Abstract
Parkinson's disease (PD) is characterized by a progressive degeneration of substantia nigra dopaminergic neurons projecting to the striatum. Restoration of dopamine transmission by l-DOPA relieves symptoms of PD but causes prominent side effects. There is a strong serotonin innervation of the striatum by serotonergic neurons that remains relatively preserved in PD. The study of this innervation has been largely neglected. Here, we demonstrate that chronic l-DOPA administration to 6-OHDA-lesioned rodents increases, via D1 receptors, the levels of the 5-HT1B receptor and its adaptor protein, p11, in dopamine-denervated striatonigral neurons. Using unilaterally 6-OHDA-lesioned p11 WT and KO mice, it was found that administration of a selective 5-HT1B receptor agonist, CP94253, inhibited l-DOPA-induced rotational behavior and abnormal involuntary movements in a p11-dependent manner. These data reveal an l-DOPA-induced negative-feedback mechanism, whereby the serotonin system may influence the symptomatology of Parkinsonism.
Keywords: annexin II light chain, basal ganglia, Parkinson's disease, S100a10, serotonin
Parkinson's disease (PD) is characterized by loss of dopaminergic neurons in the substantia nigra pars compacta that innervate the striatum (1, 2). PD is treated with dopamine replacement by administration of l-DOPA and other drugs that stimulate dopaminergic neurotransmission (2–4). l-DOPA exerts its main actions via stimulation of dopamine D1 receptors (D1Rs) and D2 receptors (D2Rs) expressed in striatal GABAergic medium-sized spiny neurons. D1Rs are enriched in striatonigral neurons (i.e., the direct striatal output pathway), whereas D2Rs are enriched in striatopallidal neurons (i.e., the indirect striatal output pathway) (5). Activation of D1Rs increases cAMP formation and facilitates responses to fast neurotransmitters, whereas activation of D2Rs decreases cAMP formation and reduces neuronal excitability (6).
As PD progresses, l-DOPA therapy often results in a supersensitized, but shortened, response (3). There also is an emergence of on–off motor fluctuations, abnormal involuntary dyskinetic movements, and psychiatric complications (3). These behavioral alterations are paralleled by numerous adaptive neurochemical and physiological changes of striatal neurons. In particular, l-DOPA causes an enhancement of D1R-mediated responses in striatonigral neurons. Indeed, repeated l-DOPA treatment, in PD patients and in animal models of this disease, leads to an increased availability of D1Rs at the cell membrane (7), augmented coupling of D1Rs to G proteins (8), up-regulated Golf and Gγ7 subunit levels (9), enhanced dopamine-stimulated adenylyl cyclase (10), and increased cAMP-dependent kinase-mediated phoshorylation of Thr34-DARPP-32 (11, 12). The l-DOPA-induced supersensitivity of D1R-mediated responses also leads to elevated levels of transcription factors, including ΔFosB, and genes, such as dynorphin, selectively in striatonigral neurons (13–15) and underlies the l-DOPA-induced increase in GABA release in substantia nigra pars reticulata (SNr) (16).
Treatment with D1R-selective antagonists may counteract l-DOPA-induced side effects in PD, but it also could diminish the therapeutic efficacy of l-DOPA. There is a strong serotonin innervation of the striatum (17) that is relatively preserved in PD patients (18). In some animal models of PD, there is actually a compensatory serotonergic hyperinnervation to striatum (e.g., ref. 19). Moreover, several serotonin receptors are highly expressed in the striatum (20) and are, thus, positioned to modulate l-DOPA-mediated actions. Targeting the serotonin system may offer alternative approaches for the treatment of advanced Parkinsonism.
In the present study, we used the unilateral 6-OHDA-lesioning animal model of PD (21) to examine a possible role for 5-HT1B receptors (5-HT1BRs) in l-DOPA treatment of Parkinsonism. We provide evidence that l-DOPA increases the levels of the 5-HT1BR and its adaptor protein, p11, in striatonigral neurons. Using unilaterally 6-OHDA-lesioned p11 WT and KO mice, we found that administration of a selective 5-HT1BR agonist, CP94253 (22), inhibits l-DOPA-induced rotational behavior and abnormal involuntary movements (AIMs) in a p11-dependent manner. l-DOPA-induced levels of 5-HT1BRs and p11 may serve as a negative-feedback mechanism to counteract hyperactivity of striatonigral neurons in PD patients treated with l-DOPA.
Results
Postmortem Determination of the Efficacy of Unilateral 6-OHDA Lesioning.
The efficacy of the unilateral 6-OHDA lesions was verified by [125I-RTI-55 binding to the dopamine transporter (DAT) in the striatum. DAT levels were reduced by 91.7 ± 1.7% in mice and 94.2 ± 1.7% in rats in the dopamine-denervated hemisphere compared with the intact hemisphere. The efficacy of the unilateral 6-OHDA lesions also was verified by Western blotting of tyrosine hydroxylase (TH), showing reductions by 92.9 ± 4.0% in mice and 97.2 ± 1.0% in rats in the dopamine-denervated hemisphere.
Regulation of 5-HT1BR mRNA and p11 mRNA by l-DOPA in Striatum.
Based on previous studies of l-DOPA-induced sensitization in mice (e.g., ref. 14), unilaterally 6-OHDA-lesioned mice were treated with saline or 50 mg of l-DOPA per kg per day for 28 days. In saline-treated mice, there was no significant regulation of 5-HT1BR mRNA or p11 mRNA in striatum of the dopamine-denervated hemisphere (Fig. 1a). However, after treatment with l-DOPA, increased levels of both 5-HT1BR mRNA and p11 mRNA were found in striatum of the 6-OHDA-lesioned hemisphere (Fig. 1a). In experiments with rats, two different treatment regimens were used to study the effects of l-DOPA treatment on 5-HT1BR and p11 mRNA levels. One regimen used subchronic administration (twice daily for 5 days) of a high concentration of l-DOPA (100 mg/kg, i.p.), which has been shown to regulate gene expression in striatum (23) and to be useful for studies of l-DOPA-induced sensitization and side effects (24). Another regimen used chronic adminstration (once daily for 28 days) of a low concentration of l-DOPA (10 mg/kg, i.p.), which also induces l-DOPA-mediated sensitization and dyskinesias (12, 13). The results were very similar to those observed in mice. The unilateral 6-OHDA lesion had no significant effects on 5-HT1BR mRNA or p11 mRNA in saline-treated rats (Fig. 1 b and c). However, both regimens of l-DOPA treatment caused a significant increase in the levels of 5-HT1BR mRNA and p11 mRNA in the dopamine-denervated hemisphere compared with the intact hemisphere or saline-treated rats (Fig. 1 b and c).
Fig. 1.
Regulation of 5-HT1BR mRNA and p11 mRNA in response to l-DOPA in unilaterally 6-OHDA-lesioned mice and rats. (a–c) (Left) Brightfield autoradiograms showing the expression of 5-HT1BR and p11 in unilaterally 6-OHDA-lesioned mice treated with 50 mg/kg l-DOPA (i.p., once daily) for 28 days (a), unilaterally 6-OHDA-lesioned rats treated with 100 mg/kg l-DOPA (i.p., twice daily) for 5 days (b), and unilaterally 6-OHDA-lesioned rats treated with 10 mg/kg l-DOPA (i.p., once daily) for 28 days (c). (Right) Quantification from mice (a) and rats (b and c) in the dorsolateral striatum. Filled bars, intact hemisphere; open bars, 6-OHDA-lesioned hemisphere. Data represent means ± SEM for four to eight animals per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 between indicated treatments; one-way ANOVA followed by Newman–Keuls test.
Regulation of 5-HT1BR Protein and p11 Protein by l-DOPA in Striatum.
Western blotting methodology was used to determine the effects of l-DOPA treatment on 5-HT1BR and p11 protein levels in striatum of 6-OHDA-lesioned rodents. Consistent with the results on gene expression, dopamine denervation did not significantly alter the levels of 5-HT1BR protein or p11 protein in saline-treated mice or rats (Fig. 2). However, treatment with l-DOPA caused significant increases of 5-HT1BR protein and p11 protein levels in the dopamine-denervated hemisphere in both mice and rats (Fig. 2). In addition, chronic treatment with a low dose of l-DOPA in rats also significantly increased the 5-HT1BR protein level in the intact hemisphere (Fig. 2c).
Fig. 2.
Regulation of 5-HT1BR protein and p11 protein in the striatum in response to l-DOPA in unilaterally 6-OHDA-lesioned mice and rats. (a–c) (Upper) Western blots of 5-HT1BR, p11, TH, and actin in unilaterally 6-OHDA-lesioned mice treated with 50 mg/kg l-DOPA (i.p., once daily) for 28 days (a), unilaterally 6-OHDA-lesioned rats treated with 100 mg/kg l-DOPA (i.p., twice daily) for 5 days (b), and unilaterally 6-OHDA-lesioned rats treated with 10 mg/kg l-DOPA (i.p., once daily) for 28 days (c). Note the near-complete absence of TH in the 6-OHDA-lesioned hemispheres. (Lower) Quantification of striatal 5-HT1BR and p11 from mice (a) and rats (b and c). The 5-HT1BR and p11 levels were normalized to actin. Filled bars, intact hemisphere; open bars, 6-OHDA-lesioned hemisphere. Data represent means ± SEM for 4–10 animals per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 between indicated treatments; one-way ANOVA followed by Newman–Keuls test.
To examine further the regulation of 5-HT1BR protein levels by l-DOPA, we also examined autoradiographic binding of the selective 5-HT1BR radioligand, 125I-cyanopindolol, in striatum and its projection areas, substantia nigra pars reticulata (SNr) and globus pallidus (GP). In saline-treated mice and rats, the unilateral 6-OHDA lesion had no significant effects on 125I-cyanopindolol binding in any of the regions studied (Fig. 3). After chronic treatment with l-DOPA in mice and rats, there was increased binding of 125I-cyanopindolol in the striatum and SNr, but not in GP (Fig. 3 a and c). In rats subchronically treated with a high dose of l-DOPA, 125I-cyanopindolol binding was increased in the striatum, SNr, and GP (Fig. 3b). The specific 125I-cyanopindolol binding in the dorsal hippocampus (Fig. 3) was not changed by any treatment (data not shown).
Fig. 3.
Regulation of 125I-cyanopindolol binding to 5-HT1BRs in response to l-DOPA in unilaterally 6-OHDA-lesioned mice and rats. (a–c) (Left) Brightfield autoradiograms showing 125I-cyanopindolol binding in striatum (Str), GP, and SNr in unilaterally 6-OHDA-lesioned mice treated with 50 mg/kg l-DOPA (i.p., once daily) for 28 days (a), unilaterally 6-OHDA-lesioned rats treated with 100 mg/kg l-DOPA (i.p., twice daily) for 5 days (b), and unilaterally 6-OHDA-lesioned rats treated with 10 mg/kg l-DOPA (i.p., once daily) for 28 days (c). (Right) Quantification from mice (a) and rats (b and c) in the dorsolateral striatum, GP, and SNr. Filled bars, intact hemisphere; open bars, 6-OHDA-lesioned hemisphere. Data represent means ± SEM for four to eight animals per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 between indicated treatments; one-way ANOVA followed by Newman–Keuls test.
Effects of l-DOPA on 5-HT1BR Agonist-Stimulated [35S]GTPγS Autoradiography.
To determine whether the l-DOPA-mediated up-regulation of 5-HT1BRs corresponded to an increased 5-HT1BR agonist-mediated activation of associated Gi proteins, we examined the ability of the 5-HT1BR agonist, CP93139 (25), to stimulate [35S]GTPγS binding detected by autoradiography. In saline-treated mice and rats, dopamine denervation had no effect on CP93139-stimulated [35S]GTPγS binding in striatum, SNr, or GP (data not shown). However, after treatment with l-DOPA in mice or rats, there was an increased CP93139-stimulated [35S]GTPγS binding in striatum and SNr but not in GP (data not shown). This lack of effect in GP, even in rats treated with the higher concentration of l-DOPA, indicates that the up-regulation of functional 5-HT1BRs by l-DOPA is restricted to striatonigral neurons.
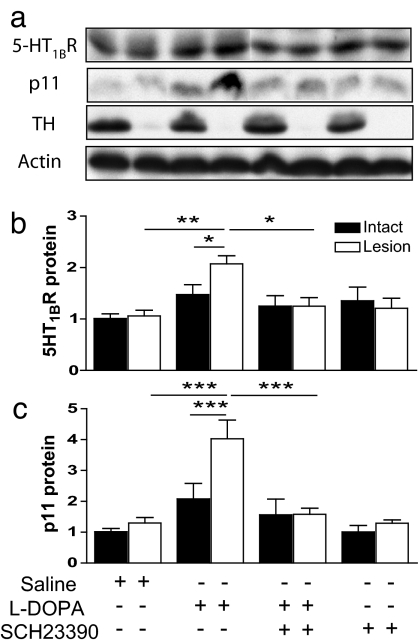
Effect of a D1R Antagonist, SCH23390, on l-DOPA-Induced Levels of 5-HT1BR Protein and p11 Protein.
Based on the existing literature and the above-mentioned results, we hypothesized that the l-DOPA-mediated up-regulation of 5-HT1BR and p11 proteins depends on D1R stimulation. To test this possibility directly, we treated rats with saline, 100 mg/kg l-DOPA (i.p.), or 0.5 mg/kg SCH23390 (a selective D1R antagonist) alone or in combination twice daily for 5 days. We confirmed that dopamine denervation had no effect on 5-HT1BR or p11 protein levels in saline-treated animals (Fig. 4) but that l-DOPA treatment caused a significant induction of both 5-HT1BR and p11 protein levels in the 6-OHDA-lesioned hemisphere (Fig. 4). Cotreatment of rats with SCH23390 and l-DOPA abolished the effects on 5-HT1BR and p11 protein levels seen when animals were treated with l-DOPA alone (Fig. 4).
Fig. 4.
Regulation of 5-HT1BR protein and p11 protein in unilaterally 6-OHDA-lesioned rats treated with saline, l-DOPA, or SCH23390 alone or in combination. (a) Western blots of 5-HT1BR, p11, TH, and actin in unilaterally 6-OHDA-lesioned rats treated with saline, 100 mg/kg l-DOPA (i.p., twice daily), or 0.5 mg/kg SCH23390 (i.p., twice daily) alone or in combination for 5 days. Note the near-complete absence of TH in the 6-OHDA-lesioned hemispheres. (b and c) Quantification of striatal 5-HT1BR (b) and p11 levels (c) normalized to actin. Filled bars, intact hemisphere; open bars, 6-OHDA-lesioned hemisphere. Data represent means ± SEM for 4–10 animals per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 between indicated treatments; one-way ANOVA followed by Newman–Keuls test.
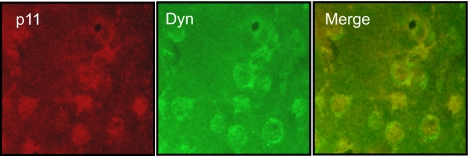
Double Immunohistochemical Detection of p11 and Prodynorphin in the Dopamine-Denervated Striatum of l-DOPA-Treated Animals.
To determine whether p11 protein levels are up-regulated in striatonigral neurons, we performed double immunohistochemical experiments with antibodies toward p11 and prodynorphin (which selectively labels striatonigral neurons) in rats treated with 10 mg/kg l-DOPA (i.p., once daily) for 28 days. When p11-positive neurons were counted in sections from three different rats, 61 of 63 neurons also were positive for prodynorphin (Fig. 5). Likewise, 70 of 73 neurons that were positive for prodynorphin also were positive for p11. Thus, p11 appears to be almost exclusively up-regulated in striatonigral neurons.
Fig. 5.
Colocalization of p11 and prodynorphin proteins in dorsolateral striatum from unilaterally 6-OHDA-lesioned rats treated with l-DOPA. Immunostaining for p11 (red) and prodynorphin (green) and their codistribution (yellow) in the dopamine denervated hemisphere in a rat treated with 10 mg/kg l-DOPA (i.p., once daily) for 28 days.
Effect of a 5-HT1BR Agonist, CP94253, on l-DOPA-Induced Rotations and AIMs in p11 WT and KO Mice.
To study the role of 5-HT1BR and p11 in l-DOPA-induced behaviors, p11 WT and KO mice were cotreated with the 5-HT1BR agonist, CP94253, which is an analog of CP93139 with an improved blood–brain barrier penetration (22). Both CP93139 and CP94253 lack affinities for dopamine receptors (22, 25). Unilaterally 6-OHDA-lesioned P11 WT and KO mice were treated with 10 mg/kg l-DOPA (i.p.) for 2 weeks and then with 50 mg/kg l-DOPA (i.p.). As shown in Fig. 6, rotational behavior was measured on days 1 and 7 of 50 mg/kg l-DOPA i.p. treatment and AIMs on day 7. On day 8, the 50 mg/kg l-DOPA i.p. treatment was combined with the 5-HT1BR agonist, CP94253 (2.5 mg/kg; i.p.), and rotational behavior was measured on days 8 and 15 and AIMs on day 15. After cotreatment with CP94253 and l-DOPA, there were significantly fewer rotations compared with treatment with l-DOPA alone (i.e., day 7 vs. day 8 or 15) in p11 WT mice (Fig. 6a). Moreover, on day 16, when mice were treated with l-DOPA alone again, the number of rotations in p11 WT mice significantly exceeded the number of rotations found in mice cotreated with CP94253 and l-DOPA. In contrast, in p11 KO mice, these effects on motor activity by CP94253 were abolished (Fig. 6b). Cotreatment with CP94253 and l-DOPA also counteracted l-DOPA-induced AIMs (i.e., day 7 vs. 15) in p11 WT mice (Fig. 6c) but not in p11 KO mice (Fig. 6d).
Fig. 6.
5-HT1BR agonist-mediated inhibition of l-DOPA-induced rotations and AIMs in p11 WT versus KO mice. p11 WT and KO mice were treated with 50 mg/kg l-DOPA (i.p.) alone or together with the 5-HT1BR agonist, CP94253 (2.5 mg/kg, i.p.) as indicated. (a and b) Effects of l-DOPA alone (days 1, 7, and 16) or together with CP94253 (days 8 and 15) on contralateral turns in p11 WT mice (filled bars) (a) and p11 KO mice (open bars) (b). (c and d) Effects of l-DOPA alone (day 7) and CP94253+l-DOPA (day 15) on AIMs in p11 WT mice (filled bars) (c) or p11 KO mice (open bars) (d). Data represent means ± SEM for 9–11 animals per group. *, P < 0.05 between indicated treatments; one-way ANOVA, followed by Newman–Keuls test. ##, P < 0.01 between l-DOPA- and CP94253 plus l-DOPA-treated mice; Student's t test.
Discussion
5-HT1BRs are expressed in most, if not all, striatal medium-sized spiny GABAergic neurons, i.e., both striatonigral and striatopallidal neurons (26). Our present data demonstrate that l-DOPA treatment increases the levels of 5-HT1BRs in dopamine-denervated striatonigral neurons in unilaterally 6-OHDA-lesioned rodents. The efficacy of 5-HT1BRs is regulated by its interacting adaptor protein p11 (27). p11 increases localization of 5-HT1BRs at the cell surface, and studies in cell lines and p11 KO mice have shown that p11 modulates biochemical, electrophysiological, and behavioral responses to 5-HT1BR stimulation. p11 is increased in rodent brains by antidepressants therapies but is decreased in an animal model of depression and in depressed patients (27). Our present observation that l-DOPA treatment increases p11 in the dopamine-denervated striatum of unilaterally 6-OHDA-lesioned rodents provides additional evidence for a dynamic regulation of this protein. It is interesting that l-DOPA is an antidepressant in some unipolar depressed patients (37). The l-DOPA-mediated up-regulation of p11 occurs in prodynorphin-containing neurons and represents another example of an aberrantly up-regulated protein in striatonigral neurons in response to l-DOPA treatment in PD.
During the preparation of this manuscript, it was reported that the 5-HT1BR agonist, CP94253, significantly counteracts l-DOPA-induced AIMs in unilaterally 6-OHDA-lesioned rats (28). Our data confirm that CP94253 counteracts l-DOPA-induced AIMs and also show that CP94253 counteracts l-DOPA-induced rotational behavior in WT mice. Furthermore, we demonstrate that the inhibitory effects of CP94253 on l-DOPA-induced rotations and AIMs involve p11 because they are not found in p11 KO mice.
Because the 5-HT1BR and p11 are found in several brain regions (26, 27), multiple mechanisms may underlie the CP94253-mediated reduction of l-DOPA-induced behaviors. 5-HT1BRs serve as autoreceptors on serotonin neurons originating from the raphe nuclei (29). Serotonergic neurons have the capacity to synthesize dopamine, as a false neurotransmitter, from l-DOPA (e.g., ref. 30). There is evidence that the inhibitory effect of CP94253 on l-DOPA-induced AIMs involves 5-HT1BR-mediated inhibition of dopamine release from serotonergic nerve terminals (28). The turnover of serotonin is increased in p11 KO mice (27) presumably because of a decreased autoinhibition by 5-HT1BRs on the serotonergic nerve terminals. A decreased efficacy of 5-HT1B autoreceptors also may contribute to the lack of inhibition of CP94253 on l-DOPA-induced rotational behavior in p11 KO mice.
Based on our observation that l-DOPA increases the levels of 5-HT1BRs and p11 in striatonigral neurons, we propose a postsynaptic mechanism whereby CP94253 counteracts l-DOPA-induced rotations and AIMs. Stimulation of 5-HT1BRs inhibits cAMP formation (31) and reduces GABA release in striatum and SNr (32). In contrast, stimulation of D1Rs increases cAMP formation (6) and GABA release in striatum and SNr (33). Because D1R supersensitivity in striatonigral neurons appears to underlie l-DOPA-mediated rotations and AIMs, it is reasonable to believe that the inhibitory effects of a 5-HT1BR agonist on these behaviors involves an inhibitory influence on D1R-mediated cAMP formation and/or GABA release.
The possibility that 5-HT1BR agonists may counteract l-DOPA-induced behaviors by diminishing D1R-mediated increases of GABA release from striatonigral neurons is in agreement with current knowledge on the role of GABA in l-DOPA supersensitivity. Repeated treatment with l-DOPA to animal models of PD up-regulates genes for GABA synthesis in striatonigral neurons (13) and increases GABA levels in the SNr (but not in the GP) (16).
In conclusion, the present data demonstrate that repeated l-DOPA treatment to unilaterally dopamine-denervated rodents increases the level of the 5-HT1BR and its adaptor protein, p11, in striatonigral neurons. Furthermore, administration of a selective 5-HT1BR agonist, CP94253, inhibits l-DOPA-induced rotational behavior and AIMs in a p11-dependent manner. Because the serotonergic innervation of the striatum remains relatively preserved in PD patients, inhibitory modulation of striatonigral neurons by 5-HT1BRs/p11 may be particularly important in advanced PD. Because the blockade of D1Rs is not a treatment option for l-DOPA-induced side effects and because it would diminish the therapeutic efficacy of l-DOPA, the use of 5-HT1BR agonists to modulate signaling in striatonigral neurons may offer an alternative approach. These data also indicate that an adjunctive treatment with 5-HT1BR ligands might be used to modify the therapeutic dose window of l-DOPA treatment.
Materials and Methods
Animals, Surgery, and Pharmacological Treatment.
Adult male C57Bl6 mice, p11 WT and KO (129SV/C57BL/6) (27), and Sprague–Dawley rats were used. Experiments were performed in agreement with the European Communities Council (86/609/EEC) and were approved by the ethical committee at Karolinska Institute (N282/06).
Mice were anesthetized with 80 mg/kg ketamine (i.p.; Parke-Davis) and 5 mg/kg xylazine (i.p.; Bayer), pretreated with 25 mg/kg desipramine (i.p.; Sigma–Aldrich) and 5 mg/kg pargyline (i.p.; Sigma–Aldrich), placed in a stereotaxic frame, and injected, over 2 min, with 3 μg of 6-OHDA in 0.01% ascorbate (Sigma–Aldrich) into the median forebrain bundle (MFB) of the right hemisphere. The coordinates for injection were AP, −1.1 mm; ML, −1.1 mm; and DV, −4.75 mm relative to bregma and the dural surface (34). Rats were anesthetized and immobilized as the mice and injected with 12.5 μg of 6-OHDA into the right MFB. The coordinates for injection were AP, −2.8 mm; ML, −2.0 mm; and DV, −9.0 mm relative to bregma and the dural surface (35).
Two weeks after unilateral 6-OHDA lesioning, rodents were administered 1 mg/kg apomorphine (i.p; Sigma–Aldrich). Only mice rotating >50 turns per 30 min and rats rotating >100 turns per 30 min were included in further experiments.
Four weeks after surgery, WT mice used for biochemical experiments were treated with saline or 50/12.5 mg/kg l-DOPA/benserazide (i.p.; Sigma–Aldrich) once daily for 28 days. Animals were killed 1 h after the last injection. In another series of experiments, with behavioral measurements, p11 WT and KO mice were treated with 10/7.5 mg/kg l-DOPA/benserazide (i.p., once daily) for 2 weeks, 50/12.5 mg/kg l-DOPA/benserazide (i.p., once daily) for 1 week, 2.5 mg/kg CP94253 (i.p.; Tocris, a 5-HT1BR agonist) plus 50/12.5 mg/kg l-DOPA/benserazide (i.p., once daily) for 1 week, and 50/12.5 mg/kg l-DOPA/benserazide (i.p., once daily) for 1 day.
Likewise, 4 weeks after the surgery, rats were treated with saline or 10/7.5 mg/kg l-DOPA/benserazide (i.p., once daily) for 28 days. Another set of rats was treated with saline or 100/25 mg/kg l-DOPA/benserazide (i.p., twice daily) for 5 days. A third set of rats was treated with saline, 100/25 mg/kg l-DOPA/benserazide (i.p.), or 0.5 mg/kg SCH23390 (i.p., a D1R antagonist; Sigma–Aldrich), alone or in combination, twice daily, for 5 days. Animals were killed 1 h after the last injection. For brievity, l-DOPA/benserazide treatments are solely labeled l-DOPA throughout this article.
In Situ Hybridization.
To detect 5-HT1BR and p11 mRNAs, radioactive riboprobe in situ hybridization experiments were carried out as described previously (27).
Western Blotting.
To detect 5-HT1BR, p11, TH, and actin in striatal tissue samples, Western blotting was performed as described previously (27).
Ligand-Binding Autoradiography.
Cryostat sections for the detection of DAT were incubated in 50 mM Tris·HCl, 120 mM NaCl, 50 pM 125I-RTI-55 (PerkinElmer), and 1 μM fluoxetine (Sigma) for 60 min. For nonspecific binding, 100 μM nomifensine (Sigma–Aldrich) was added. The slides were washed in ice-cold binding buffer, dried, and exposed together with 2.2–160 nCi/mg 125I-Microscales (GE Healthcare) on Kodak Biomax MR films for 4 days.
To label the 5-HT1BRs, 125I-cyanopindolol binding was performed as described previously (27).
5-HT1BR Agonist-Stimulated [35S]GTPγS Autoradiography.
To analyze functional G protein coupling of 5-HT1BRs, 5-HT1BR agonist-stimulated [35S]GTPγS binding was performed as described previously (27) with minor modifications.
Immunohistochemistry.
Rats were anesthetized and perfused transcardially with 4% paraformaldehyde (PFA). Brains were postfixed overnight in 4% PFA, cryoprotected in 30% sucrose, and cut into 40-μm sections. Sections were preincubated with PBS, 2 N HCl, and PBS/0.3% Triton X-100/3% BSA before primary antibodies against p11 (1:200; R&D Diagnostics) and prodynorphin (1:200; Bachem) were added overnight at 4°C. Sections were then washed in PBS/0.3% Triton X-100 before Alexa Fluor 568 donkey anti-goat (1:200; Molecular Probes) and Alexa Fluor 488 goat anti-rabbit (1:200) secondary antibodies were added for 1 h. After washing in PBS/0.3% Triton X-100, the sections were mounted and coverslipped. Flourescent images were captured by using a Nikon Eclipse E600 microscope connected to a Nikon digital sight DS-U1 camera and merged with the NIS-Elements F 2.20 software.
Behavioral Experiments.
Unilaterally 6-OHDA-lesioned p11 WT or KO mice were treated with saline, l-DOPA, and/or CP94253 as indicated, and the number of ipsi- and contralateral rotations was counted for 30 min. Immediately after the quantification of rotational behaviors, the incidence of AIMs, classified into forelimb, orofacial, axial, and locomotive behaviors, was quantified for 5 min according to published mouse scales (14, 36).
Statistical Analyses.
Films from the in situ hybridization, Western blotting, and autoradiographic experiments were digitized, and the brain regions were identified with brain atlases (34, 35). Optical density values were measured by using Scion Image. For ligand-binding autoradiographic experiments, optical densities were converted to pmol per g wet weight based on coexposed standards. Biochemical data are presented as relative level toward the intact hemisphere in saline-treated animals. Statistical analyses of biochemical and behavioral data were made by using one-way ANOVA, followed by a Newman–Keul test for pairwise comparisons or Student's t test.
ACKNOWLEDGMENTS.
We thank Dr. Jennifer Warner-Schmidt and Martin Egeland for advice on the immunohistochemical experiments and Hongshi Qi for help with the behavioral experiments. This work was supported by the Picower Foundation, the Peter Jay Sharp Foundation, the Simons Foundation, the F. M. Kirby Foundation, the Micrael Sterns Parkinson's Research Foundation, National Institutes of Health Grants MH074866 and DA10044 (to P.G.), and Vetenskapsrådet, Hjärnfonden, Svenska Läkarsällskapet, Torsten och Ragnar Söderbergs stiftelse (to P.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 2.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 4.Cotzias GC. l-Dopa for Parkinsonism. N Engl J Med. 1968;278:630. doi: 10.1056/nejm196803142781127. [DOI] [PubMed] [Google Scholar]
- 5.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 6.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 7.Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E. Altered D (1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Dis. 2007;26:452–463. doi: 10.1016/j.nbd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Aubert I, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- 9.Corvol JC, et al. Persistent increase in olfactory type G-protein α subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24:7007–7014. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pifl C, Nanoff C, Schingnitz G, Schutz W, Hornykiewicz O. Sensitization of dopamine-stimulated adenylyl cyclase in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys and patients with idiopathic Parkinson's disease. J Neurochem. 1992;58:1997–2004. doi: 10.1111/j.1471-4159.1992.tb10939.x. [DOI] [PubMed] [Google Scholar]
- 11.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 12.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci MA, Lee CS, Björklund A. l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- 14.Pavón N, Martín AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with l-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormalinvoluntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 16.Mela F, et al. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson's disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 17.Soghomonian JJ, Doucet G, Descarries L. Serotonin innervation in adult rat neostriatum. I. Quantified regional distribution. Brain Res. 1987;425:85–100. doi: 10.1016/0006-8993(87)90486-0. [DOI] [PubMed] [Google Scholar]
- 18.Kish SJ, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar P, Febvret A, Colombo J. Serotonergic sprouting in primate MTP-induced hemiparkinsonism. Exp Brain Res. 1993;93:100–106. doi: 10.1007/BF00230443. [DOI] [PubMed] [Google Scholar]
- 20.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 21.Ungerstedt U. 6-Hydroxydopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 22.Koe KB, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- 23.Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 1991;552:113–118. doi: 10.1016/0006-8993(91)90667-k. [DOI] [PubMed] [Google Scholar]
- 24.Johnston TH, Lee J, Gomez-Ramirez J, Fox SH, Brotchie JM. A simple rodent assay for the in vivo identification of agents with potential to reduce levodopa-induced dyskinesia in Parkinson's disease. Exp Neurol. 2005;191:243–250. doi: 10.1016/j.expneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Macor JE, et al. 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: A potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J Med Chem. 1990;33:2087–2093. doi: 10.1021/jm00170a007. [DOI] [PubMed] [Google Scholar]
- 26.Maroteaux L, et al. Mouse 5HT1B serotonin receptor: Cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svenningsson P, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 28.Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 29.Knobelman DA, Kung HF, Lucki I. Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT(1A) and 5-HT(1B) receptors. J Pharmacol Exp Ther. 2000;292:1111–1117. [PubMed] [Google Scholar]
- 30.Ng KY, Chase TN, Colburn RW, Kopin IJ. Dopamine: Stimulation-induced release from central neurons. Science. 1971;172:487–489. doi: 10.1126/science.172.3982.487. [DOI] [PubMed] [Google Scholar]
- 31.Bouhelal R, Smounya L, Bockaert J. 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substantia nigra. Eur J Pharmacol. 1988;151:189–196. doi: 10.1016/0014-2999(88)90799-6. [DOI] [PubMed] [Google Scholar]
- 32.Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aceves J, et al. Activation of D1 receptors stimulates accumulation of γ-aminobutyric acid in slices of the pars reticulata of 6-hydroxydopamine-lesioned rats. Neurosci Lett. 1992;145:40–42. doi: 10.1016/0304-3940(92)90198-g. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 2001. [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. San Diego: Academic; 1998. [Google Scholar]
- 36.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin FK, Brodie HK, Murphy DL, Bunney WE., Jr Administration of a peripheral decarboxylase inhibitor with l-dopa to depressed patients. Lancet. 1970;1:908–911. doi: 10.1016/s0140-6736(70)91044-5. [DOI] [PubMed] [Google Scholar]