of PTC124 to Cftr−/− mice expressing a human CFTR-G542X transgene suppressed the G542X nonsense mutation and restored a significant amount of human (h)CFTR protein and function. Translational readthrough of the premature stop codon was demonstrated in this mouse model in two ways. First, immunofluorescence staining showed that PTC124 treatment resulted in the appearance of hCFTR protein at the apical surface of intestinal glands in Cftr−/− hCFTR-G542X mice. In addition, functional assays demonstrated that PTC124 treatment restored 24–29% of the average cAMP-stimulated transepithelial chloride currents observed in wild-type mice. These results indicate that PTC124 can effectively suppress the hCFTR-G542X nonsense mutation in vivo. In light of its oral bioavailability, safety toxicology profile in animal studies, and efficacy with other nonsense alleles, PTC124 has the potential to be an important therapeutic agent for the treatment of inherited diseases caused by nonsense mutations.
Keywords: genetic diseases, readthrough, stop mutations
Cystic fibrosis (CF) is a common autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR gene encodes a cAMP-activated chloride channel that is expressed in epithelial cells of the lung, pancreas, intestine, and male reproductive system (1). Mutations in the CFTR gene frequently lead to severe and chronic respiratory infections that result in respiratory failure and premature death. Although the CFTR-ΔF508 mutation is found in approximately two-thirds of CF patients, ≈10% of CF patients carry an in-frame nonsense mutation that promotes premature termination of translation of the CFTR mRNA (2). Of these mutations, CFTR-G542X is the most common.
Nonsense mutations have been implicated in numerous other inherited diseases and several cancers (3). Given the large number of individuals collectively afflicted by the consequences of these mutations, a therapeutic approach to their suppression could be of considerable benefit to a patient population that has a substantial unmet medical need. Previous studies have demonstrated that aminoglycoside antibiotics have nonsense-suppressing activity. G418 and gentamicin were shown to suppress common CFTR nonsense mutations in cultured human cells (4–6), and gentamicin and amikacin suppressed a human CFTR nonsense allele expressed in a transgenic mouse model for CF (7, 8). Three pilot clinical trials have also provided evidence that gentamicin can suppress nonsense mutations in CF patients (9–11). Despite these promising results, significant limitations are associated with the use of aminoglycoside therapy for nonsense mutations that cause inherited diseases (12, 13).
The need for compounds that can provide safe and convenient suppression of nonsense mutations that cause human diseases recently led to the development of PTC124 (14). This chemical entity, an orally bioavailable 284 d-1,2,4-oxadiazole, induces dose-dependent suppression of premature translational termination without concomitant effects on normal termination or mRNA decay (14). PTC124 treatment elicits little off-target activity, and a comprehensive pharmacological and toxicological analysis found that PTC124 was well tolerated in human subjects at plasma levels in excess of those required for the suppression of premature translational termination in cell culture and in animal models (15).
The ability of PTC124 to restore the in vivo functional activity of genes harboring nonsense mutations was originally tested in mice and humans with mutations in the dystrophin gene (14). To establish firmly that this compound treats the underlying gene expression defect and that PTC124 treatment could be applicable to numerous other nonsense mutation-mediated inherited diseases, it is essential to examine other models of such diseases. Accordingly, in the present work we show that subcutaneous (s.c.) or oral administration of PTC124 suppressed the G542X mutation in a CF mouse model that expressed a human CFTR-G542X transgene in a Cftr−/− background, leading to a significant restoration of CFTR expression and function. These results indicate that PTC124 is not only a chemical agent for the suppression of nonsense mutations that cause CF and Duchenne muscular dystrophy, but that this investigational drug may also have broader clinical applicability for the subset of patients that suffer from other inherited disorders attributable to nonsense mutations.
Results
Subcutaneous Injection of PTC124 Suppresses Nonsense Mutations and Induces hCFTR Expression in Cftr−/− hCFTR-G542X Mice.
We constructed a CF transgenic mouse model that expressed a human CFTR (hCFTR) cDNA containing the G542X premature stop mutation in a mouse line that carried a knockout of the endogenous Cftr locus (referred to hereafter as the Cftr−/− hCFTR-G542X mouse line) (8). Although chronic lung infections are the primary cause of morbidity in CF patients (16), lung pathology is not observed in CF mouse models. Instead, the primary morbidity observed in CF mice is intestinal blockage that leads to high mortality after weaning (17). Because of these differences, we used the compact, intestine-specific rat fatty acid-binding protein (FABP) promoter to drive expression of the CFTR-G542X transgene. This Cftr−/− hCFTR-G542X mouse model was used to show that both gentamicin and amikacin could suppress the hCFTR-G542X mutation and partially restore CFTR protein expression and function (7, 8).
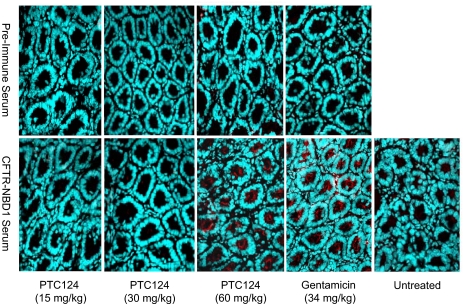
To examine the ability of PTC124 to suppress the hCFTR-G542X mutation in vivo, once daily s.c. injections of PTC124 were administered to Cftr−/− hCFTR-G542X mice at dosages of 60, 30, or 15 mg/kg body weight for 14–21 days. Previous studies with i.p. injection of PTC124 in mdx mice suggested that single doses in this range would lead to plasma levels of the drug that were transiently within the lower range at which suppression was observed (14). The mice were then killed, and intestinal tissues were harvested for immunofluorescence staining to determine whether hCFTR protein could be detected. Earlier work with this mouse model showed that gentamicin or amikacin treatment resulted in the appearance of hCFTR protein primarily at the apical surface of epithelial cells of submucosal glands in the duodenum (7, 8). As shown in Fig. 1, no hCFTR protein was detected in this area (or any other area of the intestine) in samples analyzed with preimmune serum. Similarly, no hCFTR protein was detected in intestinal tissues from untreated Cftr−/− hCFTR-G542X mice with hCFTR-specific antiserum. However, strong hCFTR staining was observed at the apical surface of epithelial cells in submucosal glands from Cftr−/− hCFTR-G542X mice treated with 60 mg/kg PTC124 and, as observed, in tissues from mice treated with 34 mg/kg gentamicin. Much weaker staining was detected in submucosal glands from mice treated with 30 mg/kg PTC124, whereas no signal could be detected in mice treated with 15 mg/kg PTC124. These results indicate that PTC124 can suppress the G542X mutation and partially restore hCFTR protein expression in Cftr−/− hCFTR-G542X mice.
Fig. 1.
hCFTR expression in submucosal glands of intestinal tissues from mice treated with PTC124 by once daily s.c. injections. Samples were prepared from the duodenum of untreated Cftr−/− hCFTR-G542X mice and Cftr−/− hCFTR-G542X mice treated with 15, 30, or 60 mg/kg PTC124. Intestinal tissues from Cftr−/− hCFTR-G542X mice treated with 34 mg/kg gentamicin by the same administration protocol were also examined as a positive control. For immunofluorescence assays, samples were incubated with either preimmune or hCFTR-NBD1 serum. After incubation of the sample with a fluorescent secondary antibody, the samples were visualized by fluorescence microscopy. (Magnification, ×400.)
Subcutaneous Injection of PTC124 Partially Restores cAMP-Stimulated Chloride Channel Activity in Cftr−/− hCFTR-G542X Mice.
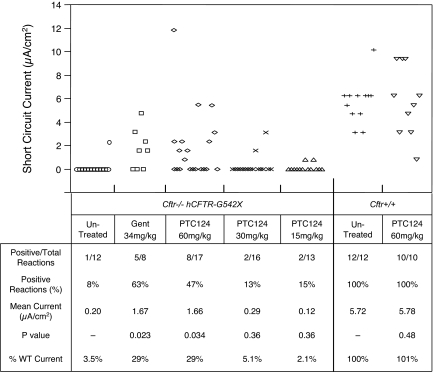
The hCFTR protein is a cAMP-activated chloride channel that facilitates transepithelial chloride conductance. We reported that cAMP-dependent transepithelial chloride conductance appeared in intestinal tissues of Cftr−/− hCFTR-G542X mice after treatment with 34 mg/kg gentamicin (7, 8). Hence, we asked whether the s.c. administration of 60 mg/kg PTC124 could also induce the appearance of cAMP-dependent transepithelial chloride currents in intestinal tissues from these mice. Supporting information (SI) Fig. 7 shows representative short-circuit current tracings obtained from Cftr+/+ mice, untreated Cftr−/− hCFTR-G542X mice, and Cftr−/− hCFTR-G542X mice treated with once daily s.c. injections of either 60 mg/kg PTC124 or 34 mg/kg gentamicin. The intestinal tissues harvested from untreated Cftr−/− hCFTR-G542X mice showed no change in short-circuit currents after the addition of forskolin, a cAMP agonist. In contrast, forskolin addition frequently induced an increase in short-circuit current in intestinal tissues from Cftr−/− hCFTR-G542X mice injected with 60 mg/kg PTC124 or 34 mg/kg gentamicin once a day for 14–21 days.
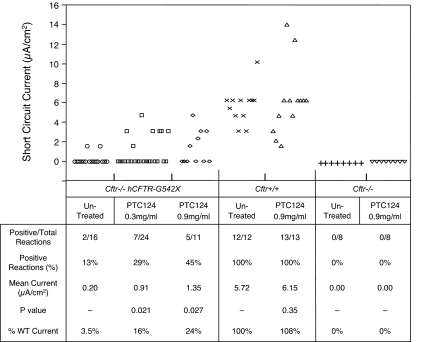
Fig. 2 summarizes the data collected from short-circuit current measurements from intestinal tissues harvested from untreated Cftr−/− hCFTR-G542X mice, Cftr−/− hCFTR-G542X mice treated with three different dosages of PTC124 for 14–21 days, or Cftr−/− hCFTR-G542X mice treated with 34 mg/kg gentamicin for 14–21 days. In all cases, PTC124 or gentamicin was administered once daily by s.c. injection. In untreated Cftr−/− hCFTR-G542X mice, we detected cAMP-stimulated short-circuit currents in only 8% of samples (1 of 12), resulting in an average current of 0.20 μA/cm2. In the Cftr−/− hCFTR-G542X mice treated with 60 mg/kg PTC124, 47% of samples (8 of 17) showed a positive reaction after the addition of forskolin, resulting in an average current of 1.66 μA/cm2. Similarly, Cftr−/− hCFTR-G542X mice treated with 34 mg/kg gentamicin manifested cAMP-stimulated short-circuit currents in 63% of samples (5 of 8), resulting in an average current of 1.67 μA/cm2. These results indicate that the administration of 60 mg/kg PTC124 to Cftr−/− hCFTR-G542X mice once daily by s.c. injection produced a statistically significant increase (P < 0.05) in cAMP-stimulated transepithelial chloride currents in intestinal tissues relative to untreated controls, resulting in 29% of the mean cAMP-stimulated short currents measured in wild-type mice. These results are consistent with our finding that the same dose of PTC124 restored hCFTR protein expression as indicated by immunofluorescence experiments. The s.c. administration of PTC124 at 30 or 15 mg/kg once daily did not show a significant increase in cAMP-stimulated short currents after forskolin addition relative to untreated Cftr−/− hCFTR-G542X mice, indicating that 60 mg/kg PTC124 is the minimal effective dose with this administration protocol. Finally, we also treated Cftr+/+ mice with 60 mg/kg PTC124 by s.c. injection once daily for 14–21 days. We found that 100% of samples (10 of 10) provided positive cAMP-stimulated short-circuit currents, with an average current of 5.78 μA/cm2. Because these values were not significantly different from untreated Cftr+/+ mice, we conclude that the administration of PTC124 does not alter the magnitude of cAMP-stimulated transepithelial chloride currents resulting from the endogenous mouse CFTR protein.
Fig. 2.
Effect of PTC124 s.c. injection on cAMP-stimulated transepithelial chloride currents in intestinal tissues from Cftr−/− hCFTR-G542X and Cftr+/+ mice. (Upper) Individual data points from short-circuit current measurements. (Lower) Analysis of the data. P values comparing all data points from treated and untreated mice were calculated with Student's t test. P < 0.05 was considered significant.
Oral Administration of PTC124 Partially Restores hCFTR Protein Expression and cAMP-Stimulated Chloride Channel Activity in Cftr−/− hCFTR-G542X Mice.
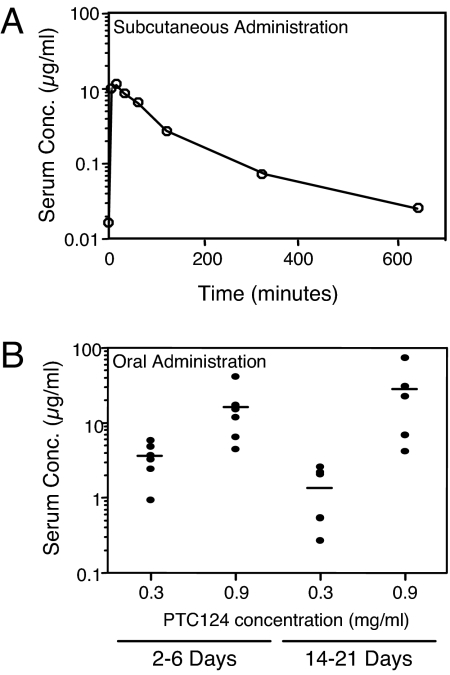
The results above indicated that once daily s.c. injection of 60 mg/kg PTC124 suppressed the G542X mutation in Cftr−/− hCFTR-G542X mice and restored the expression of functional hCFTR protein. To determine serum levels resulting from this administration protocol, age-matched Cftr+/− hCFTR-G542X mice were injected with 60 mg/kg PTC124, and orbital blood was collected at various times after injection (Fig. 3A). We observed a PTC124 peak serum concentration of 10 μg/ml 15 min after injection that dropped to 0.1 μg/ml (a 100-fold decrease) by 5 h (300 min) after injection. Because previous studies with mdx mice demonstrated that optimal nonsense suppression occurred when plasma concentrations of PTC124 were maintained at 5–10 μg/ml (14), these results both provided an explanation for the weak nonsense suppression activity of the 30 and 15 mg/kg doses and suggested that an alternative dosing regimen might prove more effective.
Fig. 3.
Comparison of serum levels of PTC124 after s.c. vs. oral administration. (A) Blood was taken from different age-matched Cftr+/− hCFTR-G542X mice at the indicated times after s.c. injection of 60 mg/kg PTC124. (B) Blood samples were taken after feeding Cftr+/− hCFTR-G542X mice a liquid diet containing 0.3 or 0.9 mg/ml PTC124 for 2–6 days or Cftr−/− hCFTR-G542X mice a liquid diet containing 0.3 or 0.9 mg/ml PTC124 for 14–21 days. The concentration of PTC124 in each serum sample was determined by liquid chromatography/tandem mass spectrometry (for details, see Materials and Methods).
Because of the potential for intestinal blockage in Cftr−/− hCFTR-G542X mice, these animals are routinely fed a liquid complete diet (Peptamen Complete Elemental Diet; Nestlé) upon weaning to promote survival. To identify an oral dose of PTC124 that might provide a therapeutic benefit, the compound was dissolved in the liquid diet at two concentrations (0.3 and 0.9 mg/ml) and given to Cftr+/− hCFTR-G542X mice as the sole source of food and water for 2–6 days. Blood was then collected from six mice treated with each drug concentration, and the serum levels of PTC124 were determined (Fig. 3B, Left). In mice fed the liquid diet with 0.3 mg/ml PTC124, serum levels ranged from 0.9 to 5.9 μg/ml, with an average serum concentration of 3.6 μg/ml. In mice provided the liquid diet with 0.9 mg/ml PTC124, the serum concentration ranged from 4.5 to 42.2 μg/ml, with an average of 16.4 μg/ml.
Because the average serum levels obtained by oral administration with these doses of PTC124 effectively bracketed the peak serum levels obtained immediately after s.c. injection and the optimal dosing established in the mdx studies, Cftr−/− hCFTR-G542X mice were fed the Peptamen liquid diet containing 0.3 or 0.9 mg/ml PTC124 for 14–21 days to determine whether this dosing protocol could lead to effective suppression of the G542X mutation. Once the treatment period was completed, blood was collected from five mice treated with each drug concentration, and the serum levels of PTC124 were again determined (Fig. 3B, Right). In mice fed a liquid diet containing 0.3 mg/ml PTC124, serum levels ranged from 0.3 to 2.2 μg/ml, with an average serum level of 1.4 μg/ml. In mice maintained on a liquid diet with 0.9 mg/ml PTC124, the serum concentration ranged from 4.2 to 74.1 μg/ml, with an average of 28.0 μg/ml. The variability in serum levels of PTC124 observed under each condition was probably caused by the mice consuming different amounts of the liquid diet (and corresponding differences in the amount of PTC124) before serum collection.
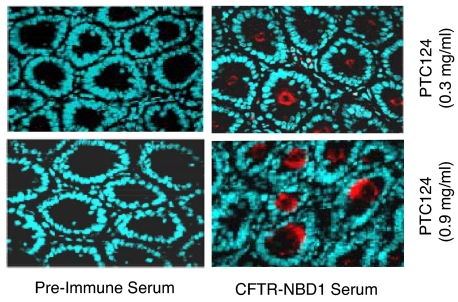
To determine whether hCFTR protein could be detected after oral administration of PTC124, intestinal tissues were harvested and assayed by immunofluorescence with either preimmune serum or an hCFTR-specific polyclonal antiserum (Fig. 4). No hCFTR protein was detected in the intestines of treated Cftr−/− hCFTR-G542X mice with preimmune serum. However, strong hCFTR protein staining was detected at the apical surface of epithelial cells in the submucosal glands from mice treated with either 0.3 or 0.9 mg/ml PTC124 and hCFTR-specific antiserum. These results indicated that oral administration of PTC124 could partially restore hCFTR protein expression in Cftr−/− hCFTR-G542X mice.
Fig. 4.
Immunofluorescence of hCFTR in the submucosal glands of intestinal tissues from mice fed a liquid diet containing PTC124. Samples were from the duodenum of Cftr−/− hCFTR-G542X mice treated with 0.3 or 0.9 mg/ml PTC124 in the Peptamen liquid diet. Samples were incubated with either preimmune or hCFTR-NBD1 serum as indicated. After incubation of the sample with a fluorescent secondary antibody, the samples were visualized by fluorescence microscopy.
We also examined cAMP-activated transepithelial chloride currents in intestinal tissues from mice that were fed the Peptamen liquid diet containing 0.3 mg/ml or 0.9 mg/ml PTC124 for 14–21 days (Fig. 5). We found that intestinal tissues harvested from Cftr−/− hCFTR-G542X mice fed the liquid diet with 0.3 mg/ml PTC124 yielded cAMP-stimulated short-circuit currents in 29% of intestinal tissues assayed (7 of 24), resulting in an average currents of 0.91 μA/cm2. When mice were fed the Peptamen diet containing 0.9 mg/ml PTC124, cAMP-activated short-circuit currents were observed in 45% of intestinal samples (5 of 11), resulting in an average current of 1.35 μA/cm2. These results indicate that both doses of PTC124 resulted in statistically significant increases (P value <0.05) in cAMP-stimulated transepithelial chloride currents relative to untreated controls, consistent with the increased hCFTR protein expression observed in the immunofluorescence assays. The mean cAMP-stimulated short-circuit currents observed in Cftr−/− hCFTR-G542X mice fed the liquid diet with 0.9 mg/ml PTC124 was 24% of the average cAMP-stimulated currents measured in wild-type mice. This level of correction relative to wild-type mice was close to that observed when these mice were administered either 60 mg/ml PTC124 or 34 mg/kg gentamicin by once daily s.c. injection. We also examined the effects of feeding Cftr+/+ mice the Peptamen diet containing 0.9 mg/ml PTC124 for 14–21 days and found that 100% of samples (13 of 13) exhibited positive cAMP-stimulated short-circuit currents, with an average current of 6.15 μA/cm2. This value was not significantly different from the cAMP-stimulated short-circuit currents observed in untreated Cftr+/+ mice (5.72 μA/cm2). Finally, cAMP-stimulated short-circuit currents were not observed in Cftr−/− knockout mice that lacked the hCFTR-G542X transgene, regardless of whether they were untreated or treated with 0.9 mg/ml PTC124. This result confirmed that the CFTR function observed after PTC124 treatment depends on the presence of the hCFTR-G542X transgene, consistent with a role for PTC124 in inducing readthrough of premature translation termination codons.
Fig. 5.
Effect of PTC124 oral administration on cAMP-stimulated transepithelial chloride currents in intestinal tissues from Cftr−/− hCFTR-G542X, Cftr+/+, and Cftr−/− mice. (Upper) Individual data points from short-circuit current measurements. (Lower) Analysis of the data. P values comparing all data points from treated and untreated mice were calculated with Student's t test. P < 0.05 was considered significant.
mRNA Levels from the hCFTR Transgene After PTC124 Administration.
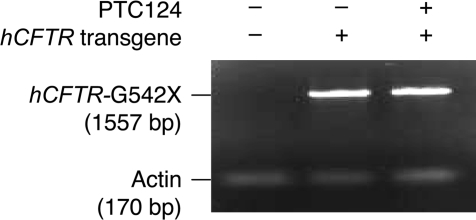
Premature translation termination frequently promotes rapid mRNA destabilization by the process of nonsense-mediated mRNA decay (NMD) (18). Hence, although PTC124 is thought to have a direct effect on nonsense codon readthrough (14), it is formally possible that the ability of the drug to suppress the hCFTR-G542X mutation is attributable to an effect on mRNA stability. However, RT-PCR analysis demonstrated that there were comparable levels of mRNA from the hCFTR-G542X transgene in untreated mice and in mice treated with 0.9 mg/ml PTC124 in the liquid diet (Fig. 6). This result is consistent with the notion that PTC124 selectively promotes translational readthrough of premature nonsense codons without any effect on NMD or other aspects of mRNA stability (14).
Fig. 6.
RT-PCR detection of hCFTR mRNA in untreated Cftr−/− hCFTR-G542X mice and Cftr−/− hCFTR-G542X mice treated with an oral dose of 0.9 mg/ml PTC124.
Discussion
PTC124 is a compound recently shown to be a potential therapeutic for genetic disorders caused by nonsense mutations (14). However, the ability of PTC124 to restore gene function has so far only been shown with dystrophin and LUC reporter nonsense alleles. Here, we have provided additional evidence that PTC124 functions more broadly in promoting nonsense suppression. In this work, we found that the administration of 60 mg/kg PTC124 to Cftr−/− hCFTR-G542X mice by s.c. injection restored 29% of the normal intestinal transepithelial cAMP-stimulated short-circuit currents observed in Cftr+/+ mice. Similarly, feeding these mice a liquid Peptamen diet that contained 0.9 mg/ml PTC124 restored 24% of the normal intestinal transepithelial cAMP-stimulated short-circuit currents observed in Cftr+/+ mice. Although these levels of hCFTR activity were similar to the 29% of cAMP-stimulated transepithelial chloride currents restored by the administration of 34 mg/kg gentamicin, it should be noted that this dose of gentamicin has been shown to produce a peak serum level (≈60 μg/ml) that is well beyond that recommended for human use.
Our data indicate that the human CFTR protein produced by suppression of the nonsense mutation in Cftr−/− hCFTR-G542X mice is located primarily at the epithelial surface of the submucosal glands in the duodenum. In contrast, murine CFTR is normally found at the epithelial surface of the crypts of Lieberkuhn in the colon, ileum, and jejunum. To determine whether the suppressed level of CFTR expression can prevent the frequent intestinal blockage associated with the lack of functional CFTR protein, we are currently making a knockin Cftr-G542X mouse that will have a normal distribution of CFTR expression.
We observed an occasional weak cAMP-stimulated current in intestinal tissues from Cftr−/− hCFTR-G542X mice in the absence of any treatment but not in Cftr−/− knockout mice that lacked the transgene (see Fig. 5). These data suggest that there may be a low level of endogenous readthrough of the premature stop codon in Cftr−/− hCFTR-G542X mice that we occasionally detect because such residual activity would not be observed in Cftr−/− knockout mice that lack the hCFTR-G542X transgene. Previous studies have shown that some nonsense codons (particularly UGA codons like that encoded by the G542X mutation) have a higher basal level of readthrough and are more susceptible to readthrough induced by aminoglycosides than other stop codons (19–21). This finding suggests that such nonsense mutations should not necessarily be considered to be complete “null” alleles in this (or other) animal disease models.
PTC124 has two clear advantages as a potential therapeutic for the suppression of nonsense mutations. First, PTC124 has thus far demonstrated an appropriate safety toxicology profile in animal models and in human subjects (14, 15). Second, because pharmacological suppression of premature stop mutations as a therapy to treat genetic disease will require administration of the compound at regular intervals throughout a patient's life, the oral bioavailability of PTC124 provides another clear advantage over aminoglycosides. The latter are not absorbed through the digestive system and must be administered by intramuscular or s.c. injection.
In conclusion, our results demonstrate that PTC124 induces readthrough of the premature translation termination codon encoded by the hCFTR-G542X nonsense mutation, resulting in the partial restoration of CFTR protein and cAMP-activated chloride currents in the intestines of Cftr−/− hCFTR-G542X transgenic mice. These results begin to demonstrate the potential applicability of this investigational drug to both Duchenne muscular dystrophy and cystic fibrosis and suggest that PTC124 represents an important chemical entity for the potential treatment of genetic disorders caused by nonsense mutations.
Materials and Methods
Transgenic Mice.
The rat FABP promoter was used to drive expression of the hCFTR transgene that contained the G542X (UGA) premature stop mutation. The plasmid construction of the FABP-hCFTR-G542X transgene and generation of the Cftr−/− hCFTR-G542X mouse were described in refs. 7 and 8. Control Cftr+/+ mice were C57BL/6J, whereas control Cftr−/− mice were C57BL/6 Cftrtm1Unc.
Administration Protocols.
Subcutaneous injections with the indicated doses of PTC124 or gentamicin were made in the hind limb of age-matched Cftr−/− hCFTR-G542X mice. Treatments were initiated 16 days after birth (1 week before weaning) and continued once daily until the animals were killed for analysis. Whenever possible, littermates were divided into treatment and control groups. Because of the potential for intestinal blockage in Cftr−/− hCFTR-G542X mice, they were maintained on a liquid diet (Peptamen) after weaning, and other food and water were withheld. Control mice were maintained in a similar manner. This treatment was continued for the indicated time period, and mice were then killed for analysis.
Analysis of PTC124 Levels in Blood.
Orbital blood was obtained from age-matched mice at various times after s.c. or oral administration of PTC124 for the indicated time period. To determine serum levels, PTC124 and an internal standard were extracted from plasma by solid-phase extraction on a hydrophilic–lipophilic balanced support in a 96-well plate format. The extraction plate was washed with organic solvent and then conditioned with water. Samples were loaded, washed with water, and then eluted with methanol. The extracts were analyzed by liquid chromatography/tandem mass spectrometry. Concentrations of PTC124 were determined by a least-squares linear regression with 1/concentration2 as a weighing factor. The standard curve for the analysis ranged from 5 ng/ml to 100 ng/ml for PTC124 based on 50 μl of mouse serum. These values were fixed as the lower and upper limits of quantitation, respectively. Samples were diluted to ensure that the final concentration was within the linear range of quantitation.
Immunofluorescence.
Immunofluorescence experiments were carried out essentially as described in refs. 7 and 8. Immediately after the mice were killed, their intestinal tissue was placed in a cryomold (Miles Laboratories) containing optimum cutting temperature (OCT) embedding medium. Samples were flash-frozen by immersion in a metal cup filled with 2-methylbutane prechilled with liquid nitrogen. Frozen blocks were then sectioned with a cryostat. Five-micrometer sections were collected and fixed in 3% formaldehyde in PBS for 45 min at room temperature. After blocking with 50–100% normal goat serum for 1 h at room temperature to reduce nonspecific antibody binding, samples were incubated with a 1/200 dilution of either the hCFTR polyclonal rabbit antiserum 4562 or preimmune serum for 1 h at 37°C. The samples were washed with four changes of PBS and again blocked with 50–100% normal goat serum for 1 h at room temperature. The samples were then incubated for 1 h at 37°C with 25 μg/ml goat anti-rabbit IgG conjugated to Alexa Fluor-488 (A-11001;Molecular Probes). Finally, samples were washed twice with PBS and incubated with 20 μg/ml Hoechst 33258 for 4 min at room temperature. This CFTR-specific antiserum was raised against an antigen that included hCFTR-NBD1 and a portion of the R domain (hCFTR amino acids 521–828) fused to the Escherichia coli TrpE protein (22).
Short-Circuit Current Measurements.
Four intestinal tissue segments of ≈5 mm in length (one from duodenum, two from jejunum, and one from ileum) were placed in a PBS solution containing tetrodotoxin (3.3 × 10−4 μM) for at least 10 min to block sodium channels activated by action potentials. The intestinal segments were mounted as a flat sheet in a modified Ussing chamber (area of ≈0.16 cm2), and short-circuit recordings were made as described (7, 8, 23). The mucosal bathing solution (37°C, pH 7.4) contained 167.2 mM Na+, 5 mM K+, 6 mM Cl−, 1.2 mM Ca2+, 1.2 mM Mg2+, 25 mM HCO3−, 4.2 mM PO4, and 10.8 mM d-glucose. The serosal surface of the tissue was bathed in a Ringer's solution (37°C, pH 7.4) containing 145 mM Na+, 5 mM K+, 124.8 mM Cl−, 1.2 mM Ca2+, 1.2 mM Mg2+, 25 mM HCO3−, 4.2 mM PO42−, and 10 mM d-glucose. Both the mucosal and serosal solutions were constantly circulated by bubbling 95% O2/5% CO2 through the solutions. Forskolin (10 μm) was added to both the mucosal and serosal solutions for at least 10 min while the short-circuit current was continuously monitored. In all experiments, the current measurements obtained immediately before and 10 min after forskolin addition were used to calculate the current change in each sample. See also SI Materials and Methods.
Detection of hCFTR mRNA Expression.
To monitor expression of the hCFTR-G542X transgene, mRNA was isolated from intestinal tissues of untreated Cftr−/− hCFTR-G542X mice and Cftr−/− hCFTR-G542X mice treated with 0.9 mg/ml PTC124 in the liquid diet for 17 days. RT-PCR analysis of hCFTR-G542X mRNA was done by using a SuperScript III one-step RT-PCR system with platinum Taq polymerase (Invitrogen). The thermal cycler program (Applied Biosystems 9700) included a cDNA synthesis step (52°C for 30 min), a denaturation step (95°C for 5 min), 35 cycles for PCR amplification (95°C for 0.5 min, 60°C for 1 min, 72°C for 1.3 min), and a final extension step (68°C for 7 min). The primers used to amplify a 1,557-bp fragment from the hCFTR-G542X mRNA were DB985 (5′-CAAGATAGAA AGAGGACAGT TGTT-3′) and DB986 (5′-TTGAGGGTTG ACATAGGTGC TTGAA-3′). The primers used to amplify a 170-bp actin fragment were DB2783 (5′-CTTCTGCATC CTGTCAGCAA T-3′) and 2784 (5′-GAGGCTCTTT TCCAGCCTTC C-3′).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Kim Keeling for helpful discussions and for critically reading the manuscript, Allan Jacobson for helpful discussions and assistance in all phases of this study, Shirley Yeh for technical assistance, and the University of Alabam at Birmingham Cystic Fibrosis Research Center for the use of the CFTR assay core facility. This work was supported by National Institutes of Health Grant P50 DK53090.
Footnotes
Conflict of interest statement: E.M.W., S.H., and S.W.P. are employees of PTC Therapeutics, Inc. and D.M.B. is a consultant for PTC Therapeutics, Inc. No other authors have a conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711795105/DC1.
References
- 1.Riordan JR, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Genetic Analysis Consortium. Population variation of common cystic fibrosis mutations. Hum Mutat. 1994;4:167–177. doi: 10.1002/humu.1380040302. [DOI] [PubMed] [Google Scholar]
- 3.Culbertson MR. RNA surveillance: Unforeseen consequences for gene expression, inherited genetic disorders, and cancer. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 4.Zsembery A, et al. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3− secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95–104. doi: 10.1053/jhep.2002.30423. [DOI] [PubMed] [Google Scholar]
- 5.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2:467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 6.Bedwell DM, et al. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat Med. 1997;3:1280–1284. doi: 10.1038/nm1197-1280. [DOI] [PubMed] [Google Scholar]
- 7.Du M, et al. Clinical doses of amikacin provide more effective suppression of the human CFTR-G542X stop mutation than gentamicin in a transgenic CF mouse model. J Mol Med. 2006;84:573–582. doi: 10.1007/s00109-006-0045-5. [DOI] [PubMed] [Google Scholar]
- 8.Du M, et al. Aminoglycoside suppression of a premature stop mutation in a Cftr−/− mouse carrying a human CFTR-G542X transgene. J Mol Med. 2002;80:595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilschanski M, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med. 2003;349:1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 10.Wilschanski M, et al. A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am J Respir Crit Care Med. 2000;161:860–865. doi: 10.1164/ajrccm.161.3.9904116. [DOI] [PubMed] [Google Scholar]
- 11.Clancy JP, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;163:1683–1692. doi: 10.1164/ajrccm.163.7.2004001. [DOI] [PubMed] [Google Scholar]
- 12.Guan MX, Fischel-Ghodsian N, Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum Mol Genet. 2000;9:1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- 13.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 15.Hirawat S, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, after single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47:430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 16.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 17.Snouwaert JN, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 18.Amrani N, Sachs MS, Jacobson A. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol. 2006;7:415–425. doi: 10.1038/nrm1942. [DOI] [PubMed] [Google Scholar]
- 19.Bonetti B, Fu L, Moon J, Bedwell DM. The efficiency of translation termination is determined by a synergistic interplay between upstream and downstream sequences in Saccharomyces cerevisiae. J Mol Biol. 1995;251:334–345. doi: 10.1006/jmbi.1995.0438. [DOI] [PubMed] [Google Scholar]
- 20.Keeling KM, Bedwell DM. Clinically relevant aminoglycosides can suppress disease-associated premature stop mutations in the IDUA and P53 cDNAs in a mammalian translation system. J Mol Med. 2002;80:367–376. doi: 10.1007/s00109-001-0317-z. [DOI] [PubMed] [Google Scholar]
- 21.Manuvakhova M, Keeling K, Bedwell DM. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA. 2000;6:1044–1055. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koerner TJ, Hill JE, Myers AM, Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- 23.Dawson DC. Na and Cl transport across the isolated turtle colon: Parallel pathways for transmural ion movement. J Membr Biol. 1977;37:213–233. doi: 10.1007/BF01940933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.