Abstract
The neural underpinnings of age-related memory impairment remain to be fully elucidated. Using a subsequent memory face–name functional MRI (fMRI) paradigm, young and old adults showed a similar magnitude and extent of hippocampal activation during successful associative encoding. Young adults demonstrated greater deactivation (task-induced decrease in BOLD signal) in medial parietal regions during successful compared with failed encoding, whereas old adults as a group did not demonstrate a differential pattern of deactivation between trial types. The failure of deactivation was particularly evident in old adults who performed poorly on the memory task. These low-performing old adults demonstrated greater hippocampal and prefrontal activation to achieve successful encoding trials, possibly as a compensatory response. Findings suggest that successful encoding requires the coordination of neural activity in hippocampal, prefrontal, and parietal regions, and that age-related memory impairment may be primarily related to a loss of deactivation in medial parietal regions.
Keywords: aging, fMRI, hippocampus, default network, Alzheimer's disease
The process of successful memory formation likely requires coordinated patterns of neural activity among a distributed network of brain regions. Multiple functional MRI (fMRI) studies in young adults have reported a set of brain regions, including the hippocampus and related structures within the medial temporal lobe (MTL) and prefrontal cortices, that consistently demonstrate greater activity during the encoding of stimuli that will be subsequently remembered successfully compared with those that are subsequently forgotten (1–4). Studies in old adults have had much less consistent findings, particularly with regard to MTL activation (5–8).
Recent work in young adults also suggests that specific brain regions may need to “turn off” or deactivate [i.e., decrease in blood oxygen level-dependent (BOLD) signal] during successful encoding (9). This set of regions, including medial and lateral parietal cortices, are critical components of the “default mode network” (10, 11), because these regions consistently show higher levels of activity in the absence of focused cognitive processing. fMRI studies using a variety of cognitive tasks with block-design paradigms have demonstrated failure of deactivation in these regions in old adults, particularly prominent in those with mild cognitive impairment (MCI) and Alzheimer's disease (AD), compared with young adults (12–16). Moreover, there is recent evidence to suggest that the MTL memory system and the default mode network are part of a large distributed neural network that supports memory processing (10, 13, 17, 18) and that reciprocal alterations in these systems may underlie memory impairment in early AD (12).
Although recent studies have begun to investigate differences between young and old adults during memory processes, it remains unknown whether age-related memory impairment is accompanied by alterations in activation, deactivation, or in the coordination of activity within large-scale memory networks. In particular, the importance of deactivation during successful encoding in old adults has not been fully explored. Given that there are age-related alterations in the pattern of deactivation using block-design paradigms with other cognitive tasks (14, 16, 19), and that young adults deactivate specific neural structures to a greater degree during the encoding of subsequently remembered information (9), it is possible that age-related decline in memory performance is primarily related to a failure of suppression of default mode activity during memory formation.
Old adults who are considered cognitively normal can display a wide range of performance on memory tasks, compared with young adults; thus, it is important to study neural correlates in the context of memory performance. Previous comparisons of high-performing and low-performing old adults have suggested that increases in activation among old adults may reflect a need to compensate for age-related decline in memory systems (6, 7, 20–24). This effect of compensatory frontal activation has been reported both in high-performing old adults (20) and in elderly with declining cognition (24). Additionally, previous work has suggested that old adults with very mild cognitive impairment may demonstrate hyperactivation in the MTL to maintain memory performance (12, 25). However, other work has also suggested that not all increased activation observed in old adults is compensatory, but may also be “nonselective,” indicating old adults' failure of efficient cognitive processing (26) or decreased sensitivity to modulate brain responses according to task demands (16). Thus, it is important to study the impact of memory performance on both positive and negative modulation of fMRI activity and during both successful and failed memory encoding.
The present study, therefore, had three main aims: (i) to explore whether young and old adults differed in the pattern of activation and/or deactivation during successful associative encoding; (ii) to determine whether patterns of activation or deactivation were related to overall memory performance; and (iii) to investigate whether there was evidence of compensatory activation in the setting of failure to deactivate specific regions of the default mode network. Based on previous block-design studies (14, 19), and previous work in cognitively intact old adults (8, 27), we hypothesized that the greatest age-related differences during successful encoding would be seen in deactivation, particularly in medial parietal regions, rather than differences in MTL activation. Furthermore, it was predicted that these differences in deactivation would be related to differences in overall memory performance. Finally, we hypothesized that successful memory encoding requires the contribution of both task-related “activating nodes” and “deactivating nodes” within a distributed memory network (28), specifically involving coordinated activity between the MTL and parietal cortices. Given previous evidence that hyperactivation of the MTL might serve as a compensatory mechanism (12, 25), we hypothesized that failure of deactivation during successful encoding would be associated with greater hippocampal activation to achieve successful memory formation.
To investigate these hypotheses, we used a face–name associative memory paradigm (2), because difficulty remembering proper names is the most common memory complaint in old individuals (29). Seventeen young and 17 healthy old adults were scanned during encoding of unfamiliar faces paired with first names, followed by a postscan associative memory recognition test. To compare young and old adults on successful associative encoding, BOLD fMRI responses during those face–name pairs that were subsequently remembered correctly with high confidence were compared with the BOLD responses of those face–name pairs that were subsequently forgotten. To investigate the contribution of overall memory performance, we performed between-group analyses based on a median split of postscan memory test performance (18, 20) and correlational analyses using both whole-brain and region-of-interest (ROI) approaches.
Results
Behavioral Results.
Behavioral results on the postscan recognition memory test are presented in Tables 1 and 2. Notably, old adults performed significantly worse than young adults, correctly identifying 60.2% (SD = 5.5%) of trials overall, and correctly identifying 28.9% (SD = 16.9%) of trials overall with high confidence, t(32) = 5.2, P < 0.01 and t(32) = 3.2, P < 0.01, respectively. No significant age-related differences emerged in the percentage of face–name pairs remembered with low confidence or forgotten with low or high confidence.
Table 1.
Behavioral results by age group
| Young adults, M (SD) | Old adults, M (SD) | |
|---|---|---|
| Total hits, % | 73.3 (8.7) | 60.3 (5.5)* |
| HC-hits, % | 44.4 (10.3) | 28.9 (16.9)* |
| LC-hits, % | 28.9 (8.9) | 31.5 (15.8) |
| HC-misses, % | 8.2 (6.6) | 14.0 (11.3) |
| LC-misses, % | 17.9 (6.7) | 24.5 (11.8) |
Mean (M) percentage of face–name pairs that were classified as high-confidence hits, low-confidence hits, high-confidence misses, and low-confidence misses on the post scan memory test for young and older adults.
*Significant difference between young and older adults at P < 0.05.
Table 2.
Behavioral results by age and performance groups
| Age group/performance |
||||
|---|---|---|---|---|
| Young/high, M (SD) | Young/low, M (SD) | Old/high, M (SD) | Old/low, M (SD) | |
| Percentage of trials | ||||
| Total hits, % | 80.5 (5.6) | 66.0 (4.7) | 64.8 (4.0) | 55.8 (2.9)* |
| HC-hits, % | 48.9 (5.2) | 39.1 (12.5) | 38.0 (10.7) | 23.1 (17.5) |
| LC-hits, % | 31.6 (5.6) | 26.9 (11.5) | 26.9 (10.8) | 32.8 (18.2) |
| HC-misses, % | 4.2 (3.0) | 12.0 (7.5) | 15.5 (7.4) | 14.2 (14.5) |
| LC-misses, % | 14.9 (4.4) | 21.3 (7.6) | 18.2 (6.0) | 29.1 (13.8) |
| Reaction time | ||||
| Total hits, s | 2.2 (0.3) | 2.2 (0.4) | 2.2 (0.3) | 2.8 (1.5) |
| Total misses, s | 2.2 (0.2) | 2.2 (0.4) | 2.4 (0.5) | 2.8 (1.2) |
| HC-hits, s | 2.2 (0.3) | 2.2 (0.4) | 2.3 (0.2) | 3.1 (2.0) |
| LC-hits, s | 2.2 (0.3) | 2.2 (0.4) | 2.2 (0.3) | 2.8 (1.4) |
| HC-misses, s | 2.3 (0.3) | 2.3 (0.5) | 2.4 (0.6) | 3.0 (0.9) |
| LC-misses, s | 2.1 (0.2) | 2.2 (0.4) | 2.3 (0.5) | 2.7 (1.3) |
Mean (M) percentage of face–name pairs classified into the different trial types for high- and low-performing young and older adults. Mean reaction times (i.e., seconds until button press) during encoding of face–name pairs for each trial type and group.
*, P < 0.05 difference from all other groups.
To examine the contribution of memory performance, we divided young and old subject groups on the basis of a median split by postscan associative recognition performance (Hits), creating four groups: high-performing (HP) young, low-performing (LP) young, HP old, and LP old. We performed a 2 (age) × 2 (performance) ANOVA on percentage of trials correctly identified (hits) and percentage of trials correctly identified with high confidence (HC-hits). For both measures, there were main effects of age and performance (P < 0.01).
LP old adults performed worse than all other groups on the percentage of trials correctly identified (Table 2). Although LP old adults had relatively fewer HC-hits, a much higher percentage of high-confidence responses were hits than misses, t(7) = 3.9, P < 0.01, suggesting that HC-hit responses can be interpreted as “true learning” even among the LP individuals.
Old adults tended to be slower in responding to the stimuli than young adults; however, the differences were not significant for any trial type (Table 2). Importantly, reaction times did not differ between HC-hits and misses for old adults, t(11) = 0.6, P = 0.56, or for young adults, t(16) = −0.7, P = 0.51. Additionally, HP old adults and LP old adults did not differ in reaction times for any trial type.
Within-Group Activations.
We first examined whole-brain analyses for young and old groups separately, comparing event-related fMRI activity during encoding of face–name pairs that were subsequently remembered with high confidence (HC-hits) to forgotten pairs (misses). Both young and old groups demonstrated significant activation in anterior and middle regions of the hippocampus bilaterally (see Fig. 1; peak MNI coordinates x, y, z: young left: −24, −15, −18; young right: 21, −12, −21; old left: −18, −21, −12; old right: 30, −21, −21) and in bilateral inferior frontal regions (young left: −48, 6, 21; young right: 45, 33, 6; old left: −42, 24, 27; old right: 36, 27, −18) during the encoding of HC-hits compared with misses. Full tables of activation for each group are available as supporting information (SI).
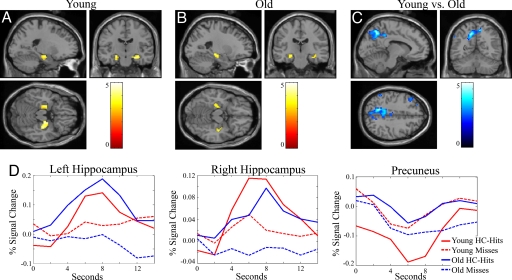
Fig. 1.
Hippocampal activation and medial parietal deactivation during successful encoding in young and old adults. (A and B) SPM2 random effects analysis demonstrates that young (A) and old (B) adults activate the hippocampus bilaterally (P < 0.005 minimum threshold). (C) The medial parietal region (peak MNI coordinates x, y, z: −6, −75, 45) shows significantly greater deactivation during successful encoding for young adults than old adults. (D) Estimated hemodynamic responses for HC-hits (solid lines) and misses (dashed lines) demonstrate that old adults (blue) show similar MR responses to that of young adults (red) during HC-hits (solid lines) and misses trials (dashed lines) in the bilateral hippocampus but not in medial parietal region.
Within-Group Deactivations.
We examined the opposite contrast, misses greater than HC-hits, to investigate whether HC-hits were associated with differential patterns of deactivation. Young adults demonstrated a significant decrease in BOLD signal during HC-hits compared with misses in the left middle frontal gyrus, left middle temporal gyrus, left inferior parietal lobule, and precuneus (−12, −57, 45). Examination of time courses for these clusters revealed BOLD signal decreasing below baseline during HC-hits, suggesting that these differences were driven by deactivation during successful encoding. As a group, old adults did not reveal any regions exhibiting differential deactivation during HC-hits compared with misses.
Age-Related Differences in Activations and Deactivations.
To compare differences in activation/deactivation between young and old groups, whole-brain two-sample map-wise comparisons of HC-hits greater than misses were performed. First, compared with old adults, young adults showed greater activation during HC-hits compared with misses in the left inferior temporal gyrus and right fusiform gyrus. No significant differences between age groups were found in the hippocampus in the whole-brain map-wise comparison. Additionally, performing a region of interest (ROI) analysis revealed no significant differences between young and old in either extent or magnitude of hippocampal activation. Full statistical tables for the ROI analyses are available as SI.
We then investigated areas where, compared with old adults, young adults showed less BOLD fMRI signal during HC-hits than during misses. The most significant age-related differences were found in medial parietal regions, in particular the precuneus bilaterally (left: −6, −75, 45 and right: 6, −72, 39). Investigation of the BOLD signal time courses within these regions revealed that young adults demonstrated greater deactivation (i.e., a larger magnitude of below-baseline BOLD signal during HC-hits) than old adults (see Fig. 1). A whole-brain map-wise ANOVA with trial type (HC-hits vs. misses) as a within-subject factor and age group (young vs. old adults) as a between-subjects factor similarly revealed significant interactions between trial type and age group in the precuneus (-15, −63, 36) and superior/middle temporal cortices (−54, −6, −9). An ANOVA of the percent signal change estimates from the precuneus ROI for all four trial types (HC-hits, low-confidence (LC)-hits, HC-misses, LC-misses) revealed a significant age × trial type interaction, F(3,30) = 9.4, P < 0.01. Follow-up analyses demonstrated a significant difference between young and old adults only for HC-hits [t(32) = 3.5, P < 0.01], demonstrating a greater decrease in BOLD signal in the young adults. No significant differences emerged for the other trial types.
Between-group differences were also found in left prefrontal regions (left superior frontal gyrus: −18, 57, 27 and left middle frontal gyrus: −39, 27, 42). ROI analyses revealed that group differences in these prefrontal regions were driven by greater activation in the old adults (i.e., old adults showed a larger magnitude of above-baseline BOLD signal during HC-hits) than young adults.
Relationship of Memory Performance to fMRI Activity.
To determine whether overall face–name memory performance was related to patterns of fMRI activity during successful encoding, we performed three separate analyses: (i) ROI analyses of the groups divided based on the median split of memory performance, (ii) ROI analyses correlating MR signal with performance both within and across groups, and (iii) map-wise correlational analyses.
The median split ROI 2 (age) × 2 (performance) ANOVA of the HC-hits percent signal change extracted from the precuneus revealed a trend toward an interaction between age and performance, F(1,28) = 2.8, P = 0.10. Further analysis revealed that HP old adults showed greater deactivation than LP old adults, t(14) = 2.7, P = 0.02; there was no difference between HP and LP young adults, t(14) = 0.25, P = 0.80. In fact, the LP old adults were the only group that did not show a significant decrease in BOLD fMRI signal below zero during trials of HC-hits, t(7) = 1.0, P = 0.33 (see Fig. 2). We did not find significant effects of performance for any of the other clusters that showed significant group differences between young and old adults.
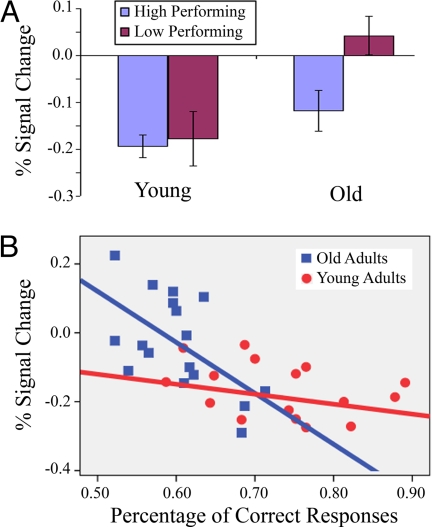
Fig. 2.
Medial parietal deactivation by age and performance. (A) HC-hits percent signal change estimates extracted from the medial parietal cluster show significant differences in deactivation between young and old adults. (B) Additional analysis reveals significant correlations between memory performance and HC-hit percent signal change estimates extracted from the precuneus among old adults (r = −0.59, P < 0.05) but not among young adults.
We then performed correlations between memory performance and percent signal change estimates extracted from the precuneus ROI. Across both young and old adults, there was a significant correlation between memory performance and HC-hits percent signal change, r = −0.58, P < 0.01, indicating that greater deactivation during successful encoding was related to better overall memory performance. No significant differences emerged for the other trial types (LC-hits, HC-misses, LC-misses). Next, we separately performed these correlations for young and old adults. Consistent with the median split analyses, old adults showed a significant correlation between HC-hits percent signal change estimates extracted from the precuneus and overall memory performance, r = −0.59, P = 0.01, but young adults did not demonstrate a significant correlation within group, r = −0.21, P = 0.42 (see Fig. 2). Moreover, no significant correlations emerged for percent signal change estimates of LC-hits, HC-misses, or LC-misses for either young or older adults.
Lastly, at a map-wise level, we correlated memory performance with successful encoding (HC-hits > misses). Across young and older adults, there was a significant correlation in the precuneus (−9, −63, 33) such that greater deactivation during HC-hits relative to misses was correlated with better overall memory performance. Full tables of clusters showing significant correlations to performance are available as SI.
Beneficial “Hyperactivation” in the Hippocampus and Prefrontal Cortex.
To explore the hypothesis that, in the setting of deactivation failure, increased activation in the hippocampus might represent a compensatory response, we examined MR signal response across the median split performance groups. To get an unbiased estimate of activation from the hippocampus for both young and older adults, we created an ROI that was based on the overlap of group-level activation in the hippocampus between young and old adults and then extracted the HC-hits percent signal change estimate for the four groups. A 2 (age) × 2 (performance) ANOVA revealed a significant effect of performance in the right hippocampus, F(1,28) = 4.1, P = 0.05, such that LP individuals activated the right hippocampus to a greater degree than HP individuals during successful encoding. No significant effect of age emerged, F(1,28) = 0.3, P = 0.61, again suggesting that young and old adults activate the hippocampus to a similar degree. Among old adults, LPs showed a marginally significant greater activation than HPs in the right hippocampus, t(14) = 2.0, P = 0.06. Although a similar relationship between performance and greater hippocampal activation was seen in the left hippocampus, this did not reach statistical significance. No significant effects emerged for percent signal change estimates extracted from LC-hits, HC-misses, or LC-misses for any of the groups.
We then explored whether any of the other regions that old adults activated during successful encoding might relate to overall memory performance (percentage of correct responses) in the old-adult groups. As can be seen in Fig. 3, comparison of the LP and HP old groups revealed a significantly greater BOLD fMRI signal response in the right hippocampus, t(14) = 2.2, P = 0.04, the right superior frontal cortex, t(14) = 2.9, P = 0.01, and a marginally significant effect in the left hippocampus, t(14) = 1.9, P = 0.08. Additionally, a correlational analysis across the entire old group revealed significant correlations between memory performance and BOLD signal during HC-hits in the right hippocampus (r = −0.51, P = 0.04) and the right superior frontal region (r = −0.64, P < 0.01). Importantly, we did not find significant correlations between memory performance and fMRI responses during misses in either region (right hippocampus: r = −0.23, P = 0.36; right superior frontal region: r = −0.30, P = 0.23). We also examined the hippocampal and prefrontal time courses for evidence of age-related differences in temporal features (16, 30). There was a trend in the right superior frontal region (P = 0.06) toward old adults taking longer to peak than young adults.
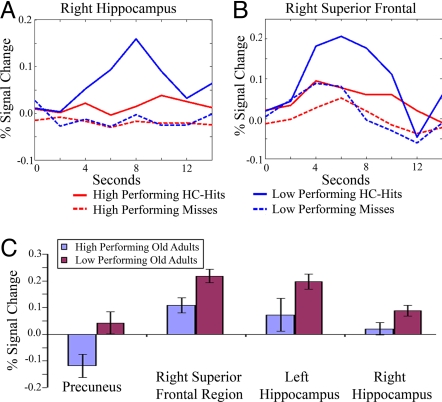
Fig. 3.
Activation and deactivation among old adults by performance. (A and B) Low-performing old adults (blue) show significantly increased MR responses to that of high-performing old adults (red) during HC-hits (solid lines), but not misses (dashed lines), in the right hippocampus (A) and right superior frontal region (B). (C) Low-performing old adults also demonstrate reduced deactivation (below baseline HC-hits percent signal change) in the precuneus.
Lastly, to investigate the hypothesis that successful memory formation is subserved by coordinated fMRI activity in a distributed memory network, we examined inverse correlations between precuneus deactivation and hippocampal and right superior frontal activation among old adults. Medial parietal deactivation was significantly inversely correlated with left hippocampal activation (r = −0.53, P = 0.03), right hippocampal activation (r = −0.63, P < 0.01), and right superior frontal activation (r = −0.55, P = 0.02) such that less deactivation during successful encoding was correlated with greater hippocampal and right superior frontal activation. In contrast, no significant correlations were found in the MR signal extracted during misses from the left hippocampus (r = −0.19, P = 0.48), right hippocampus (r = −0.01, P = 0.99), or right superior frontal region (r = −0.31, P = 0.22).
Discussion
Our study provides evidence that successful memory formation requires a coordinated pattern of activation and deactivation in a distributed memory network that is altered by the process of aging. During successful periods of encoding, healthy young individuals demonstrated a reciprocal relationship of MTL activation and parietal deactivation. Old adults, as a group, activated the hippocampus to a similar degree as young adults during successful associative encoding. Interestingly, the greatest age-related differences were found in the pattern of deactivation in the precuneus. Furthermore, decreased parietal deactivation was associated with more impaired memory performance on the postscan testing. Finally, successful memory formation in LP adults, who showed the most prominent failure of parietal deactivation, appeared to require increased hippocampal and inferior frontal activation, perhaps as a compensatory response. These findings suggest that a failure in the ability to deactivate specific regions in the default mode network and consequent disruption of reciprocal neural activity between MTL and parietal memory systems may underlie age-associated memory impairment.
Both block and event-related fMRI studies have suggested that the anterior hippocampus is critical for associative encoding (2, 31, 32). Our previous studies in healthy old adults (8, 12, 27) have also demonstrated intact anterior hippocampal activation in old adults, although these earlier studies did not directly compare successful to failed encoding. Recent studies with old adults from other groups have reported somewhat variable results regarding hippocampal activation (6, 7). These studies have focused primarily on the encoding of single stimuli instead of associative encoding and/or compared successful encoding to fixation rather than successful encoding to failed encoding. Our results provide evidence that to successfully form lasting cross-modal associations, old adults activate their hippocampus to a similar degree as young adults.
The patterns of deactivation that we observed in young adults are consistent with recent studies demonstrating “beneficial” deactivation in the precuneus and posterior cingulate during successful encoding (9, 33). These medial parietal regions are hypothesized to be a part of a “default mode” network that is active during periods when a person is awake but not engaged in a specific cognitive task (34). Multiple studies have shown this network of regions to deactivate when a person is given a specific goal-related cognitive task on which they must focus their attention (16, 35–37). Thus, deactivating parietal regions may allow individuals to reallocate their cognitive resources to focus more on the task at hand and thus be more successful during periods of encoding. Recent research even suggests that young and old adults demonstrate similar reductions in deactivation during relatively easy repetition priming tasks (38). It is possible that our findings regarding deactivation in old adults reflect a failure to fully attend to the stimuli as suggested by other recent work (25); however, we believe that this is unlikely to fully account for our results, given the finding of increased hippocampal and prefrontal activation in the setting of failed deactivation.
Medial parietal regions have also been characterized as part of a “retrosplenial memory system” involved in the successful retrieval of information (17). Once the information has been retrieved from memory, these regions are also involved in the evaluation of that information. Previous research has demonstrated that the posterior cingulate and some lateral parietal regions are more active during “remember” than “know” conditions (39). Furthermore, our own studies have suggested that these regions are involved in assessment of one's own memory performance (40), consistent with other reports that medial parietal regions activate to a greater degree during self-reflection and -assessment (41). Thus, it is possible that the degree of deactivation of these medial parietal regions during successful encoding is related to the degree of activation that would be observed during the successful retrieval of that information, particularly if the retrieval is associated with a subjective judgment about the memory retrieval.
Our findings are also consistent with previous research supporting the importance of a reciprocal relationship between parietal deactivation and hippocampal activation. There is evidence to suggest that the process of successful memory formation requires coordinated neural activity in both the activating nodes and deactivating nodes of a distributed memory network that includes both medial temporal lobe and parietal structures (28). There is also supporting data from resting fMRI studies that suggest that the hippocampus and parietal regions are functionally connected even when not engaged in a specific task (13, 42, 43). One recent study examining differences between young and old adults during memory retrieval also suggests a potential age-related decrease in connectivity within the hippocampal-parietal network during retrieval processes (18). Our data suggest that the reciprocal relationship between these regions during encoding is similarly altered by the process of aging.
Although all of the old adults in the current study are considered cognitively normal, the LP-old group did perform poorly, overall, on the postscan task. We specifically studied the pattern of activation and deactivation during HC-correct response to investigate “true learning” rather than responses that might be correct by chance. Given the low performance in this group, our findings have potential implications for age-related neurodegenerative diseases, such as early AD. The striking anatomic overlap between the failure of deactivation in aging and AD, with the pattern of FDG hypometabolism and amyloid deposition on PET imaging in medial and lateral parietal regions has been recently noted (17). Our previous work using block-design fMRI paradigms has furthermore suggested that there are parallel alterations in activation and deactivation memory systems that evolve over the course of MCI and AD (12). Similarly, another study found that normal old adults failed to deactivate medial parietal regions compared with young adults, and AD patients actually activated (rather than deactivated) these regions (14). These studies, in conjunction with our findings that the lowest performing old adults were also the least likely to deactivate, suggest that an inability to deactivate the medial parietal area could be one of the earliest signs of cognitive impairment that may herald incipient AD. In addition, the current study's findings may relate to the compensatory hypothesis raised in previous work. Consistent with our findings, it has recently been shown that old individuals with longitudinal decline in episodic memory performance demonstrated the greatest increases in frontal activation (24). Moreover, our previous work in MCI subjects (12, 25, 44) and studies from other groups in asymptomatic genetic at-risk individuals (45–47) have also suggested that hyperactivation of the hippocampus and prefrontal cortices may serve as a compensatory mechanism to maintain memory performance in the setting of early AD pathology.
In summary, our study provides evidence that the process of successful memory formation requires coordinated neural activity in both “activating nodes” and “deactivating nodes” of a distributed memory network that includes both medial temporal lobe and parietal structures. Both young and old adults engage the hippocampus during successful encoding, but old adults, particularly those on the lower end of memory performance within the continuum of normal aging, failed to deactivate medial parietal regions. Furthermore, our data suggest that increased activation in regions that are beneficial for memory encoding may be required to compensate for failure of deactivation during successful encoding. Future studies with larger sample sizes and longitudinal clinical follow-up will be required to determine whether these alterations in parietal deactivation are harbingers of further cognitive decline.
Materials and Methods
Subjects.
Seventeen right-handed, healthy young adults (5 males, 12 females; mean age: 23.9 years, range: 20–29) and 17 right-handed, healthy old adults (10 males, 7 females; mean age: 74.9 years, range: 58–82) consented to participate in this study. All subjects were screened for neurological and psychiatric illnesses, as well as any medications with central nervous system effects. Young adults were recruited via a web-based advertisement. Old adults were recruited from ongoing longitudinal studies on aging. All old subjects had been followed for at least 1 year before scanning and remained cognitively normal [Clinical Dementia Rating (CDR) = 0.0 and memory performance within 1.0 standard deviation of age- and education-adjusted normative scores] over the course of that year. The study procedures were approved by the Human Research Committee at Brigham and Women's Hospital.
Procedure.
The face–name associative encoding paradigm was a slower version of that previously published for young adults (2). Subjects were scanned during the encoding of 230 face–name pairs. Faces were displayed against a black background with a fictional first name printed in white underneath the face for 3.75 s. During the presentation of each face–name pair, subjects were asked to press a button indicating a purely subjective decision about whether the name was a good “fit” for the face or not. Before each run, subjects were explicitly instructed to try to remember the name associated with the face. Face–name stimuli were randomly intermixed with trials of visual fixation (a white crosshair centered on a black background) varying in length from 0.25 to 10 s with a mean fixation length of 2.84 s. Subjects were instructed to focus their attention on this cross while it appeared on the screen.
After scanning, subjects were shown each of the faces seen during scanning paired with two names written underneath: one that was correctly paired with the face and one that was paired with a different face during scanning. Subjects were asked to indicate which of two names was correctly paired with each face and to indicate how confident they were in their decision (high vs. low).
fMRI Scanning.
Subjects were scanned on a GE 3.0 Tesla scanner with a single channel head coil, with a T2*-weighted gradient-echo echo-planar imaging sequence [repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, and flip angle = 90°]. Twenty-eight slices (5-mm thickness; 1-mm interslice gap) were acquired in an oblique coronal orientation, perpendicular to the anterior commissure-posterior commissure line. Five functional runs were acquired for each subject with 145 time points per run.
fMRI Data Analysis.
fMRI data were preprocessed and analyzed by using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London) for Matlab (Mathworks). The data were motion corrected and normalized to the standard SPM2 EPI template, resliced into 3 × 3 × 3-mm3 resolution in Montreal Neurological Institute (MNI) space and smoothed with a Gaussian kernel of 8 mm. Event trials were modeled with the canonical hemodynamic response function only. No scaling was implemented for global effects. A high pass filter of 84 s was used to filter out low-frequency variations.
Trials were categorized by recognition accuracy (hit vs. miss) and confidence (high vs. low), allowing for four possible conditions: HC-hit, HC-miss, LC-hit, and LC-miss. The event-related analysis was based on a mixed-effects general linear model in SPM2. For each subject, all runs were concatenated and regressors added to account for signal differences between runs. For each subject, an HC-hits vs. misses contrast and misses vs. HC-hits contrast were created to compare successful and failed encoding trials.
One-sample t tests were run separately on the HC-hits vs. misses and misses vs. HC-hits contrasts in young and old groups. Two-sample t tests were then run to compare young and old adults on the HC-hits vs. misses and misses vs. HC-hits contrasts. All results were considered significant at P < 0.005 uncorrected with an extent threshold of 30 voxels. Additionally, given our a priori interest pertaining to the hippocampal and medial parietal regions, we also applied a small-volume-corrected threshold of P < 0.05 to the within-group, between-group, and correlational results regarding these areas by using SPM2 MarsBaR structural hippocampal and precuneal ROIs. All hippocampal and precuneal results of the present study that exceeded the whole-brain threshold of uncorrected P < 0.005 combined with the extent threshold of ≥30 voxels also exceeded the regional small-volume correction. Time courses were extracted from the corresponding significant clusters. Percent signal change estimates for each trial type were extracted from each cluster that previously showed a significant difference between young and old adults.
For hippocampal ROI analyses, extent of activation (number of voxels significant at P < 0.05 uncorrected) and magnitude of activation (percent signal change) for the HC-hits vs. misses contrast at the individual level were calculated within hippocampal MNI Marsbar ROI's based on the structural standard MNI template for each subject and entered into two-sample t tests. Two-way ANOVAs were run on the percent signal change estimates extracted from clusters showing significant differences between young and old adults during successful encoding. Percent signal change estimates extracted from the precuneus were correlated with percentage of overall correct responses (hits). Results from all ANOVAs, t tests, and correlational analyses of percent signal change estimates were considered significant at P < 0.05. ANOVAs were run on the percent signal change estimates extracted from unbiased hippocampal ROIs that were created by taking the intersection of the young and old group level activation maps (HC-hits > misses at P < 0.005 with an extent of 30).
Supplementary Material
ACKNOWLEDGMENTS.
This study was supported by National Institute on Aging Grants RO1-AG027435 and P50-AG00513421, the American Federation in Aging Research, and Harvard NeuroDiscovery.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706818105/DC1.
References
- 1.Kirchhoff BA, Wagner AD, Maril A, Stern CE. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uncapher MR, Otten LJ, Rugg MD. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 5.Daselaar SM, Veltman DJ, Rombouts SA, Lazeron RH, Raaijmakers JG, Jonker C. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 6.Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. J Cognit Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 7.Morcom AM, Good CD, Frackowiak RS, Rugg MD. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 8.Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Daselaar SM, Prince SE, Cabeza R. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Greicius MD, Krasnow B, Reiss AL, Menon V. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celone KA, Calhoun VD, Dickerson BD, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe D, Blacker D, et al. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius MD, Srivastava G, Reiss AL, Menon V. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. J Cognit Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- 17.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, et al. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady CL, Spring MV, Hongwanishkul D, McIntosh AR, Winocur G. J Cognit Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 20.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 21.Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 22.Dennis NA, Daselaar SM, Cabeza R. Neurobiol Aging. 2006;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Cereb Cortex. 2007 Oct 8; doi: 10.1093/cercor/bhm155. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson BC, Salat D, Greve D, Chua E, Rand-Giovannetti E, Rentz D, Bertram L, Mullin K, Tanzi R, Blacker D, et al. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 27.Sperling R, Bates J, Chua E, Cocchiarella A, Schacter DL, Rosen B, Albert M. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelinski EM, Gilewski MJ. Psychopharmacol Bull. 1988;24:523–529. [PubMed] [Google Scholar]
- 30.Handwerker DA, Gazzaley A, Inglis BA, D'Esposito M. Hum Brain Mapp. 2007;28:846–859. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson O, III, Shacter DL. NeuroImage. 2004;1:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 32.Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- 33.Otten LJ, Rugg MD. Curr Biol. 2001;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- 34.Gusnard DA, Raichle ME. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 35.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 36.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 37.Cavanna AE, Trimble MR. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 38.Soldan A, Gazes E, Hilton HJ, Stern Y. J Cognit Neurosci. in press. [Google Scholar]
- 39.Wheeler ME, Buckner RL. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. NeuroImage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 42.Greicius MD, Menon V. J Cognit Neurosci. 2004;16:1481–1483. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 43.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 44.Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondi MW, Houston WS, Eyler LT, Brown GG. Neurology. 2005;65:1514–1515. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. N Engl J Med. 2000;17:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mondadori CR, Buchmann A, Mustovic H, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Streffer J, Henke K. Brain. 2006;129:2908–29022. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.