Abstract
The vacuolated lens (vl) mouse mutant causes congenital cataracts and neural tube defects (NTDs), with the NTDs being caused by abnormal neural fold apposition and fusion. Our positional cloning of vl indicates these phenotypes result from a deletion mutation in an uncharacterized orphan G protein-coupled receptor (GPCR), Gpr161. Gpr161 displays restricted expression to the lateral neural folds, developing lens, retina, limb, and CNS. Characterization of the vl mutation indicates that C-terminal tail of Gpr161 is truncated, leading to multiple effects on the protein, including reduced receptor-mediated endocytosis. We have also mapped three modifier quantitative trait loci (QTL) that affect the incidence of either the vl cataract or NTD phenotypes. Bioinformatic, sequence, genetic, and functional data have determined that Foxe3, a key regulator of lens development, is a gene responsible for the vl cataract-modifying phenotype. These studies have extended our understanding of the vl locus in three significant ways. One, the cloning of the vl locus has identified a previously uncharacterized GPCR-ligand pathway necessary for neural fold fusion and lens development, providing insight into the molecular regulation of these developmental processes. Two, our QTL analysis has established vl as a mouse model for studying the multigenic basis of NTDs and cataracts. Three, we have identified Foxe3 as a genetic modifier that interacts with Gpr161 to regulate lens development.
Keywords: cataracts, Foxe3, spina bifida
Cataract and neural tube defects (NTDs) are two common human disorders. Both have a multifactorial basis with genetics and environment contributing to increased risk (1–3). Age-related cataract affects ≈20.5 million Americans over the age of 40, whereas cataract is the leading cause of childhood blindness worldwide (4, 5). NTDs affect the formation of the neural tube during neurulation and are the second most common human birth defect, occurring in ≈1/1,000 American Caucasian live births (1). Mouse mutants have been useful tools for studying human disease, but few of the >300 NTDs and cataract mutations model the multifactorial basis of these human diseases.
The vacuolated lens (vl) mutation arose spontaneously on the C3H/HeSnJ background, and vl/vl displays both congenital cataracts and NTDs. Vacuoles in the lens at birth have been described by Dickie (6), but no embryological assessment of the developing lens has been reported. Later studies discovered that vl mutant embryos exhibit two different neural tube phenotypes (7, 8). Approximately 50% of vl/vl embryos display lumbar-sacral spina bifida. In the other vl/vl embryos, the neural tube closes; however, dorsal phenotypes are observed, including a thinning of the midline neuroepithelium and epidermis, dilation of the dorsal ventricle, and the presence of ectopic neuroepithelial cells in the ventricle. All of these phenotypes have also been documented in a closed human NTD called embryonic hydromyelia (9). The vl mutation results in lethality in ≈50% of the vl/vl. The cause of the lethality is unknown, but because mouse NTD mutants typically die before birth (10, 11), one likely possibility is the lumbar-sacral spina bifida. All surviving adult vl/vl mice display congenital cataracts and do not exhibit any obvious signs of spina bifida.
The neurulation phenotypes of vl embryos have been extensively studied by Wilson and Wyatt (7, 8, 12–14). Histological assessment of the dorsal midline phenotypes, ultrastructural EM studies of the neural folds, and cultures of mutant embryos indicate that the vl mutation affects the last step of neurulation, apposition, and fusion of the neural folds. The molecular regulation of neural fold fusion is not well understood. One reason is that mouse mutants defective at this last step of neurulation have been difficult to identify because unfused neural folds rapidly splay apart, which mimics defects in the elevation of the neural plate (10). Although there are >200 mouse mutants that affect neural tube closure (www.jax.org), vl is currently one of three mouse mutants considered to be defective in neural fold fusion (11). Thus the vl mutant provides a unique opportunity for studying this final stage of neurulation.
G protein-coupled receptors (GPCRs) constitute a large superfamily of proteins that are commonly used by cells to sense and respond to their environment. There are >360 nonsensory GPCRs in the human genome. The ligand for ≈200 of these receptors have been identified, whereas the remaining 160 receptors are orphan GPCRs because their endogenous ligands are not known (15). The binding of ligands to GPCRs activates cytoplasmic G proteins, allowing the receptors to transduce extracellular signals across the plasma membrane into the cell. These heterotrimeric G proteins then regulate the cellular response to the extracellular signal through numerous second-messenger cascades. Attenuation of GPCR signaling is also important and is achieved by phosphorylation of the receptor, which results in either a conformational change that affects G protein binding or reduced cell surface expression through receptor-mediated endocytosis (15, 16).
Here, we report that the vl phenotypes are caused by a mutation in an orphan GPCR called Gpr161. We also describe Gpr161 expression during early embryonic development, the effect of the vl mutation on Gpr161 expression and subcellular localization, the mapping of three modifier loci that affect the penetrance of the vl NTD and cataract phenotypes, and the identification of Foxe3 as a gene responsible for the vl cataract-modifying effect.
Results
Positional Cloning of the vl Locus.
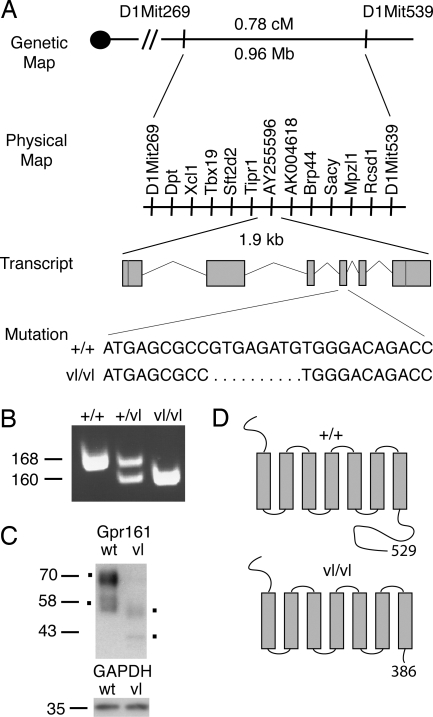
A single allele of the vl locus arose spontaneously on the C3H/HeSnJ background. In a previous C57BL/6J intercross the vl locus was mapped to a 5-cM region on distal mouse chromosome 1 (17). To fine-map the vl locus and identify the gene responsible for the vl phenotypes, we performed a MOLF/Ei intersubspecific intercross. A total of 854 F2 mice were genotyped for simple sequence length polymorphism (SSLP) markers located in the vl region. Three informative recombinants of 193 F2 vl/vl mice were identified, which localized the vl critical interval to a 0.96-Mb region between markers D1Mit269 and D1Mit539 (Fig. 1A). A separate CAST/Ei intercross with 58 vl/vl F2 mice confirmed this map position.
Fig. 1.
Positional cloning of the vl mutation. (A) To clone the gene responsible for the vl mutation, MOLF/Ei and CAST/Ei intercrosses were performed. Recombinant mapping delimited the vl critical interval to 0.78 cM or 0.96 Mb. Genes within this interval are illustrated and were sequenced in +/+ and vl/vl C3H/HeSnJ to identify the 8-bp deletion in exon 4 of Gpr161. The genomic structure of Gpr161 transcript is depicted with red lines representing the position of start and stop codons. The nucleotide sequence surrounding the vl mutation is shown. (B) PCR across the deletion reveals a 160-bp amplicon in vl/vl compared with a 168-bp amplicon in +/+ and both amplicons in +/vl. (C) Western analysis for N-terminal myc tagged WT and vlGpr161 is shown and demonstrates the predicted smaller size for vlGpr161. vlGpr161 levels are also reduced compared with GAPDH. (D) Schematic representation of wt and vlGpr161 protein with the predicted site of the C-terminal tail truncation illustrated.
Inspection of the mouse genome identified 11 transcripts, nine previously annotated genes and two spliced ESTs, in the vl critical region (Fig. 1A). The coding region of all 11 transcripts in the vl critical interval was sequenced from +/+ C3H/HeSnJ and vl/vl DNA to identify mutations. An 8-bp exonic deletion was identified in EST AY255596 in vl/vl but not +/+ DNA (data not shown). PCR across the deletion generated a smaller amplicon, confirming the presence of the mutation (Fig. 1B). The 8-bp deletion was not observed in 19 additional inbred mouse strains, including all C3H substrains (see Materials and Methods), consistent with this deletion not being a polymorphism. Sequence and expression analysis of other transcripts in the vl critical interval did not identify any changes that were consistent with these genes encoding the vl locus (data not shown and see Materials and Methods). In addition, the 8-bp deletion segregated with the vl phenotypes in >1,000 meioses in +/vl × +/vl and vl/vl × +/vl matings in our C3H/HeSnJ colony, where no +/+ or +/vl mice displayed a NTD or cataracts phenotype and all phenotypically mutant embryos and postnatal mice were homozygous for the deletion (data not shown). These genetic data provide evidence that the 8-bp deletion in EST AY255596 is the mutation responsible for the vl phenotypes.
EST AY255596 is a partial EST that contains coding sequence for a putative orphan GPCR. Inspection of the mouse genome identified two sets of nonoverlapping ESTs (genome.ucsc.edu). RT-PCR using embryonic day (E) 8.5 cDNA and primers to the 5′ portion and 3′ portion of each set of ESTs generated a 1.9-kb product, which was then sequenced. Blastn and Blastp homology searches with our full-length mouse cDNA and predicted protein sequence demonstrated that the cloned gene was 87% and 92% identical on a nucleotide and amino acid level to a human orphan GPCR, GPR161. These results indicate that a previously uncharacterized orphan GPCR, Gpr161, is the gene responsible for the vl mutant phenotypes.
BLAT searches with the Gpr161 cDNA sequence demonstrated the transcript is encoded by six exons (genome.ucsc.edu) (Fig. 1A). The predicted ORF is 1,587 nt or 529 aa in length with an estimated molecular mass of 58.5 kDa (ca.expasy.org). The vl mutation (8-bp deletion) is located in exon 4 of the transcript and is expected to cause a frameshift and premature stop codon 50 nt 3′ of the deletion. The deletion in the incorporation of 16 novel amino acids (GAHGRRTVPGTQQQHR) and truncation of the GPCR at residue 386, deleting 143 (of 203) amino acids of the C-terminal tail (Fig. 1D). The mutant protein is predicted to be ≈15 kDa smaller than WT Gpr161 (ca.expasy.org). Western analysis performed on lysates isolated from cells transfected with N-terminal myc-tagged WT and mutant (vl) Gpr161 identified two major isoforms, with the smallest band migrating at the predicted size (≈58 kDa) and a larger band at ≈70 kDa (Fig. 1C). This larger band likely represents a modified version of Gpr161 (ca.expasy.org; McVector version 9.0). The mutation reduces both protein products by ≈15 kDa, consistent with the mutation truncating the C-terminal tail. Reduced steady-state levels of the mutant isoforms were also observed (Fig. 1C).
Gpr161 Expression Analysis.
We next investigated the developmental expression pattern of Gpr161. RT-PCR demonstrated that Gpr161 was expressed from E8.5 to E11.5 [supporting information (SI) Fig. 5A]. In situ hybridization (ISH)-determined Gpr161 expression was restricted to the lateral neural folds of the neural plate along the A-P axis (E8.0-E9.5) (Fig. 2 A and B and SI Fig. 5 B and C), consistent with vl affecting neural fold fusion (7, 8, 12–14). Gpr161 is expressed at all examined stages of lens development: lens pit (E10.5), lens vesicle (E11.5), primary lens fiber cells (E12.5), and differentiating secondary lens fiber cells (E14.5). At E12.5 and E14.5, Gpr161 transcripts are restricted to differentiating lens fiber cells and are absent from the proliferating anterior lens epithelium. Gpr161 expression is highest at the lens pit stage and differentiating secondary lens fiber cells and is weakly expressed in the lens vesicle and primary lens fiber cells (Fig. 2 C–E and SI Fig. 5 D and E). Gpr161 is also expressed in a number of other structures from E9.5 to E12.5, including the ventricular zone of the developing CNS (E9.5-E11.5), the fore and hindlimbs (E12.5), and the retina (E10.5-E14.5), suggesting a role for Gpr161 in their development (Fig. 2 C–G and SI Fig. 5 D–G; data not shown). Finally, RT-PCR and ISH (E9.5) demonstrated no difference in Gpr161 expression in vl/vl embryos (data not shown).
Fig. 2.
Gpr161 expression. (A and B) Whole-mount and section ISHs for Gpr161 demonstrate restricted expression to the lateral neural folds of the neural plate (arrow). An E8.0 dorsal view at the mid-hindbrain region and the posterior E9.5 tail bud region is shown in A and B, respectively. (C–E) Gpr161 is expressed in the developing lens: lens pit (C) (E10.5), lens vesicle (D) (E11.5), and primary lens fiber cells (E) (E12.5). At E12.5 Gpr161 is not expressed in the developing cornea (arrowhead) or proliferating anterior lens epithelium (arrow). Gpr161 is also expressed in the developing retina from E10.5 to E12.5 (C and D and data not shown). (F and G) Whole-mount and section ISHs demonstrating Gpr161 expression at E11.5 and in the forelimb at E12.5. lf, primary lens fiber cells; lp, lens pit; lv, lens vesicle; r, retina. (Magnification: A, ×6.3; B, ×40; C–E, ×20; F, ×2; G, ×10.)
Vl Mutation Affects Gpr161 Receptor-Mediated Endocytosis.
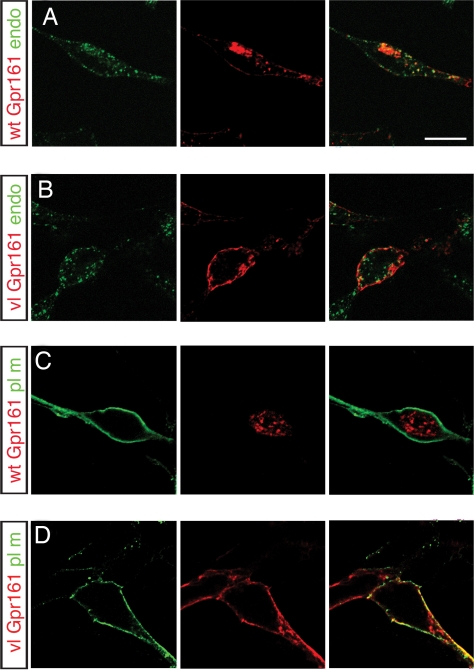
Receptor-mediated endocytosis is a common mechanism by which GPCR signaling is attenuated and is regulated by C-terminal tail phosphorylation (15, 16, 18, 19). To investigate the effects of the mutation on Gpr161 plasma membrane targeting and intracellular localization, WT and vl-myc-Gpr161 constructs were transfected into HEK293T cells. To distinguish cell surface versus intracellular receptors, these studies were performed under nonpermeabilized and permeabilized conditions. In nonpermeabilized cells, both WT and vlGpr161 were targeted to the plasma membrane. In permeabilized cells, a different staining pattern was detected with wtGpr161 displaying an intracellular puctate pattern and vlGpr161 localized to the cell surface (SI Fig. 6). To characterize this difference further, WT and vlGpr161 plasmids were either cotransfected with expression constructs that target GFP to different subcellular compartments [plasma membrane, endoplasmic reticulum (ER), nucleus] or the transfected cells were incubated with FITC-labeled transferrin, an endosome marker. For wtGpr161 significant colocalization with transferrin was observed, indicating that Gpr161 is present in endosomes. Minimal colocalization was observed with GFP targeted to the plasma membrane (Fig. 3 and SI Fig. 7), whereas no overlap with ER or nuclear-GFP was observed (data not shown). For vlGpr161, colocalization was detected for plasma membrane targeted GFP but not with FITC-transferrin (Fig. 3 and SI Fig. 7), ER-GFP, or nuclear-GFP (data not shown). These results demonstrate that vl affects Gpr161 intracellular localization and are consistent with vl disrupting Gpr161 internalization from the plasma membrane into the endosome compartment.
Fig. 3.
vl subcellular localization. Double labeling for WT (A and C) and vlGpr161 (B and D) with either the endosome marker, FITC-transferrin (A and B), or plasma membrane-targeted GFP (C and D) was performed for permeabilized transiently transfected HEK293T cells. wtGpr161 is localized to the endosome compartment, whereas vlGpr161 remains on the plasma membrane, consistent with the C-terminal tail truncation affecting receptor-mediated endocytosis of the Gpr161. Confocal microscopy of ≈0.5-μm optical sections through transfected cells is shown. (Scale bar: 10 μm.)
Vl Quantitative Trait Locus (QTL) Analysis.
Human NTDs and cataracts are multigenic disorders (1–3). Our intercrosses demonstrated that genetic background significantly affected the penetrance of the vl NTD and cataract phenotypes. On the B6/C3H and CAST/C3H backgrounds, ≈50% of adult F2 vl/vl mice display spina bifida and hindlimb paralysis (B6/C3H: 57/105; CAST/C3H: 42/94), which is never observed on the C3H background. Humans with spina bifida consistently display these phenotypes but to our knowledge are not observed in any other mouse NTD mutant (10, 11, 20), establishing vl/vl B6 and CAST/C3H mice as an important mouse model for studying human spina bifida and associated abnormalities. On the MOLF/C3H background adult vl/vl mice with spina bifida are not observed but the incidence of cataracts is decreased by 85% (19/126 F2 vl/vl).
Genome scans using 60–80 SSLP markers spaced evenly throughout the genome were performed on F2 progeny from all three crosses. QTL analysis identified three modifiers of the vl phenotypes (Modvl1–3) (Table 1). For the B6 cross when the spina bifida phenotype was used as the covariate, one significant QTL (Modvl1) was identified (SI Fig. 8). Modvl1 maps to chromosome 5 [44 cM, logarithm of odds (LOD) 3.7], and the allele effect at the peak marker, D5Mit509, demonstrated that the C3H background contributed to the spina bifida phenotype in a dominant fashion. For the CAST cross, when spina bifida was used as a covariate, Modvl2 was mapped to chromosome 1 (26 cM, LOD 3.2). The C3H allele of Modvl2 contributed to the spina bifida phenotype in an additive fashion (SI Fig. 9). Modvl1 and Modvl2 account for 15.9% and 13.1% of F2 phenotypic variance in their respective crosses.
Table 1.
Summary of modifier loci for the vl mutation (Modvl)
| Cross | QTL | Chromosome (cM) | Peak marker | LOD | 95% C.I., cM | Phenotype | High allele | Mode of inheritance |
|---|---|---|---|---|---|---|---|---|
| B6 | Modvl1 | 5 (44) | D5Mit309 | 3.7 | 38–50 | Spina bifida | C3H | Dominant |
| CAST/Ei | Modvl2 | 1 (26) | D1Mit236 | 3.3 | 0–36 | Spina bifida | C3H | Additive |
| MOLF/Ei | Modvl3 | 4 (51) | D4Mit168 | 4.2 | 45–61 | Cataract | MOLF | Additive |
For the MOLF cross, a cataract modifier, Modvl3, was mapped to chromosome 4 (51 cM, LOD 4.2). Even though our vl MOLF intercross reduced the penetrance of cataract, the allele effect for Modvl3 demonstrated that the MOLF background contributed to the cataract phenotype in an additive fashion. This finding is consistent with Modvl3 and the vl mutation being responsible for cataracts on the mixed C3H/MOLF background. Other unidentified MOLF modifiers likely reduce the penetrance of the cataracts phenotype but these were not mapped because of their heterogeneity or low penetrance. Modvl3 accounts for 9.9% of the F2 phenotypic variance (SI Fig. 10). These data demonstrate that the penetrance of the vl spina bifida and cataract phenotypes are influenced by unlinked modifiers, establishing vl as a mouse model for studying the multigenic inheritance of these disease phenotypes.
Foxe3 and Modvl3 Cataract-Modifying Phenotype.
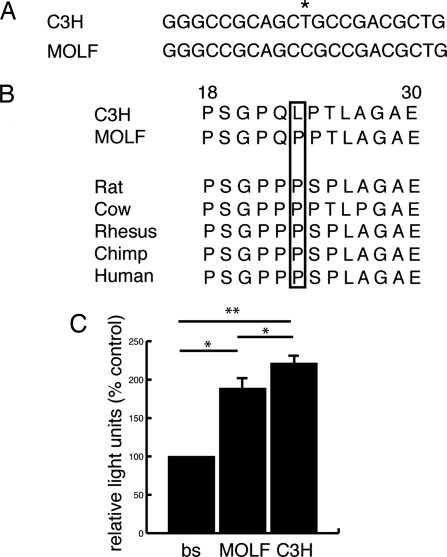
To identify candidate genes that may contribute to the modifying effects of Modvl1–3, the 95% C.I. of Modvl1–3 were scanned for biologically relevant candidates based on expression and disease phenotypes. This analysis identified Foxe3 as a biologically relevant candidate for Modvl3. Foxe3 is a winged helix forkhead transcription factor expressed in the developing lens and when mutated causes cataract and other lens-associated diseases in humans and mice. Knockdown of Foxe3 in zebrafish also leads to a lens phenotype (21–25). Foxe3C3H and FoxeMOLF were sequenced and two unique SNPs (mouse dbSNP build 127) were identified: a T-to-C transition at base pair 68 and an A-to-C transversion at base pair 499. The transversion does not result in an amino acid change, whereas the TC3H to CMOLF transition replaces a leucine (LC3H) with a proline (PMOLF) at amino acid 23 in the N terminus of the protein (Fig. 4A and data not shown).
Fig. 4.
Foxe3C3H and FoxeMOLF allelic differences. (A) The TC3H-to-CMOLF SNP at base pair 68 (*) is shown with flanking sequence. (B) The TC3H-to-CMOLF transition replaces a leucine (LC3H) with a proline (PMOLF) at amino acid 23 in the N terminus of the protein. This amino acid change plus flanking amino acids (18–30 in mouse) is shown for C3H, MOLF, and other vertebrate species. The P23 allele (boxed) is evolutionarily conserved from MOLF to humans. (C) Transient cotransfection assays revealed a functional difference in transcriptional activity between Foxe3C3H and Foxe3MOLF. Relative light units as percent control is shown for the Foxe3 binding site-luc construct transfected individually (bs), with Foxe3MOLF (MOLF) or Foxe3C3H (C3H). *, P < 0.02; **, P < 0.007; one tailed paired Student's t test; n = 3.
This region of Foxe3 was then sequenced in 22 other mouse strains. Eighteen of the strains had the A allele (L23) at base pair 68, whereas only four had a C allele (P23) (SI Fig. 11A). Although P23 is not commonly observed in different mouse strains it is evolutionarily conserved in rat, cow, rhesus, chimp, and human (Fig. 4B). Given that proline commonly disrupts protein secondary structure, bioinformatics for Foxe3C3H and Foxe3MOLF were performed. For Foxe3C3H a β-sheet is predicted to extend from amino acids 22–28 followed by an α-helix from amino acids 28–34. The P23 substitution in Foxe3MOLF shortens the β-sheet and inserts a turn at amino acid 28, preventing the formation of the α-helix (SI Fig. 11B). These bioinformatic data along with the evolutionary conservation of the proline suggest that the L23 to P23 alteration could functionally alter Foxe3.
If this amino acid change were responsible for the cataract-modifying effect, we would predict that genetic backgrounds with the L23 allele would not affect the penetrance of the vl cataract phenotype. To investigate this possibility, a vl BALB/c intercross was then performed. A total of 109 F2 C3H/Balb progeny were generated (32 +/+, 63 +/vl, 13 vl/vl) with 100% of the vl/vl mice displaying an obvious cataract. These findings indicate that the BALB/c background does not modify the vl cataract phenotype, consistent with the P23 allele in Foxe3 contributing to the Modvl3-modifying effect.
We then investigated whether the L23-to-P23 alteration affects the activity of Foxe3. Because the N terminus of other forkhead transcription factors function as transactivators, Foxe3C3H and Foxe3MOLF were cotransfected with a luciferase (luc) construct driven by a consensus Foxe3 binding site (26). To test the effect of the L23-to-P23 alteration, these constructs were transfected into HEK293T cells, which do not express endogenous Foxe3 (data not shown and cgap.nci.nih.gov/SAGE). Both Foxe3C3H and Foxe3MOLF increased luc activity over the binding site alone. Moreover, Foxe3MOLF resulted in significantly lower luc activity than Foxe3C3H, indicating that the P23 allele functionally alters the transcriptional activity of Foxe3 (Fig. 4C). The lower activity of Foxe3MOLF is also consistent with Modvl3 enhancing the cataract phenotype. Thus, we provide sequence, protein modeling, genetic, and functional data supporting Foxe3 as a gene responsible for the cataract-modifying effect of Modvl3.
Discussion
Our positional cloning of the vl locus has identified Gpr161, an uncharacterized orphan GPCR, as one of the first genes necessary for neural fold apposition and fusion. We have also demonstrated that Gpr161 is expressed in the lateral neural folds during neurulation and the vl mutation affects receptor-mediated endocytosis, a common mechanism used to attenuate GPCR signaling. These data suggest that Gpr161 signaling normally regulates downstream pathways necessary for neural fold apposition and fusion. This possibility is consistent with previous vl phenotypic analysis. Embryonic cultures of vl mutants demonstrated normal elevation and bending of the neural plate but apposition and fusion are abnormal (14), indicating that vl affects the pathways required for this last step of neurulation. In addition, EM studies in normal embryos have determined that cellular protrusions extend from the apical neural folds, which then interdigitate upon contact during neural fold fusion (27). In vl these cellular protrusions have an abnormal ultrastructural morphology (12). Future experiments should use vl to identify the molecular and cellular pathways regulated by Gpr161 during neural fold apposition and fusion.
It is well established that extracellular signals are essential for neurulation. Explant experiments have demonstrated that medial bending is induced by the notochord through Shh signaling (28) while the adjacent lateral surface ectoderm is required for elevation, dorso-lateral bending and formation and fusion of the neural folds. It has been suggested that the surface ectoderm like the notochord is a source of extrinsic factors important for neurulation (28, 29). Our results support this possibility and imply that a previously uncharacterized small molecule ligand is present in the neural environment, which binds and activates Gpr161 in the lateral neural folds during neurulation and is an important regulator of neural fold apposition and fusion.
Our ISH analysis has demonstrated that Gpr161 is expressed at all stage of lens development. Interestingly, our phenotypic data has detected an obvious vl lens phenotype only after E14.5 (SI Fig. 12), suggesting that either this later stage is more sensitive to the vl mutation or subtle defects occur throughout lens development with a more severe phenotype being obvious only at later ages. The ocular environment is also known to contain secreted factors that regulate all stages of lens development. Although various secreted proteins (insulin-like growth factor 1, FGFs, Wnts, bone morphogenetic proteins) have been identified that coordinate these developmental processes (30), our results indicate that the small-molecule ligand for Gpr161 is another important regulator of lens development.
The vl mutation specifically deletes the C-terminal tail of Gpr161. Numerous mutagenesis studies have demonstrated that phosphorylation of serine and threonine residues in the C-terminal tail initiates receptor-mediated endocytosis (15, 16, 18, 19). Nine putative S/T phosphorylation sites are deleted by the vl mutation (ca.expasy.org; McVector version 9.0), consistent with our endocytosis phenotype. The C-terminal tail of GPCRs also serves as a scaffold for the binding of GPCR-interacting proteins (GIPs) that regulate receptor signaling (31). The vl mutation is likely to perturb the binding of these GIPs, which could affect additional aspects of Gpr161 activity. The mutation is then likely to have multiple effects on the Gpr161 protein including: reduced levels, decreased attenuation of receptor signaling, and altered binding of regulatory GIPs to the C-terminal tail. Together, these effects could lead to a complex Gpr161 signaling phenotype that may vary between cell types depending on the expression of different G proteins, kinases, and GIPs. In addition, the expression of Gpr161 in vl/vl embryos and the targeting of the mutant receptor to the plasma membrane indicate that Gpr161vl is likely not a null allele. Conditional loss-of-function Gpr161 alleles should be generated in the future to investigate whether Gpr161 has additional functions during neurulation and lens development and other embryonic structures expressing Gpr161.
We have also demonstrated that genetic background significantly affects the penetrance of the vl cataracts and spina bifida phenotype, enabling us to map the position of three vl modifiers. In our crosses, ≈50% of adult vl/vl-B6/C3H and CAST/C3H mice display a lumbar-sacral lesion and hind-limb paralysis, phenocopying important aspects of the human disorder and making it an valuable mouse model for studying the causes and effects of human spina bifida (20). It will be important in the future to examine the lumbar-sacral lesion in these mice to determine whether it has a similar or different neuropathology to what has been reported in humans and to investigate the cause of the hind-limb paralysis. The genetic loci responsible for this adult spina bifida phenotype have now been mapped to chromosome 5 (Modvl1-B6) and chromosome 1 (Modvl2-CAST/Ei). Because the B6 or CAST/Ei alleles of these QTL segregate with an absence of spina bifida, it will be interesting in future congenic experiments to determine whether these loci are sufficient to rescue the spina bifida phenotype.
One cataract-specific QTL, Modvl3, was mapped to chromosome 4 in our MOLF/Ei cross, and we have identified the lens transcription factor, Foxe3, as a gene that contributes to this modifying effect. In 2003 The Complex Trait Consortium established eight criteria for identifying modifier genes, requiring more than two to be fulfilled for positive identification (32). Our experiments fulfill four criteria: a previously unidentified coding polymorphism predicted to structurally alter the Foxe3 protein; Foxe3 expression in the lens, the structure affected by Modvl3 (22); mutations or knockdown of Foxe3 result in lens phenotypes in three different species (21, 23–26), and a functional difference in transcriptional activity between Foxe3C3H and Foxe3MOLF demonstrated by in vitro studies. The SNP also alters an evolutionarily conserved amino acid, consistent with the structural and functional differences observed between Foxe3C3H and Foxe3MOLF. Finally, crossing vl to BALB/c, a strain with the L23 allele, did not affect the penetrance of cataract. These data provide considerable support for Foxe3 as a gene responsible for the Modvl3-modifying effect. Consistent with this possibility Foxe3 plays a central role in lens development, regulating numerous pathways including lens vesicle closure, proliferation of anterior epithelial cells, fiber cell differentiation, and αA-crystallin transcription (22). Thus, reduced transcriptional activity of Foxe3MOLF is likely to affect the expression of many downstream genes, which in combination with the Gpr161 vl mutation contributes to the cataract phenotype on the C3H/MOLF background. It remains possible that additional MOLF variants in the 95% C.I. for Modvl3 also contribute to the cataract phenotype. To test whether the L23-to-P23 amino acid change is sufficient to enhance the vl cataract phenotype would require knock-ins not currently possible for the modifying strains. Foxe3MOLF also likely interacts with unmapped MOLF modifiers that decrease cataract penetrance, adding another level of complexity that would not be recapitulated in the knock-in.
Because the phenotypic effect of Modvl3 is observed only in the presence of the vl mutation and is not sufficient to cause cataract on a WT background, it is likely that Foxe3 and Gpr161 function in the same or interacting pathways to regulate lens development. Coexpression of Foxe3 and Gpr161 in the same cell types during lens morphogenesis supports this possibility (E9.5–E10.5) (21, 23). Future experiments will help determine the functional relationship between Foxe3 and Gpr161 by examining their expression in respective mouse mutants and the lens phenotype in compound mutants as well as determining whether Gpr161 functions upstream or downstream of Foxe3 through a series of cell culture and in vitro experiments. Because these vl modifiers likely function in the same pathway as Gpr161, future analysis also should be directed at identifying genes for Modvl1 and 2. These genes likely function with Gpr161 to regulate the signaling pathways necessary for neural fold apposition and fusion and may also help identify the small-molecule ligand that binds and activates Gpr161 during neurulation and lens development.
Finally, vl provides an important resource for investigating the biological basis of human cataracts and NTDs. Several rare human disorders also have been reported to display both congenital cataracts and NTDs (33–35). GPR161 is then an appropriate candidate for future mutational analysis for these disorders and human embryonic hydromyelia and congenital cataracts. This approach has been successful for congenital cataracts, where several genes that are mutated in the mouse are also affected in humans with the disease (PAX6, PITX3, FOXE3) (36). Given the modifiability of the mutant phenotypes, vl is also a useful mouse model for studying the more common forms of these diseases like lumbar-sacral spina bifida and age-related cataracts. Future association analysis can test whether GPR161 and the vl modifiers are susceptibility loci, and together with the identification of the extracellular ligand for Gpr161, these studies may provide insight into the multifactorial basis of NTDs and cataracts.
Materials and Methods
Positional Cloning and Expression Analysis of Gpr161.
The vl locus was mapped by intersubspecific intercrosses to MOLF/Ei and CAST/Ei. F2 vl/vl were identified by mutant phenotype, and recombinants delimited the vl locus to a 0.96-Mb region. Eleven genes mapped to the vl minimal region, and each exon was sequenced, identifying the 8-bp deletion in Gpr161. Nineteen additional inbred strains were sequenced to investigate whether the deletion was a polymorphism. The 8-bp deletion was confirmed by PCR, which was subsequently used as a genotyping assay. Standard RT-PCR and ISHs were used to determine Gpr161 expression in +/+ and vl/vl E8.0–E14.5 embryos.
Western Analysis and Immunocytochemistry.
Full-length +/+ and vl/vl Gpr161 was cloned 3′ of an N-terminal myc epitope tag and transiently transfected into HEK293T cells. Standard Western protocols were used with cMyc (1:1,000 dilution; Cell Signaling) and GAPDH (1:300 dilution; Chemicon) antibodies. For subcellular colocalization studies, the above constructs were transfected into HEK293T cells and after 18–20 h were fixed and immunostained by using standard protocols [primary antibody, cMyc (Cell Signaling); secondary antibody, Alexa-Fluor 568 (Molecular Probes)] under permeabilized (0.1% Triton X-100) and nonpermeabilized conditions. To further investigate the difference in subcellular localization, the constructs were cotransfected with GFP expression vectors targeted to the ER, plasma membrane, and nucleus (pEYFP-ER; pEYFP-Nuc; pEYFP-Mem; Clontech) followed by immunostaining [primary antibody, GFP (Molecular Probes); secondary antibody, Alexa-Fluor 488 (zcomMolecular Probes)]. For endosome colocalization, standard protocols were followed (37); the HEK293T cells were serum starved for 2 h and then incubated with transferrin-Alexa-Fluor 488 (Molecular Probes) for 5 or 15 min before fixation and immunostaining.
QTL Analysis.
Phenotypic and nonphenotypic F2 vl/vl B6, CAST, and MOLF/C3H mice were used for QTL analysis. These mice were identified by their C3H/C3H genotype for multiple chromosome 1 microsatellitle markers flanking the vl locus (see SI Text). For the B6 and CAST crosses, the spina bifida, but not the cataract, phenotype was recorded, whereas for the MOLF cross all phenotypes were recorded. A total of 86–132 F2 vl/vl depending on cross were genotyped for 60–80 SSLP markers evenly spaced throughout the genome. QTL analysis was performed as described (38).
Foxe3 Analysis.
OMIM and expression databases (genome.ucsc.edu) identified Foxe3 as a candidate gene. Sequence analysis was performed as described above. Bioinformatic evolutionary analysis and protein modeling was performed with McVector version 9.0, with the later using both Robson-Garnier (SI Fig. 11B) and Chou-Fasman algorithms. The vl BALB/c intercross was performed as described above, and cataract was noted by an obvious opacity of the lens. To test for a functional difference between Foxe3C3H and Foxe3MOLF, both versions of the gene were cloned into the BamHI site of pCMV-Tag3 expression vector after PCR amplification and sequence verification. HEK293T cells were transfected with 1.6 μg of the luc reporter, Foxe3 expression constructs, and 10 ng of phRL-null vector by using Lipofectamine 2000. Twenty-four hours after transfection, standard protocols for calculating normalized luciferase values were conducted.
See SI Text for more details.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Theolyn Gilley for technical support. This work was supported by New Jersey Commission on Spinal Cord Research Grants 02-3016-SCR-S-0 and 04-2901-SCR-E-0 (to J.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the Genbank database (accession no. EF197953).
This article contains supporting information online at www.pnas.org/cgi/content/full/0705657105/DC1.
References
- 1.Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Neurotoxicol Teratol. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond CJ, Duncan DD, Snieder H, de Lange M, West SK, Spector TD, Gilbert CE. Invest Ophthalmol Visual Sci. 2001;42:601–605. [PubMed] [Google Scholar]
- 3.Hammond CJ, Snieder H, Spector TD, Gilbert CE. N Engl J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 4.Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O'Colmain B, Wu SY, Taylor HR. Arch Ophthalmol. 2004;122:487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Ezegwui IR, Umeh RE, Ezepue UF. Br J Ophthalmol. 2003;87:20–23. doi: 10.1136/bjo.87.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickie M. Mouse News Lett. 1967;36:39–40. [Google Scholar]
- 7.Wilson DB, Wyatt DP. J Neuropathol Exp Neurol. 1986;45:43–55. doi: 10.1097/00005072-198601000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DB, Wyatt DP. Anat Embryol. 1988;178:559–563. doi: 10.1007/BF00305044. [DOI] [PubMed] [Google Scholar]
- 9.Ikenouchi J, Uwabe C, Nakatsu T, Hirose M, Shiota K. Acta Neuropathol. 2002;103:248–254. doi: 10.1007/s00401-001-0465-9. [DOI] [PubMed] [Google Scholar]
- 10.Juriloff DM, Harris MJ. Hum Mol Genet. 2000;9:993–1000. doi: 10.1093/hmg/9.6.993. [DOI] [PubMed] [Google Scholar]
- 11.Copp AJ, Greene ND, Murdoch JN. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DB, Wyatt DP. Acta Neuropathol. 1989;79:94–100. doi: 10.1007/BF00308963. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DB, Wyatt DP. Teratology. 1992;45:105–112. doi: 10.1002/tera.1420450110. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DB, Wyatt DP. J Neuropathol Exp Neurol. 1993;52:253–259. doi: 10.1097/00005072-199305000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 16.Prossnitz ER. Life Sci. 2004;75:893–899. doi: 10.1016/j.lfs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Mu J, Gilley T, Turner R, Paigen B. Mamm Genome. 1996;7:770. doi: 10.1007/s003359900229. [DOI] [PubMed] [Google Scholar]
- 18.Koch T, Schulz S, Schroder H, Wolf R, Raulf E, Hollt V. J Biol Chem. 1998;273:13652–13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- 19.Roth A, Kreienkamp HJ, Nehring RB, Roosterman D, Meyerhof W, Richter D. DNA Cell Biol. 1997;16:111–119. doi: 10.1089/dna.1997.16.111. [DOI] [PubMed] [Google Scholar]
- 20.Volpe J. Neurology of the Newborn. 3rd Ed. Philadelphia: Saunders; 1995. pp. 5–21. [Google Scholar]
- 21.Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- 22.Medina-Martinez O, Jamrich M. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- 23.Brownell I, Dirksen M, Jamrich M. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- 25.Valleix S, Niel F, Nedelec B, Algros MP, Schwarz C, Delbosc B, Delpech M, Kantelip B. Am J Hum Genet. 2006;79:358–364. doi: 10.1086/505654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ormestad M, Blixt A, Churchill A, Martinsson T, Enerback S, Carlsson P. Invest Ophthalmol Visual Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- 27.Geelen JA, Langman J. Anat Embryol. 1979;156:73–88. doi: 10.1007/BF00315716. [DOI] [PubMed] [Google Scholar]
- 28.Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- 29.Moury JD, Jacobson AG. Dev Biol. 1989;133:44–57. doi: 10.1016/0012-1606(89)90295-9. [DOI] [PubMed] [Google Scholar]
- 30.Lovicu FJ, McAvoy JW. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Bockaert J, Fagni L, Dumuis A, Marin P. Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Abiola O, Angel JM, Avner P, Bachmanov AA, Belknap JK, Bennett B, Blankenhorn EP, Blizard DA, Bolivar V, Brockmann GA, et al. Nat Rev. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobyns WB, Pagon RA, Armstrong D, Curry CJ, Greenberg F, Grix A, Holmes LB, Laxova R, Michels VV, Robinow M, et al. Am J Med Genet. 1989;32:195–210. doi: 10.1002/ajmg.1320320213. [DOI] [PubMed] [Google Scholar]
- 34.Siegel-Bartlet J, Levin A, Teebi AS, Kennedy SJ. J Med Genet. 2002;39:145–148. doi: 10.1136/jmg.39.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sniderman LC, Koenekoop RK, O'Gorman AM, Usher RH, Sufrategui MR, Moroz B, Watters GV, Der Kaloustian VM. Am J Med Genet. 2000;90:146–149. [PubMed] [Google Scholar]
- 36.Graw J. Int J Dev Biol. 2004;48:1031–1044. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- 37.Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. J Cell Sci. 2003;116:1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.