Abstract
The endoplasmic reticulum (ER) in animal cells uses microtubule motor proteins to adopt and maintain its extended, reticular organization. Although the orientation of microtubules in many somatic cell types predicts that the ER should move toward microtubule plus ends, motor-dependent ER motility reconstituted in extracts of Xenopus laevis eggs is exclusively a minus end-directed, cytoplasmic dynein-driven process. We have used Xenopus egg, embryo, and somatic Xenopus tissue culture cell (XTC) extracts to study ER motility during embryonic development in Xenopus by video-enhanced differential interference contrast microscopy. Our results demonstrate that cytoplasmic dynein is the sole motor for microtubule-based ER motility throughout the early stages of development (up to at least the fifth embryonic interphase). When egg-derived ER membranes were incubated in somatic XTC cytosol, however, ER tubules moved in both directions along microtubules. Data from directionality assays suggest that plus end-directed ER tubule extensions contribute ∼19% of the total microtubule-based ER motility under these conditions. In XTC extracts, the rate of ER tubule extensions toward microtubule plus ends is lower (∼0.4 μm/s) than minus end-directed motility (∼1.3 μm/s), and plus end-directed motility is eliminated by a function-blocking anti-conventional kinesin heavy chain antibody (SUK4). In addition, we provide evidence that the initiation of plus end-directed ER motility in somatic cytosol is likely to occur via activation of membrane-associated kinesin.
INTRODUCTION
In animal cells, microtubule motors are used for establishing and maintaining the localization of organelles, as well as for transporting material from one organelle to another. Understanding the regulation of organelle-associated microtubule motors therefore remains central to our knowledge of membrane traffic within animal cells. Although many classes of microtubule motors have been documented, and the membrane cargoes have been identified for some of these, we still know little about how these motors are regulated and how the activities of opposing motors are coordinated on an individual organelle (for review, see Lane and Allan, 1998).
The inherent polarity of microtubules, with their fast-growing and -shrinking plus ends located at the cell periphery in many animal cell types, coupled with the unidirectional nature of all microtubule motors characterized so far, suggests that any organelle capable of moving in either direction along microtubules must possess at least two functional, opposing microtubule motors (although not necessarily at the same instant). The organelle-based microtubule motors already identified fall into two classes: those moving toward microtubule plus ends include conventional kinesin and the majority of other organelle-associated kinesin-related proteins (Lane and Allan, 1998), whereas minus end-directed organelle motors include cytoplasmic dynein (Paschal et al., 1987; Paschal and Vallee, 1987; Schroer et al., 1989) and potentially at least one type of kinesin-related protein, KIFC2 (Hanlon et al., 1997; Saito et al., 1997).
The endoplasmic reticulum (ER) has long been known to use the microtubule cytoskeleton as a framework for extending its reticular network of interconnecting tubules and lamellae in animal cells (Terasaki et al., 1986; Lee et al., 1989). Live cell studies using the lipophilic dye 3,3′-dihexyloxacarbocyanine iodide have revealed the ER to be a dynamic, motile organelle organized by motor-dependent tubule extension coupled with membrane fusion (e.g., Lee and Chen, 1988; Lee et al., 1989; Waterman-Storer and Salmon, 1998). The same processes have been observed in Xenopus egg extracts, which support the formation of ER networks in vitro, and in these extracts the motor protein responsible for ER motility has been identified as the minus end-directed motor cytoplasmic dynein (Allan, 1995; Niclas et al., 1996). This presents a paradox, because in many somatic cell types, such as those used in live cell studies, the arrangement of microtubules and the ER implicate a plus end-directed motor (i.e., not cytoplasmic dynein) in ER expansion toward the cell periphery (Lee et al., 1989; Waterman-Storer and Salmon, 1998). The aim of this study was to begin to understand the molecular basis for the different directions of microtubule-based ER movement observed in Xenopus eggs and in other species and/or cells.
The orientation of microtubules within the Xenopus egg is essentially similar to that observed in many somatic cell types (with microtubule plus ends outermost; Houliston and Elinson, 1991), so the fact that they use different motors to drive ER motility cannot simply be because their microtubules are arranged differently. Until now, extracts of Xenopus eggs used for motility studies have always been prepared during the first embryonic interphase. This is a very specialized stage of embryogenesis in Xenopus, when several important developmental processes are known to take place. One of these events, pronuclear migration, describes the dynein-driven movement of the female pronucleus along the microtubules of the sperm aster (Reinsch and Karsenti, 1997). Because the ER and the nuclear envelope are contiguous, it is possible that the sole reason for cytoplasmic dynein-driven ER movement in Xenopus eggs is to aid pronuclear migration. If that were the case, then extracts prepared from embryos at the second interphase and after might use a plus end-directed motor instead. Alternatively, the relatively large size of the Xenopus egg may require the ER to be organized differently from that observed within smaller, somatic cells; for instance, the ER might use cytoplasmic dynein to extend toward the center of the cell from the ER-rich egg cortex (Allan, 1996). It may only be as the embryonic cells approach midblastula transition (MBT), when they enter the somatic cell cycle and achieve a more somatic nuclear-to-cytoplasmic volume ratio, that they activate plus end-directed ER movement.
Antisense inhibition studies indicate that conventional kinesin may be the motor for plus end-directed ER motility in astrocytes and in neurons (Feiguin et al., 1994), and an antibody to kinectin, the putative membrane receptor for kinesin, labels an ER-like structure in chick embryo fibroblasts (Toyoshima et al., 1992). Interestingly, although immunofluorescence studies suggest that kinesin may colocalize with the ER in sea urchin coelomocytes and in Xenopus eggs (Houliston and Elinson, 1991; Henson et al., 1992), microinjection of function-blocking anti-kinesin antibodies did not alter the distribution of the ER in sea urchin embryos (Wright et al., 1993). Furthermore, analysis of organelle distribution in conventional kinesin knock-out mouse cells did not reveal any alteration in ER organization (Tanaka et al., 1998). This suggests that kinesin might not drive ER movement in all cell types. An additional mechanism for ER motility occurs when ER tubules are extended by association with growing microtubule plus ends (tip attachment complexes [TACs]) both in vitro and in vivo (Waterman-Storer et al., 1995; Waterman-Storer and Salmon, 1998), but it is not yet known whether microtubule motors are involved in this process.
In this study, we use cell-free extracts from a variety of developmental stages in Xenopus to investigate whether a switch in the direction of microtubule-based ER motility occurs, with plus end-directed, motor-driven ER motility being initiated at some point during development, either in place of or in addition to dynein-dependent motility. We find that plus end-directed, microtubule-based movement is activated when Xenopus egg ER is incubated in somatic cytosol, and we provide evidence that this regulatory event occurs through activation of ER-associated conventional kinesin.
MATERIALS AND METHODS
Reagents and Buffers
Unless otherwise stated, all reagents were purchased from Sigma (Poole, United Kingdom) or BDH (Poole, United Kingdom). The protease inhibitor (PI) mixture contained leupeptin; chymostatin, pepstatin, and aprotinin, each at 10 μg/ml final concentration. Energy mix for cell-free extracts consisted of 7.5 mM creatine phosphate, 1 mM ATP, 1 mM MgCl2, and 0.1 mM EGTA, pH 7.7, final concentration. Buffers used were acetate/sucrose buffer (A/S; 100 mM K-acetate, 3 mM Mg-acetate, 5 mM EGTA, 10 mM HEPES, and 150 mM sucrose, pH 7.4), Tris-buffered saline (20 mM Tris-HCl and 150 mM NaCl, pH 7.7), PBS, extract buffer (XB; 100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 50 mM sucrose, and 10 mM HEPES, pH 7.7), and Xenopus egg/embryo buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, and 0.1 mM EGTA, pH 7.8). The cell cracker was provided by Dr Philip Woodman (University of Manchester).
Preparation of Egg Extracts and Membrane Fractions for Motility Studies
Extracts were made from freshly laid eggs of Xenopus laevis (Blades, Kent, UK) as outlined previously (Murray, 1991). For extracts at the first embryonic interphase, either cytostatic factor (CSF)-arrested extracts (Murray, 1991) or electrically activated “cycling” extracts (Murray, 1991; Minshull et al., 1994) were prepared. The release of CSF-arrested extracts into interphase, after addition of CaCl2 to 0.2 mM, was followed using histone kinase assays (Murray, 1991). For the preparation of extracts from the second embryonic interphase, cycling extracts were allowed to progress through the first embryonic mitosis in vitro before being frozen in liquid nitrogen. Analysis of the cell cycle stage of cycling extracts was carried out by observing the morphology of added sperm chromatin and by histone kinase assays (Murray, 1991).
For motility assays, cytosol and membrane fractions were prepared by dilution in 2 vol of A/S, supplemented with energy mix, followed by centrifugation for 30 min in a TLA 100 rotor (Beckman Instruments, High Wycombe, United Kingdom) at 117,000 × gav, 4°C (Allan, 1998). The cytosol was then recovered, and the ER-rich membrane fraction, which forms at the interface between the cytosol and the ribosome–glycogen pellet, was resuspended in 20 μl of A/S and used directly for motility. Alternatively, egg membranes were resuspended in 200 μl of 2 M A/S buffer and transferred to Beckman Ultraclear 5 × 41-mm centrifuge tubes, and then 200 μl of 1.5 M A/S buffer and 150 μl A/S buffer cushions were layered over them. Membranes were then recovered free from cytosolic contaminants by flotation to the interface between the 1.5 M A/S and the standard A/S buffer layers by centrifugation at 150,000 × gav in an SW55 rotor (Beckman) for 1–2 h at 4°C.
In Vitro Fertilization and Embryo Extract Preparation
To prepare cytosols from embryos at various stages of Xenopus embryonic development, freshly laid eggs were fertilized synchronously in vitro by rubbing batches of eggs with the cut edge of a freshly dissected frog testis, in a minimal volume of Xenopus egg/embryo buffer, and then flushing with several milliliters of distilled water. Fertilized eggs were dejellied as described for egg extracts (Murray, 1991). The development of dejellied embryos was assessed by observing cleavage divisions, with any eggs failing to cleave being discarded. Once the desired developmental stage was reached, 300–500 embryos were washed in 2 vol of XB containing PIs and then packed in 500-μl Eppendorf tubes in a minimum volume of XB and PIs by spinning briefly in a microfuge (∼2000 rpm). Excess XB was then removed, and the embryos were crushed using a pipette fitted with a 200-μl tip. The cytoplasmic layer was then obtained by spinning the crushed embryos for 10 min at 10,000 × gav in a chilled (4°C) microfuge. The cytoplasm was recovered, supplemented with energy mix, made to 150 mM sucrose, and snap frozen in 60-μl aliquots for storage in liquid nitrogen. Cytosol for motility assays was prepared as described for egg extracts.
Preparation of Extracts of Somatic Cells
When preparing cytosol from somatic cells, Xenopus tissue culture cells (XTCs, a tadpole-derived cell line; Pudney et al., 1973) were grown to ∼90% confluence in 80% Leibovitz L15 medium (Life Technologies, Paisley, United Kingdom), supplemented with 10% Australian origin FBS (Life Technologies), in 25 × 162-cm2 vented lid cell culture flasks. Cells were then washed and resuspended in L15 medium after trypsin treatment. Cell suspensions were then pelleted gently, washed twice with A/S buffer containing PIs, and resuspended in 6 ml of A/S containing PIs. Cells were broken on ice by passing them ∼30 times through a chilled stainless steel cell cracker (clearance, 16 μm; Balch et al., 1984). The cell suspension was then spun at 2800 × gav for 10 min in a 4K15 centrifuge (Sigma Laborzentrifugen, Osterode am Harz, Germany) to obtain a postnuclear supernatant, which was then frozen and stored in liquid nitrogen. To prepare cytosols for motility studies and biochemistry, postnuclear supernatants were defrosted, supplemented with energy mix, and then centrifuged at 117,000 × gav for 30 min in a Beckman TL100 bench-top centrifuge, using a TLA 100 rotor. Cytosol was then recovered and used on the same day for motility assays.
Motility Assays and Data Acquisition
For motility assays, cytosol and membrane fractions were recombined in microscope slide “flow cells” and observed using video-enhanced differential interference contrast (VE-DIC) microscopy on an Olympus (Tokyo, Japan) BX60 microscope as described elsewhere (Allan, 1993). To quantitate ER motility under specific conditions, random microscope fields, containing microtubules and membranes, were recorded on S-VHS videotape, the number of membrane tubule extension events counted over a given period. Determination of tubule extension rates and frame grabbing for image reproduction were carried out using the Retrac object-tracking system (Dr N. Carter, Marie Curie Research Institute, Oxted, United Kingdom). Further image processing was carried out using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Kinesin-coated Bead Assays for Analysis of Membrane Motility Directionality
Membrane movements in a given microscope field were observed by VE-DIC for several minutes while being recorded on videotape. The membrane networks and cytosol were then washed away by gently flowing through several volumes (∼30 μl total) of motility buffer (acetate buffer [A/S without sucrose] containing 50 μg/ml cytochrome c and 50 μg/ml casein) including 20 μM Taxol (LC Labs, Nottingham, United Kingdom) and 0.1% Triton X-100 (Surfact-Amps grade; Pierce, Chester, United Kingdom), taking care to maintain the same field of observation. Next, 0.1-μm carboxylated beads (Polysciences, Warrington, PA; diluted 1:100 in motility buffer) were mixed with pig brain kinesin (0.5 μl of beads and 0.5 μl of kinesin, prepared according to the method of Wagner et al., 1991) and then suspended in 9 μl of motility buffer containing 1 mM ATP before being introduced into the flow cell. Bead motility along stabilized microtubules within the field was then recorded, and the polarity of each individual microtubule was determined. The plus end-directed movement of the kinesin-coated beads was confirmed using microtubules seeded from Tetrahymena axonemal fragments.
Antibodies and Immunological Techniques
Inhibition of membrane movement by function-blocking antibodies to kinesin (SUK4; Ingold et al., 1988) was carried out as follows. First, floated egg ER fractions were incubated on ice with concentrated SUK4 solution (∼5 mg/ml; 3 μl of membrane and 1 μl of SUK4) for 45 min. The membranes were then mixed with XTC cytosol (9.5 μl of cytosol and 0.3–0.5 μl of membrane), before introduction into flow cells for motility assays. Microtubules and membrane networks were allowed to form for 30 min before ER motility was recorded by VE-DIC in random microscope fields. Mean numbers of ER tubule extensions per minute were calculated in SUK4- and control antibody-treated (mouse immunoglobulin G [IgG]) membranes, both in the presence and absence of 20 μM sodium orthovanadate.
Immunofluorescence images of fixed Xenopus XTC cells were obtained as follows. Cells, grown to subconfluence on coverslips, were fixed in 80% PBS containing 2% paraformaldehyde and 0.2% glutaraldehyde for 15 min. The cells were then washed in PBS, treated with 0.5 mg/ml sodium borohydride (three times for 5 min each) in PBS, and, after further PBS washes, permeabilized by incubation in 0.1% Triton X-100 and 0.05% SDS in PBS for 4 min. Cells were then treated with primary antibodies diluted in PBS for 1 h at room temperature: monoclonal rat anti-α-tubulin ascites (clone YL1/2, used at 1:250; a gift from Dr J. Kilmartin, Medical Research Council-Laboratory of Molecular Biology, Cambridge, United Kingdom) and mouse anti-KDEL antibody culture supernatant (1D3, used at 1:2; a gift from Dr D. Vaux, University of Oxford, Oxford, United Kingdom), which primarily recognizes protein disulfide isomerase (PDI), followed by the appropriate secondary antibodies (anti-rat cy3 and anti-mouse FITC [both from Jackson ImmunoResearch, West Grove, PA], each at 1:200) for 30 min at room temperature. Cells were viewed by fluorescence microscopy using either a Leica (Wetzlar, Germany) DMRXA microscope, from which images were obtained using a cooled, slow-scan charge-coupled device camera (CH250; Photometrics, Tucson, AZ) and IPLab Spectrum P software (Signal Analytics, Vienna, VA) or a Leica TCS NT confocal microscope.
For immunoblotting, proteins were first separated by SDS-PAGE using a mini-Protean II gel electrophoresis apparatus (Bio-Rad, Hemel Hempstead, United Kingdom) and then transferred to nitrocellulose membrane using a Genie electrotransfer apparatus (IDEA, Minneapolis, MN). Membranes were blocked and then treated for 1 h with primary antibodies diluted in Blotto (washing buffer [Tris-buffered saline and 0.1% Triton X-100] containing 5% skimmed milk powder), followed by the appropriate alkaline phosphatase–conjugated secondary antibodies (Jackson ImmunoResearch). Proteins were visualized by incubating in a solution of 0.33 mg/ml nitro blue tetrazolium and 0.17 mg/ml 5-bromo-4-chrolo-3-indolyl phosphate (Fluka, Dorset, United Kingdom). Primary antibodies used for immunoblotting were an anti-p150Glued monoclonal (Transduction Laboratories, Lexington, KY; used at 1:2000), an anti-kinesin heavy chain (KHC) polyclonal (a gift from Dr Ron Vale, University of California, San Francisco, CA; used at 1:2000; Niclas et al., 1994), an anti-dynein intermediate chain monoclonal (Chemicon, Harrow, United Kingdom; used at 1:2000); an affinity-purified anti-Xenopus dynein intermediate chain polyclonal (raised against the N-terminal 96 amino acids; Lane and Allan, our unpublished observations; used at 1:500); and an anti-PDI monoclonal antibody (CEL5C culture supernatant; a gift from Prof. Birgit Lane, University of Dundee, Dundee, United Kingdom; used at 1:5).
RESULTS
Microtubule-based ER Motility Is Driven by Cytoplasmic Dynein in Xenopus Egg and Embryo Extracts
In previous studies, microtubule motor-dependent ER motility in Xenopus egg extracts has been shown to be exclusively minus end directed and cytoplasmic dynein driven (Allan, 1995; Niclas et al., 1996), but so far, only extracts prepared during the first embryonic interphase of development have been used (e.g., Allan, 1995; Waterman-Storer et al., 1995; Steffen et al., 1997). Moreover, ER movement has only been analyzed during the first half of this extended first embryonic interphase (in extracts prepared 15 min after artificial activation). Because the early stages of the first embryonic interphase in Xenopus incorporate specialized developmental events, which may influence ER motility in vivo (namely, pronuclear migration and cortical rotation; for review see Lane and Allan, 1998), it was important to ascertain whether any change in the direction of microtubule-based ER motility occurs after the completion of these processes. We therefore prepared cytosols from extracts at various stages during first interphase by activating fresh CSF-arrested extracts in vitro, from which high-speed supernatants and membrane fractions were collected for motility assays at selected time points. CSF-arrested extracts, which are made from eggs arrested at meiotic metaphase II, maintain their metaphase block by supplementation with EGTA and can be driven into interphase by the addition of excess calcium. In histone kinase assays, fresh CSF extracts activated in vitro typically displayed a peak of kinase activity at ∼100 min (our unpublished observations), likely to represent p34cdc2/cyclin B activation at first mitosis. Although somewhat delayed, this kinase peak was a good indication that these extracts were progressing fairly normally through the first interphase. For a rapid appraisal of the motors responsible for ER motility in these extracts in vitro, motility assays were carried out in the presence of 20 μM sodium orthovanadate, which has been shown to inhibit cytoplasmic dynein without affecting kinesin-driven movement (Niclas et al., 1996). We found that ER motility was abolished by this reagent in extracts prepared up to 80 min after activation (our unpublished results), suggesting that dynein-dependent ER motility persists at least up to the first embryonic mitosis.
Because ER motility was shown to be dependent on cytoplasmic dynein within extracts prepared at different stages during the first embryonic interphase, we set out to determine whether this microtubule motor was also required for ER motility after the first embryonic mitosis. To study ER motility during the second embryonic interphase in Xenopus, we prepared motility-competent, second interphase cytosols from cycling extracts made by electrical activation of eggs (Murray, 1991; Minshull et al., 1994). The cell cycle progression of these cycling extracts was assessed by histone kinase assays and by observing the morphology of added sperm chromatin. Routinely, extracts prepared from eggs crushed 30 min after activation (Minshull et al., 1994) progressed through the first embryonic mitosis more reliably than those made 15 min after activation. To make extracts from beyond the second embryonic interphase, freshly laid Xenopus eggs were fertilized in vitro, and, by observing their cleavage divisions, cytosolic and membrane fractions were prepared from embryos at the required developmental time points. Extracts made from embryos up to the fifth interphase, and up to the point of MBT (our unpublished data), were efficient at establishing microtubule networks and supporting microtubule-based membrane movement in motility assays, as judged by VE-DIC microscopy. Allowing for the variability that exists between individual extracts, we found that extracts prepared from embryos up to the fifth interphase were equally efficient at supporting membrane network formation (as determined by counting three-way junctions), and that their capacity to form networks did not differ markedly from that of first interphase extracts.
To begin to characterize ER motility during development, we used vanadate treatment (to inhibit dynein function) and directionality assays. The direction of ER tubule movement was determined either using salt-washed Tetrahymena axonemes (which polymerize microtubules exclusively from their plus ends; Allan and Vale, 1991) or kinesin-coated bead motility assays. We developed the kinesin-coated bead assay to track direction particularly where the arrangement of microtubules and membrane networks within the field were complex. In microscope fields of this nature, it is often not possible to see a small number of seeded microtubules among a network of randomly aligned unseeded microtubules. The application of kinesin-coated beads makes it possible to determine the polarity of all microtubules within a given microscope field within which ER tubule movements had been previously recorded (Figure 1). We found that ER motility was inhibited in the presence of 20 μM vanadate in extracts prepared at the second embryonic interphase from electrically activated eggs (Figure 2A), and directionality assays demonstrated that ER tubule movements in second interphase extracts were exclusively toward the minus ends of microtubules (n = 16; Table 1). Moreover, ER motility in embryo extracts, prepared up to the fifth embryonic interphase, was also acutely sensitive to the presence of vanadate (Figure 2A). Taken together, these data show that minus end-directed, dynein-driven ER movement is not confined to the first embryonic interphase, and that the only motor active on the ER up to the fifth embryonic interphase during Xenopus development is cytoplasmic dynein.
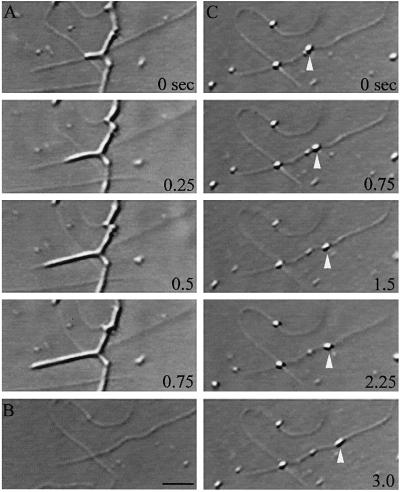
Figure 1.
Kinesin-coated bead motility for the analysis of ER tubule directionality. Membrane motility is recorded on videotape (A), and the membrane network is permeabilized and washed away while microtubule networks are stabilized by flowing through acetate buffer containing 0.1% Triton X-100 and 20 μM Taxol (B). Subsequently, carboxylated beads, precoated with pig brain kinesin, are flowed in, and the orientation of microtubules within the field is determined by observing the direction of kinesin-coated bead motility (C). Bar, 0.5 μm.
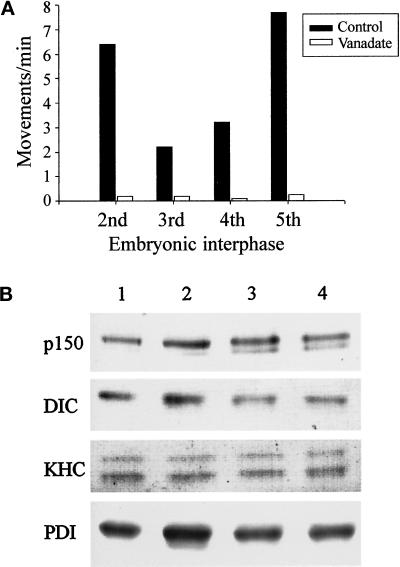
Figure 2.
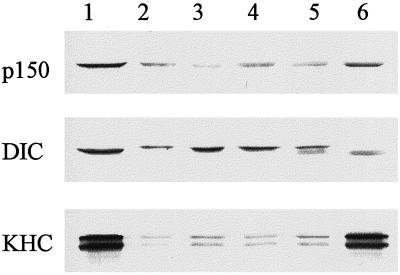
Analysis of membrane-associated motors and the sensitivity of ER movement to vanadate in extracts prepared from the first five embryonic interphases. (A) Sodium orthovanadate (20 μM) abolishes ER motility in Xenopus embryo extracts prepared from cycling extracts (second interphase) and in vitro fertilized, freshly laid eggs (third, fourth, and fifth interphases). Membrane movement was quantitated by counting the numbers of tubule extensions in 10 randomly selected microscope fields, each observed for 2 min, in the presence or absence of vanadate (average of at least two assays). (B) Analysis of ER-associated motors during Xenopus embryonic development. Floated membranes obtained from egg and embryo extracts were analyzed by immunoblotting using antibodies against p150Glued (p150), Xenopus dynein intermediate chain (DIC), KHC, and PDI (loading control). Floated membranes from eggs and embryos are shown during first interphase (lane 1), third interphase (lane 2), fourth interphase (lane 3), and fifth interphase (lane 4).
Table 1.
Characteristics of microtubule-based ER motility in X. laevis
| Assay description | Directionality summary | Rate (μm/sec) ±SD | ||
|---|---|---|---|---|
| Movement of egg ER in egg cytosol (1st interphase) | Minus end | 100% | (n > 100)a | 1.65 ± 0.29a |
| Plus end | 0% | |||
| Movement of egg ER in activated egg extracts (2nd interphase) | Minus end | 100% | (n = 16) | NDb |
| Plus end | 0% | |||
| Movement of floated egg ER in XTC cytosol | Minus end | 81% | (n = 42) | 1.29 ± 0.30 (n = 26) |
| Plus end | 19% | 0.38 ± 0.05 (n = 6) | ||
| Movement of floated egg ER in XTC cytosol + 20 μM vanadate | Minus end | 0% | (n = 17) | |
| Plus end | 100% | 0.47 ± 0.09 (n = 14) | ||
Data from Allan (1995).
ND, not determined.
Immunoblot analysis of egg and embryo membranes demonstrated that both cytoplasmic dynein intermediate chain and p150Glued (a component of the dynein regulatory complex dynactin) are present on floated egg and embryo membranes (Figure 2B). Furthermore, the relative amounts of these proteins appear unchanged on membranes derived from embryos up to the fifth embryonic interphase (Figure 2B). Interestingly, conventional KHC was also present on embryo membranes up to the fifth interphase, even though motility assays revealed that this motor is not active on membranes at these stages of development.
Xenopus Egg Membranes (ER) Exhibit Bidirectional Movement along Microtubules in XTC Extracts
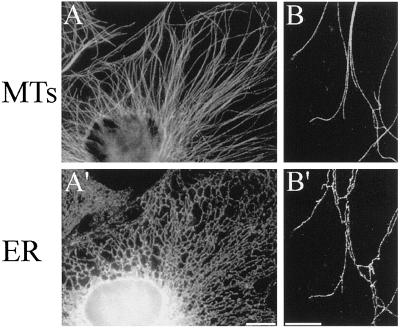
In cell types in which microtubules extend outward from an organizing center located near the middle of the cell, one would expect microtubule-based motility toward the cell periphery to be predominantly plus end directed. The distribution of microtubules and the ER as visualized by immunofluorescence of Xenopus XTC cells strongly suggests that the ER uses a plus end-directed microtubule motor for its expansion throughout the cytoplasm of this cell type (Figure 3). In the periphery of XTC cells in particular, microtubules and ER tubules are very closely aligned (Figure 3B), and discrete points of attachment can be seen both at the tips, possibly representing sites of motor activity (Allan and Vale, 1994) or TACs (Waterman-Storer et al., 1995; Waterman-Storer and Salmon, 1998), and along the lengths of ER tubules, implicating other mechanisms of ER–microtubule association such as the recently identified integral ER membrane protein p63 (Klopfenstein et al., 1998).
Figure 3.
Immunofluorescence images of fixed Xenopus XTC cells labeled with antibodies against microtubules (MTs, top) and the ER (bottom). (A and A′) Portion of a typical interphase XTC cell showing the extended, reticular morphology of the ER. Bar, 10 μm (B and B′) Confocal images of the periphery of an XTC cell showing the close association between microtubules and the ER (in most cases, microtubules and ER tubules do not coalign exactly; instead, the ER tubules appear to be associated with microtubules at discrete sites along their lengths, as well as at their tips). Bar, = 5 μm. Microtubules and the ER were visualized using a rat monoclonal antibody raised against α-tubulin (clone YL1/2) and a mouse monoclonal antibody recognizing PDI (clone 1D3), respectively, and the appropriate anti-rat and anti-mouse fluorescently labeled secondary antibodies.
To analyze the influence of somatic cytosol on ER motility, we prepared a cytoplasmic fraction from cultured XTC cells for use in motility assays. These extracts readily assembled microtubules from endogenous pools of tubulin (without supplementation with additional microtubules or the inclusion of Taxol) and also supported abundant microtubule-based membrane motility events using endogenous organelles (our unpublished observations) as well as membranes from other sources. In general, XTC extracts were a little slower to form microtubule networks than egg-derived cytosols, but microtubule-based membrane motility appeared to be similarly active in these extracts. Because the ER in somatic cells is expected to move at least partly toward microtubule plus ends (Waterman-Storer and Salmon, 1998), whereas the ER in extracts prepared from early Xenopus embryos does not (Allan, 1995; Niclas et al., 1996; this study), we decided to test what happened to the direction of egg ER movement in the presence of somatic cell cytosol. To this end, we prepared egg membranes by flotation to reduce cytosolic contamination and then combined this ER-rich egg membrane fraction with somatic cytosol derived from XTC cells.
In XTC cytosol, the floated egg membranes rapidly formed networks of tubules and lamellae by a microtubule motor-dependent process. Using VE-DIC microscopy, membrane networks were seen to form, predominantly by ER tubules being drawn outward along stationary microtubules, using motors located at their tips. Tubules eventually fused with adjacent membrane networks, forming characteristic three-way junctions, or alternatively became detached from the microtubule, presumably owing to the tension generated by tubule elongation, and then snapped back into the body of membrane. Less often, ER tubules were seen extending from the main body of membranes, attached to the growing tips of microtubules or to the tips of microtubules gliding on the coverslip surface. These sites of attachment were similar to TACs observed in egg cytosol (Waterman-Storer et al., 1995); however, the majority of tubule extensions were due to motor-driven sliding along microtubules. To confirm that these membrane networks were composed of ER, membranes were fixed and then stained with an antibody that recognizes PDI and other lumenal KDEL-containing ER proteins and labels the ER in Xenopus (Figure 3). Like the ER networks formed in egg cytosol in vitro (Allan, 1995), the membrane networks in XTC cytosol were labeled by anti-PDI (our unpublished observations) in a pattern resembling the ER of intact cells (see Figure 3).
To determine whether floated egg ER tubules were also driven exclusively by cytoplasmic dynein in XTC cytosol, motility assays were performed using these extracts in the presence of 20 μM vanadate. Unlike ER motility observed in egg and embryo cytosols, the floated egg ER fraction moved along microtubules in XTC cytosol even when vanadate was included in the assay (Figure 4). Counting ER tubule extensions along microtubules in XTC cytosol in the presence of vanadate suggested that this reagent inhibited ER motility only by ∼50% (Figure 4). This result suggested that a different microtubule motor, not cytoplasmic dynein, was driving ER motility in XTC cytosol in the presence of vanadate. We therefore decided to determine the direction of vanadate-insensitive tubule movements in XTC cytosol. Using the kinesin-coated bead directionality assay, we found that 100% of vanadate-insensitive ER tubule extensions occurred toward microtubule plus ends (n = 17; Table 1).
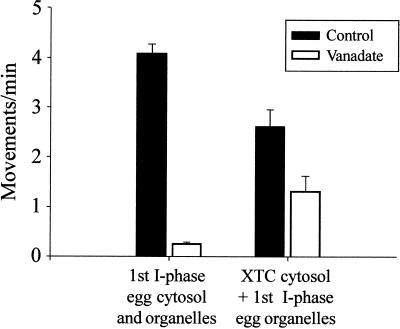
Figure 4.
ER motility is only partially inhibited by vanadate in XTC cytosol. Floated egg ER was incubated in first interphase egg cytosol or in XTC cytosol, and ER motility was quantitated by counting the numbers of membrane tubule extensions in 10 randomly selected microscope fields, each observed for 2 min, in the presence or absence of 20 μM vanadate (average of at least three assays; bars indicate SEs).
Given that ER tubules were able to move toward the plus ends of microtubules in XTC cytosol in the presence of vanadate, it seemed likely that ER networks formed in the absence of vanadate by a combination of plus and minus end-directed motility. To test whether this were true and to estimate the proportion of plus end-directed ER motility in XTC cytosol in the absence of vanadate, we recorded ER movements in individual fields of floated egg ER in XTC cytosol before carrying out kinesin-coated bead directionality assays. As expected, floated egg ER membranes were found to move in both directions along microtubules in XTC cytosol, sometimes with membrane tubules extending in opposite directions along a single microtubule (Figure 5). On one occasion membrane tubules were observed to move toward one another along the same microtubule, eventually fusing at their tips to form a continuous tubule. These events confirmed that the membrane tubules observed moving in either direction along microtubules were derived from the same organelle (ER). Using the kinesin-coated bead directionality assay, we determined that approximately one-fifth of floated egg ER tubule movements in XTC cytosol were plus end directed (19%; n = 42; Table 1), and we believe that this represents the likely contribution of the plus end-directed motor to egg ER motility in XTC cytosol more accurately than the vanadate inhibition data (for instance, in the presence of vanadate, tubules are still extended by TACs, and these events are sometimes difficult to distinguish from motor-driven movement).
Figure 5.
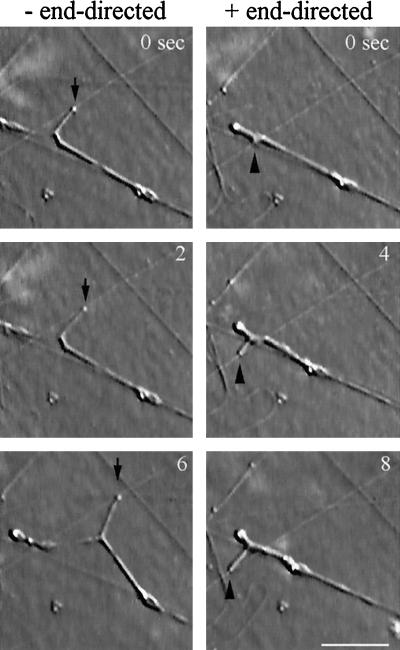
An example of plus and minus end-directed motility of floated egg ER in XTC cytosol observed by VE-DIC. ER tubules are shown extending outward from the same body of membrane, in opposite directions along the same microtubule. A bead motility assay was also performed on this field of microtubules to determine the polarity of the microtubule concerned. The minus end-directed tubule is indicated by arrows (to the left), and the plus end-directed tubule is indicate by arrowheads (to the right). Bar, 5 μm.
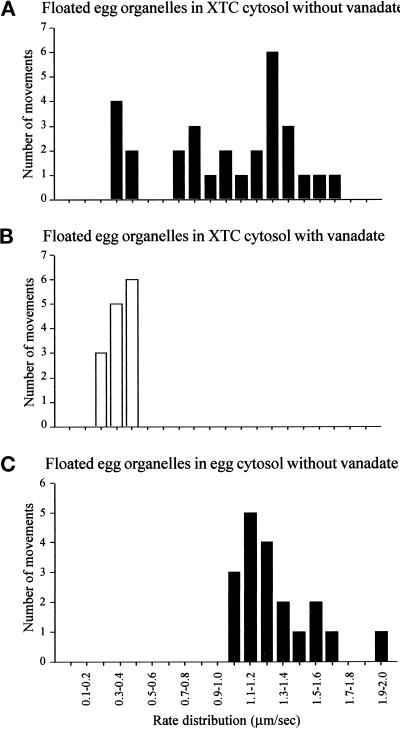
The rate of plus end-directed membrane tubule extension in the presence of vanadate was lower than the typical, vanadate-sensitive (minus end-directed) tubule movements (Table 1). This difference could not be simply due to the effects of vanadate, because plus end-directed extensions in the absence of the drug were slower (rate, 0.38 ± 0.05 μm/s; n = 6) than the minus end-directed movements in XTC cytosol (rate, 1.29 ± 0.30 μm/s; n = 26), the latter being more comparable with the rate of dynein-driven ER motility recorded previously in egg cytosol (rate, 1.65 ± 0.29 μm/s [Allan, 1995]; Table 1). We also plotted the distribution of rates of ER tubule extension in XTC versus egg cytosols (Figure 6). In the present study, the egg ER moved at an average rate of 1.41 ± 0.23 μm/s in egg cytosol (n = 19; Figure 6C), with no motility observed in the presence of vanadate. By contrast, in XTC cytosol, the same ER fraction displayed two peaks of motility rates (Figure 6A): a slower peak at ∼0.3–0.5 μm/s and a faster peak between 0.7 and 1.8 μm/s. Significantly, in the presence of vanadate only the slower peak could be distinguished (Figure 6B). In addition, we observed that plus end-directed, vanadate-insensitive movements appeared more persistent and less susceptible to the phenomenon of snapping back than minus end-directed motility. This may reflect the different biophysical properties of the two motors involved (cytoplasmic dynein and a plus end-directed motor).
Figure 6.
Analysis of the distribution of ER tubule rates in XTC and egg cytosols. (A) Floated egg organelles incubated in XTC cytosol displayed two apparent peaks of of ER extension rates: a slow rate at 0.3–0.5 μm/s, and a faster rate of between 0.7 and 1.8 μm/s. (B) When 20 μM vanadate was included, the faster peak of rates was abolished, but a slower peak of between 0.2 and 0.5 μm/s remained. (C) In egg cytosol, only a single peak of rates of egg ER motility at between 1.0 and 2.0 μm/s was observed (all motility was abolished by vanadate).
Identification of the Motor for Plus End–directed ER Motility in XTC Cytosol
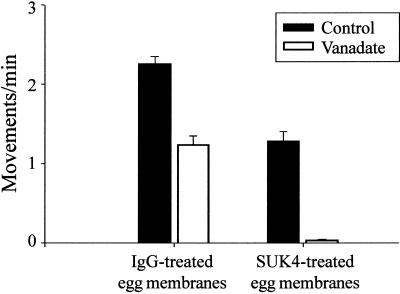
Because antisense inhibition studies in astrocytes implicate conventional kinesin as the motor driving ER motility, in this cell type at least (Feiguin et al., 1994), we tested whether plus end-directed ER motility was due to conventional kinesin in Xenopus XTC cytosol. We used the function-blocking anti-KHC antibody SUK4 (Ingold et al., 1988), which is known to inhibit conventional kinesin motility in Xenopus in vivo (Tuma et al., 1998). Motility assays were carried out in the presence and absence of 20 μM vanadate, using XTC cytosol incubated with floated egg ER that had been pretreated with SUK4 or control antibody (mouse IgG). In the presence of SUK4, vanadate-insensitive plus end-directed motility of floated egg ER in XTC cytosol was almost completely blocked (Figure 7). Under these conditions, no membrane networks were observed; instead, nonmotile membrane clumps were seen. The control antibody had no apparent affect on ER motility in XTC cytosol, and membrane networks formed normally. Furthermore, SUK4 appeared not to affect cytoplasmic dynein-driven motility observed in the absence of vanadate.
Figure 7.
Plus end-directed ER motility in XTC cytosol is inhibited by the function-blocking anti-KHC antibody SUK4. Floated egg ER membranes were preincubated either with mouse IgG (control antibody) or with SUK4 and then mixed with XTC cytosol in the presence and absence of 20 μM vanadate. Motility was recorded in 10 random microscope fields of 2 min (mean of three independent assays). In the absence of vanadate, membrane motility was reduced by SUK4 pretreatment (compare IgG-treated control with SUK4-treated control). When vanadate was included, motility of SUK4-treated ER was abolished, suggesting that the vanadate-insensitive component of egg ER motility in XTC cytosol is driven by conventional kinesin. Bars indicate SEs.
Given that egg membranes began moving using kinesin when incubated in XTC cytosol, we wanted to know how this activation occurred: did it involve the recruitment of cytosolic kinesin to the membrane or the activation of kinesin already present on the egg membranes, or was a loss or inactivation of ER-associated cytoplasmic dynein involved?
We first tested whether there were any gross changes in the amount of cytoplasmic dynein on egg-derived ER-rich membranes that had been incubated in XTC cytosol under typical motility assay conditions (Figure 8). In Xenopus egg and embryo extracts, dynein intermediate chain runs at an apparent molecular mass of 85–90 kDa (Niclas et al., 1996), whereas the somatic form of this motor is slightly more mobile by SDS-PAGE (Figure 8, compare lanes 1 and 6). After incubation of the floated egg membrane fraction in XTC cytosol, we observed a clear reduction in the amount of embryonic dynein intermediate chain on the membrane (less mobile protein; Figure 8, lane 5), whereas a small quantity of somatic cytoplasmic dynein intermediate chain was recruited to the membrane (more mobile protein; Figure 8, lane 5) from the soluble pool (Figure 8, lane 6). This result suggests that there may be some turnover of cytoplamic dynein on the egg membrane fraction in XTC cytosol but not when the membranes are incubated in buffer alone (Figure 8, compare lanes 2, 3, and 5). Similar results were obtained using an antibody to cytoplasmic dynein light intermediate chain (Addinall and Allan, unpublished data), suggesting that the whole of the cytoplasmic dynein complex, and not just dynein intermediate chain, may be exchanged upon incubation in XTC cytosol. Indeed, other studies have indicated that cytoplasmic dynein’s association with membranes may be relatively labile (for review, see Lane and Allan, 1998). In the case of the ER-rich Xenopus egg membranes, for example, an antibody to dynein intermediate chain caused a rapid dissociation of cytoplasmic dynein (and a proportion of p150Glued) from the membrane (Steffen et al., 1997). Interestingly, even though there is exchange of cytoplasmic dynein complexes, the bulk of ER movement was still dynein driven, suggesting that the newly recruited motor was active. There was no obvious change in the amount of membrane-bound p150Glued after incubation in XTC cytosol (Figure 8), but because p150Glued on egg membranes and in XTC cytosol have comparable mobilities by SDS-PAGE (Figure 8, compare lanes 1 and 6), we were unable to determine whether the cytosolic form had replaced that of the egg molecule for molecule.
Figure 8.
Analysis of ER-associated motors after incubation in egg and XTC cytosols. Floated egg ER membranes were incubated in A/S buffer, egg cytosol, or XTC cytosol and then recovered again by flotation and analyzed by immunoblotting. Lane 1, egg cytosolic proteins; lane 2, floated egg ER membranes; lane 3, egg ER after incubation in A/S buffer; lane 4, egg ER after incubation in egg cytosol; lane 5, egg ER after incubation in XTC cytosol; lane 6, XTC cytosolic proteins. In each lane, equal quantities of proteins were loaded, and membranes were probed with an antibody against a subunit of dynactin complex (p150Glued), antibodies against dynein intermediate chain (DIC; clone IC74), and KHC. This experiment was carried out three times, and on each occasion the same results were obtained.
The same samples were also immunoblotted using antibodies to KHC, and although the embryonic and somatic forms of this motor cannot be distinguished by SDS-PAGE (Figure 8, compare lanes 1 and 6), we determined that the incubation of egg membranes in buffer, egg cytosol, or XTC cytosol had no obvious effect on the amount of membrane-associated kinesin. The activation of kinesin-driven ER movement is therefore unlikely to be due to a wholesale recruitment of somatic kinesin, but using these data we cannot rule the possibility that a simple exchange of cytosolic and egg KHC had occurred during the incubation period. However, the fact that pretreating membranes with SUK4 inhibited their ability to move toward microtuble plus ends when added to untreated XTC cytosol (Figure 7) suggested strongly that the kinesin involved was already present on the egg membranes. In addition, when an equivalent concentration of SUK4 was included in the cytosol, and membranes were not pretreated with the antibody, the plus end-directed ER motility was unaffected (our unpublished results). Taken together, these results suggest that the embryonic, membrane-associated kinesin is activated in some way by somatic cytosol.
DISCUSSION
We have used an in vitro assay system to investigate why cytoplasmic dynein is the exclusive motor for ER motility in Xenopus egg extracts when a plus end-directed motor, possibly kinesin, is implicated in ER motility in somatic cells (Feiguin et al., 1994; Waterman-Storer and Salmon, 1998). An explanation might be found in the events known to occur during the first cell cycle of Xenopus embryonic development, which is when the extracts used in previous studies were prepared. One such event, pronuclear migration, is a dynein-dependent process, which, although unlikely to be driven directly by ER tubule movements (Reinsch and Karsenti, 1997), may explain why dynein is active on the contiguous ER and nuclear envelope membranes at this stage of development. If this were the case, then we might have expected to see a switch in the direction of ER motility along microtubules in Xenopus, with a plus end-directed motor being activated soon after the completion of pronuclear migration. On the contrary, we found, using vanadate treatment and directionality assays, that cytoplasmic dynein remains the sole motor for microtubule-based ER motility in extracts prepared up to at least the fifth embryonic interphase. The reasons why different motors drive ER motility in Xenopus embryos and somatic cell types are therefore unlikely to relate to specific events during the first embryonic interphase. Instead, we contend that the ER is moved exclusively by cytoplasmic dynein during early embryogenesis, perhaps until the initiation of the somatic cell cycle at around the point of MBT.
To determine which motors drive ER movement in somatic cells, we reconstituted ER motility in vitro by combining somatic cytosol from extracts of the Xenopus XTC cell line with egg-derived ER. In somatic cytosol, microtubule motor-based ER motility was not inhibited outright by 20 μM vanadate, which implicates the involvement of a motor other than cytoplasmic dynein. Furthermore, the vanadate-insensitive motility was found to be entirely plus end directed and was abolished by the function-blocking anti-KHC antibody SUK4. A role for the plus end-directed motor kinesin in ER tubule motility in vitro is supported by the observation that ER tubules extend outward toward microtubule plus ends at the periphery of somatic cells (Lee et al., 1989; Feiguin et al., 1994; Waterman-Storer and Salmon, 1998).
What evidence is there for kinesin-driven ER motility in other organisms and/or cell types? Immunofluorescence studies indicate that both kinesin (Houliston and Elinson, 1991; Henson et al., 1992) and kinectin (Toyoshima et al., 1992) are present on the ER in some cell types. However, motor location does not necessarily predict function, and there have also been several other investigations in which kinesin has not been localized to the ER (Marks et al., 1994; Lippincott-Schwartz et al., 1995). There is also good evidence that active ER motors may be concentrated in motile tip domains (Allan and Vale, 1994; Tabb et al., 1998; Waterman-Storer and Salmon, 1998), in which case motor antibodies would fail to reveal the entire, reticular organization of this organelle. A better indication of motor protein function in cells has been provided through constitutive down-regulation, although the results of these studies are inconclusive. For instance, depleting KHC in astrocytes using antisense oligonucleotides caused the ER to retract away from the cell periphery and to be incapable of centrifugal extension (Feiguin et al., 1994). By contrast, no change in ER morphology was reported after microinjection of function-blocking anti-kinesin antibodies in sea urchin embryos (Wright et al., 1993) or when kinesin function was perturbed in gene knock-out mouse cells (Tanaka et al., 1998) or in cells expressing a “rigor” kinesin mutant (Nakata and Hirokawa, 1995). Our study in Xenopus provides direct evidence for kinesin-driven ER motility, but a role for kinesin in ER motility in all animal cells remains to be confirmed.
Although we have reported that somatic Xenopus cytosol can activate kinesin-driven ER motility in vitro, ∼80% of tubule extensions were still driven by cytoplasmic dynein. Does cytoplasmic dynein also play a role in ER motility in somatic cells in vivo, perhaps providing a resisting force against which the ER is spread outward by kinesin? It is likely that cytoplasmic dynein may be at least partially responsible for membrane motility in chick embryo fibroblast extracts, because vanadate has been shown to impede the formation of these ER-like membrane networks (Dabora and Sheetz, 1988). So far, however, there have been no published examples of cytoplasmic dynein-driven ER motility in vivo. In fact, there have been some studies in which disrupting cytoplasmic dynein function appeared to have no effect on ER organization. In blastocyst cells derived from cytoplasmic dynein heavy chain 1 knock-out mouse embryos, for example, the organization of the ER was found to be unaltered (Harada et al., 1998). In addition, no change in ER morphology was described either in cells microinjected with anti-dynein antibodies (Vaisberg et al., 1996; Burkhardt et al., 1997) or in cells overexpressing p50/dynamitin, which is thought to disrupt membrane-associated dynein/dynactin Burkhardt et al., 1997; Presley et al., 1997; Roghi and Allan, unpublished results).
These negative results do not preclude a more subtle role for cytoplasmic dynein in ER movement, which would not be revealed by looking at ER morphology as a whole; in studies of the ER in living cells, practical limitations have meant that motility has only been observed at the cell periphery, meaning that any microtubule motor-driven, minus end-directed movement at the cell center may have been missed (Lee and Chen, 1988; Lee et al., 1989; Waterman-Storer and Salmon, 1998). Recently, we have confirmed, by observing the motility of rat liver ER fractions in XTC extracts, that cytoplasmic dynein does indeed contribute to the formation of somatic cell-derived ER networks in vitro and might even facilitate the maintenance of separate rough and smooth ER domains in living cells (Lane and Allan, unpublished observations). In animal cells, there is also evidence of a complementary role for the actin cytoskeleton in the retrograde flow (toward the cell center) of ER membranes in vivo (Terasaki and Reese, 1994; Waterman-Storer and Salmon, 1998), whereas in locust photoreceptor cells (Stürmer et al., 1995) and squid axoplasm (Kuznetsov et al., 1992; Kuznetsov et al., 1994; Tabb et al., 1998), the ER can translocate along actin cables as well as along microtubules. Hence, it may be that a complex combination of opposing microtubule motors and myosins contributes to the organization of the ER in animal cells.
Analysis of the rates of motor-driven organelle motility can contribute to our understanding of motor function. To date, although there are many published rates for purified or recombinant microtubule motors (for examples, see Lane and Allan, 1998), there are few examples of rates cited for organelle fractions, moving using defined motor proteins. We found that cytoplasmic dynein-driven ER motility in XTC cytosol (at 1.29 μm/s) was toward the upper range of published rates for this motor (for example, see Paschal et al., 1987; Schnapp and Reese, 1989; Schroer et al., 1989) although being somewhat slower than the rate of ER motility in egg cytosol (1.65 μm/s; Allan, 1995). So far, evidence for the rate of conventional kinesin-driven organelle translocation is sparse. We have observed an apparent rate of ∼0.4 μm/s for kinesin-driven ER motility in XTC cytosol. This is much slower than reported for Golgi membranes in rat cytosol (∼1.5 μm/s; Fullerton et al., 1998), being more akin to rates measured for purified kinesin promoting the movement of beads or microtubule gliding (Vale et al., 1985; Ingold et al., 1988; Hall et al., 1993) or of chromaffin granule ghosts (∼0.5 μm/s; Urrutia et al., 1991). In our studies, conventional kinesin must operate alongside cytoplasmic dynein on the same organelle (Figure 5; see below), so it may be that antagonism between these two motors has slowed the rate of ER motility in both directions. There are several other factors that might also have influenced the rate of ER motility, such as cytosolic protein concentration (Schnapp and Reese, 1989), load force (Hall et al., 1993; Svoboda and Block, 1994), or drag from inactive motors (Hall et al., 1993), but so far we have no evidence for any of these potential influencing factors.
As noted above, an intriguing issue that arises from this study concerns the maintenance of opposing motor activities. If conflicting motors are active on the same organelle, as is the case for the ER in this study, a “tug-of-war” may ensue. Although this would seem like a waste of energy for the cell, there are instances in which this occurs. For example, pigment granule motility may be coordinated in this manner (for review, see Lane and Allan, 1998), and squid axonal vesicles have been found to change direction when kinesin function is prevented (Muresan et al., 1996). To facilitate the coordination of opposing motors, they may exist as part of multimotor complexes. Evidence for this is provided by studies in which turning off one motor influences the function of another. For instance, antibodies to kinesin heavy and light chains have been shown to inhibit membrane movement in both directions in squid axoplasm (Brady et al., 1990; Stenoien and Brady, 1997), as have antibodies to kinectin (Kumar et al., 1995) and kinectin peptide fragments (Blocker et al., 1997) in somatic cell extracts. Furthermore, recent evidence suggests that kinesin and myosin V associate directly and in doing so may form a complex on the same organelle (Huang et al., 1999). Indeed, this may go some way toward explaining how ER-derived membranes move both on microtubules and actin in squid axoplasm and appear capable of switching between these two cytoskeletal carriageways (Kuznetsov et al., 1994; Tabb et al., 1998). However, there are also many situations where inhibiting the function of one motor protein did not affect motility in both directions (for examples, see Lane and Allan, 1998). This appears to be the case for ER motility in Xenopus described here, because when we inhibited kinesin function in vitro, dynein-driven motility persisted (see Figure 7). Whether active cytoplasmic dynein and kinesin occupy discrete regions of the ER or whether these motors are located in multimotor complexes remains elusive.
We have now established conditions for plus end-directed, microtubule-based ER motility in vitro, using Xenopus cell extracts and membranes, and have confirmed that kinesin is the motor responsible. In addition, we have provided evidence that kinesin on the ER is regulated developmentally by components present within the cytosol, being inactive in embryo extracts up to the fifth interphase at least, yet functional in somatic cytosol. This study also provides evidence that cytosols derived from distinct cell types of an individual organism regulate differently the activities of motors present on the same organelle. The cytosolic factors likely to influence the activity of membrane-associated kinesin include membrane binding/accessory proteins and regulatory enzymes. We have shown that conventional kinesin is a component of the floated egg ER fraction, which we used in motility assays, that the activation of kinesin-driven ER movement does not involve the recruitment of extra kinesin to the membrane, and that it is most likely to be the egg-derived conventional kinesin that becomes activated in somatic cytosol. Although protein phosphorylation represents the most likely way that membrane-associated kinesin is activated, this remains to be tested. Phosphorylation is known to regulate pigment granule motility, for example, although direct evidence of a role for phosphorylation in controlling motor function on other organelles is sparse (Lane and Allan, 1998). Additional studies are required to understand the processes involved in regulating the activity of kinesin and cytoplasmic dynein on the ER and indeed on most other membranes and organelles.
ACKNOWLEDGMENTS
We thank John Kilmartin, Birgit Lane, Jon Scholey, Ron Vale, and David Vaux for generously providing antibodies. We are grateful to Stephen Addinall, Pete Brown, Emma Clarke, Christian Roghi, and Philip Woodman for critical reading of the manuscript. We thank Alasdair Robertson for helpful comments and for the preparation of monoclonal antibodies from the SUK4-secreting cell line. This research was supported by Wellcome Trust grants 043846 and 045183 and the Lister Institute for Preventive Medicine. V.J.A. is a Lister Research Fellow.
Abbreviations used:
- A/S
acetate/sucrose
- CSF
cytostatic factor
- ER
endoplasmic reticulum
- IgG
immunoglobulin G
- KHC
kinesin heavy chain
- MBT
midblastula transition
- PDI
protein disulfide isomerase
- PI
protease inhibitor
- TAC
tip attachment complex
- VE-DIC
video-enhanced differential interference contrast
- XB
extract buffer
- XTC
Xenopus tissue culture cell
REFERENCES
- Allan V. Assay of membrane motility in interphase and metaphase Xenopus extracts. In: Scholey JM, editor. Methods in Cell Biology. San Diego: Academic Press; 1993. pp. 203–226. [PubMed] [Google Scholar]
- Allan V. Protein phosphatase 1 regulates the cytoplasmic dynein-driven formation of endoplasmic reticulum networks in vitro. J Cell Biol. 1995;128:879–891. doi: 10.1083/jcb.128.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Semin Cell Dev Biol. 1996;7:335–342. [Google Scholar]
- Allan V, Vale R. Movement of membrane tubules along microtubules in vitro: evidence for specialized sites of motor attachment. J Cell Sci. 1994;107:1885–1897. doi: 10.1242/jcs.107.7.1885. [DOI] [PubMed] [Google Scholar]
- Allan VJ. Organelle motility and membrane network formation in metaphase and interphase cell-free extracts. Methods Enzymol. 1998;298:339–353. doi: 10.1016/s0076-6879(98)98030-2. [DOI] [PubMed] [Google Scholar]
- Allan VJ, Vale RD. Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J Cell Biol. 1991;113:347–359. doi: 10.1083/jcb.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Blocker A, Severin F, Burkhardt J, Bingham J, Yu H, Olivo J-C, Schroer T, Hyman A, Griffiths G. Molecular requirements for bidirectional movement of phagosomes along microtubules. J Cell Biol. 1997;137:113–129. doi: 10.1083/jcb.137.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J, Echeverri C, Nilsson T, Vallee R. Overexpression of the Dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabora SL, Sheetz MP. Microtubule dependent formation of a tubularvesicular network with characteristics of the endoplasmic reticulum from cultured cell extracts. Cell. 1988;54:27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Ferreira A, Kosik KS, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. J Cell Biol. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton AT, Bau M-Y, Conrad PA, Bloom GS. In vitro reconstitution of microtubule plus end-directed, GTPγS-sensitive motility of Golgi membranes. Mol Biol Cell. 1998;9:2699–2714. doi: 10.1091/mbc.9.10.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K, Cole DG, Yeh Y, Scholey JM, Baskin RJ. Force-velocity relationships in kinesin-driven motility. Nature. 1993;364:457–459. doi: 10.1038/364457a0. [DOI] [PubMed] [Google Scholar]
- Hanlon D, Yang Z, Goldstein L. Characterization of KIFC2, a neuronal kinesin superfamily member in mouse. Neuron. 1997;18:439–451. doi: 10.1016/s0896-6273(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson JH, Nesbitt D, Wright BD, Scholey JM. Immunolocalization of kinesin in sea urchin coelomocytes. Association of kinesin with intracellular organelles. J Cell Sci. 1992;103:309–320. doi: 10.1242/jcs.103.2.309. [DOI] [PubMed] [Google Scholar]
- Houliston E, Elinson RP. Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J Cell Biol. 1991;114:1017–1028. doi: 10.1083/jcb.114.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JD, Brady ST, Richards BW, Stenoien D, Resau JH, Copeland NG, Jenkins NA. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- Ingold AL, Cohn SA, Scholey JM. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988;107:2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Yu H, Sheetz MP. Kinectin, an essential anchor for kinesin-driven vesicle motility. Science. 1995;267:1834–1837. doi: 10.1126/science.7892610. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Rivera DT, Severin FF, Weiss DG, Langford GM. Movement of axoplasmic organelles on actin filaments from skeletal muscle. Cell Motil Cytoskeleton. 1994;28:231–242. doi: 10.1002/cm.970280306. [DOI] [PubMed] [Google Scholar]
- Lane J, Allan V. Microtubule-based membrane movement. Biochim Biophys Acta. 1998;1376:27–55. doi: 10.1016/s0304-4157(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Lee C, Ferguson M, Chen LB. Construction of the endoplasmic reticulum. J Cell Biol. 1989;109:2045–2055. doi: 10.1083/jcb.109.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chen LB. Behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Larkin JM, McNiven MA. Association of kinesin with the golgi-apparatus in rat hepatocytes. J Cell Sci. 1994;107:2417–2426. doi: 10.1242/jcs.107.9.2417. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Muresan V, Godek CP, Reese TS, Schnapp BJ. Plus-end motors override minus-end motors during transport of squid axon vesicles on microtubules. J Cell Biol. 1996;135:383–397. doi: 10.1083/jcb.135.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J Cell Biol. 1995;131:1039–1053. doi: 10.1083/jcb.131.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclas J, Navone F, Hom Booher N, Vale RD. Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron. 1994;12:1059–1072. doi: 10.1016/0896-6273(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. MAP1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Vallee RB. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Pudney M, Varma MG, Leake CJ. Establishment of a cell line (XTC-2) from the South African clawed toad Xenopus laevis. Experientia. 1973;29:466–467. doi: 10.1007/BF01926785. [DOI] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E. Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol. 1997;7:211–214. doi: 10.1016/s0960-9822(97)70092-7. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA, Steuer ER, Sheetz MP. Cytoplasmic dynein is a minus-end-directed motor for membranous organelles. Cell. 1989;56:937–946. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Steffen W, Karki S, Vaughan KT, Vallee RB, Holzbaur ELF, Weiss DG, Kuznetsov SA. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenoien D, Brady S. Immunochemical analysis of kinesin light chain function. Mol Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer K, Baumann O, Walz B. Actin-dependent light-induced translocation of mitochondria and ER cisternae in the photoreceptor cells of the locust Schistocerca gregaria. J Cell Sci. 1995;108:2273–2283. doi: 10.1242/jcs.108.6.2273. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Block SM. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111:3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Reese TS. Interactions among endoplasmic reticulum, microtubules, and retrograde movements of the cell surface. Cell Motil Cytoskeleton. 1994;29:291–300. doi: 10.1002/cm.970290402. [DOI] [PubMed] [Google Scholar]
- Toyoshima I, Yu H, Steuer ER, Sheetz MP. Kinectin, a major kinesin-binding protein on ER. J Cell Biol. 1992;118:1121–1131. doi: 10.1083/jcb.118.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma MC, Zill A, Le Bot N, Vernos I, Gelfand V. Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores. J Cell Biol. 1998;143:1547–1558. doi: 10.1083/jcb.143.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R, McNiven MA, Albanesi JP, Murphy DB, Kachar B. Purified kinesin promotes vesicle motility and induces active sliding between microtubules in vitro. Proc Natl Acad Sci USA. 1991;88:6701–6705. doi: 10.1073/pnas.88.15.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MC, Pfister KK, Brady ST, Bloom GS. Purification of kinesin from bovine brain and assay of microtubule-stimulated ATPase activity. Methods Enzymol. 1991;196:157–175. doi: 10.1016/0076-6879(91)96016-k. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Gregory J, Parsons SF, Salmon ED. Membrane/microtubule tip attachment complexes (TACs) allow the assembly dynamics of plus ends to push and pull membranes into tubulovesicular networks in interphase Xenopus egg extracts. J Cell Biol. 1995;130:1161–1169. doi: 10.1083/jcb.130.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;14:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- Wright BW, Terasaki M, Scholey JM. Roles of kinesin and kinesin-like proteins in sea urchin embryonic cell division: evaluation using antibody microinjection. J Cell Biol. 1993;123:681–689. doi: 10.1083/jcb.123.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]