Abstract
The capacity to use tools is a fundamental evolutionary achievement. Its essence stands in the capacity to transfer a proximal goal (grasp a tool) to a distal goal (e.g., grasp food). Where and how does this goal transfer occur? Here, we show that, in monkeys trained to use tools, cortical motor neurons, active during hand grasping, also become active during grasping with pliers, as if the pliers were now the hand fingers. This motor embodiment occurs both for normal pliers and for “reverse pliers,” an implement that requires finger opening, instead of their closing, to grasp an object. We conclude that the capacity to use tools is based on an inherently goal-centered functional organization of primate cortical motor areas.
Keywords: neurophysiology, tool use, goal coding, motor act
The capacity to manipulate objects is a sophisticated behavior highly evolved in primates. The basic process underlying it requires coding of the objects' intrinsic properties (size and shape) and their transformation into a specific pattern of finger movements (1).
In primates, the cortical motor area crucially involved in grasping is the rostral sector of the ventral premotor cortex or area F5 (2–6). Neurons in F5 fire in association with specific types of hand shaping (3, 6), and their activity is temporally correlated with different grasping phases. Most neurons discharge in association with the last phase of grasping (“actual grasping”); others start to fire during the phase in which the hand opens and continue to discharge during the phase when the hand closes; finally a few discharge prevalently in the phase in which the hand opens. Hand grasping appears, therefore, to be coded by the joint activity of populations of neurons controlling different temporal phases of the motor act (1).
Primates are able to interact with objects not only by using their natural effectors, but also by using tools. Common tools, such as sticks, stones, and rakes, act basically as functional extensions of natural effectors (7). With practice, they become parts of the agent's body schema (8–10).
To learn tool use, its users have to associate an initial action on an object (e.g., grasp and hold a rake) with subsequent actions that tool possession offers (e.g., reach for an object). Thus, when the use of a tool is learned, a distal goal is coded on the top of the proximal one (11, 12). The aim of the present study was to investigate how the motor cortical system is able to solve this problem.
More specifically, we addressed the following questions: When an object is grasped by a tool instead of the hand, will the cortical motor neurons code the movement of the hand or the distal goal achieved by the tool? And if the distal goal is achieved using an opposite sets of movements, will the neurons still be able to code the distal goal?
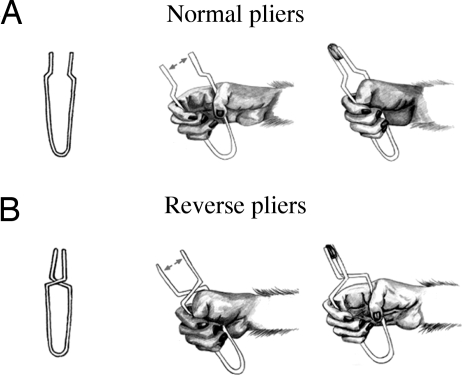
To answer these questions we trained monkeys to grasp objects using two types of tools: “normal pliers” and “reverse pliers.” With normal pliers, the object was grasped by opening the hand and then by closing it [Fig. 1A and supporting information (SI) Movies 1 and 2]. With reverse pliers, the object was grasped by using an opposite movement sequence: The hand was first closed and then opened (Fig. 1B and SI Movies 3 and 4). Once the monkeys learned the task, we tested neurons of areas F5 and F1 (primary motor cortex) during grasping performed by using the two types of pliers.
Fig. 1.
Schematic illustration of the experimental paradigm used. (A) Normal pliers. (B) Reverse pliers. To grasp the object with normal pliers, the monkey has to close its hand (A), whereas with the reverse pliers, the monkey has to open its hand (B). The arrows indicate the direction of the motion of the pliers tips.
Results
Recorded Neurons and Histology.
The activity of 113 neurons, recorded after monkeys learned to use tools, is the focus of the present report. All of these neurons discharged in association with hand grasping movements. Fifty-five of them were recorded from area F5 and 58 from area F1. Both areas were identified on the basis of their neuron-discharge properties (2, 3, 6) (see also SI Text) and of intracortical microstimulation (13–15) (see Methods). Histological controls confirmed the location of the recorded sites. The sectors of F5 and F1 from which neurons were recorded in Monkey 1 are shown in SI Fig. 6A. The electrode penetrations in Monkey 2 had similar locations. SI Fig. 6B illustrates the reconstruction of a series of penetrations in areas F5 and F1 of Monkey 1.
Activity of Area F5 Neurons.
All F5 neurons of the present sample discharged during grasping done with normal and reverse pliers. As when the grasping was performed by using the hand, the neuron activity during tool grasping correlated with specific temporal grasping phases. With normal pliers, 18 (32.7%) neurons began to discharge during hand opening, reaching their maximum firing rate just before or during hand closure; 28 (50.9%) neurons discharged almost exclusively during hand closure; 7 (12.7%) started to discharge with hand closure and kept firing during the subsequent holding phase. Finally, two (3.6%) neurons fired only during the hand-opening phase.
When the same neurons were tested with the reverse pliers, the temporal discharge pattern remained unchanged relative to the grasping motor act phases: Neurons that with normal pliers discharged when the hands was opening, with the reverse pliers discharged when the hand was closing, the discharge remaining linked to the initial phase of the motor act. Conversely, neurons that with normal pliers discharged when the hand was closing, with the reverse pliers discharged when the hand was opening, the discharge being related to the final phase of the motor act.
It is clear therefore that temporal organization necessary to reach the distal goal, and not the hand movement, was coded in F5 neurons object of the present study.
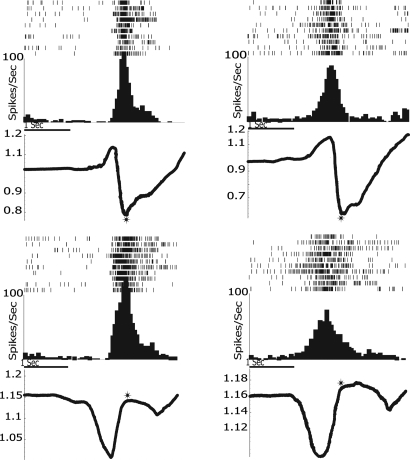
Examples of two neurons recorded during grasping with the two tools are shown in Fig. 2. As one can see, they both discharged during tool grasping with normal and reverse pliers. Most interestingly, they maintained the same relation to different phases of grasping, regardless of the fact that opposite hand movements were required to reach the distal goal.
Fig. 2.
Activity of two neurons recorded in area F5. Rasters and histograms (10 trials) illustrate the neurons' discharge recorded during grasping with normal pliers (Upper) and reverse pliers (Lower). Both rasters and histograms are aligned with the end of the grasping closure phase (asterisks). The traces below each histogram indicate the instantaneous hand position (average of the voltage changes values occurred during neuronal recording) recorded with the potentiometer and expressed as a function of the distance between the pliers handles. Trace down indicates that the hand closes, and the distance between handles decreases, whereas trace up indicates that the hand opens, and the distance between handles increases. The values shown on the vertical axes indicate the potentiometer-measured voltage. With normal pliers (Upper), Unit 210 (Left) began to fire during hand closure (trace down), reaching the maximum at approximately the moment in which the food was grasped; with the reverse pliers (Lower), this unit started to fire with the hand opening (trace up), also reaching its maximum when the food was grasped. Unit 199 (Right) started to fire in normal pliers (Upper) condition during hand opening (trace up), reaching its maximum at the beginning of the hand closure. With reverse pliers (Lower), the neuron started to fire during the hand closure (trace down), reaching its maximum during hand opening. Unit 210: peak force, averaged across 10 trials, 2.8 N and 10.2 N with normal and reverse pliers, respectively. Unit 199: peak force, averaged across 10 trials, 3.9 N and 9.3 N with normal and reverse pliers, respectively.
Activity of Area F1 Neurons.
Two distinct functional categories of neurons were found in F1. The first category was formed by neurons that, as F5 neurons, discharge in relation to the distal goal of the motor act (F1 goal-related neurons, F1g, n = 26). The other category consisted of neurons that discharged in relation to hand movements (F1 movement-related neurons, F1m, n = 32).
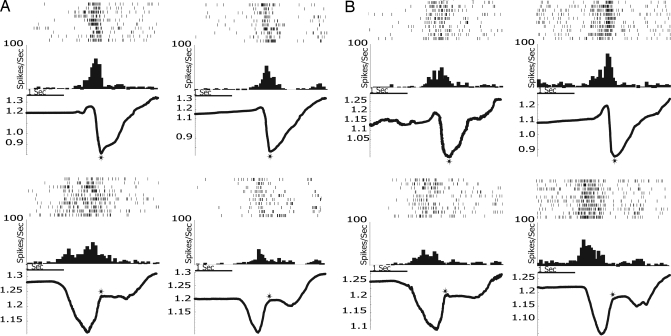
When tested with normal pliers, 12 F1g neurons (46.2%), began to discharge with hand opening, reaching their maximum during hand closure; 13 neurons (50.0%) discharged almost exclusively during hand closure; 1 neuron (3.8%) started to discharge with hand closure and kept firing also during the subsequent holding phase. When the monkeys used the reverse pliers, the temporal discharge pattern remained the same, anchored to a specific grasp phase. (For statistical analysis of the congruence between the temporal courses of neuron activity in the two conditions, see Methods). Examples of two F1g neurons are shown in Fig. 3A.
Fig. 3.
Activity of four neurons recorded in area F1. (A) Two examples of F1g neurons. (B) Two examples of F1m neurons; with normal pliers (Upper), Units 296 (Left) and 259 (Right) fired maximally when the monkey closed its hand, and the pliers' tips were closing around the food. With reverse pliers (Lower), the discharge was also related to hand closure, but, because of the structure of the reverse pliers, it was associated with the opening of the pliers' tips. The peak force, averaged across 10 trials, exerted with normal and reverse pliers were the following. Unit 252 (A Left): 2.4 N and 5.6 N; Unit 243 (A Right): 3.0 N and 7.3 N; Unit 296 (B Left): 2.5N and 6.7 N; Unit 259 (B Right): 3.0 N and 8.4 N. All conventions are as in Fig. 2.
F1m neurons showed a discharge pattern markedly different from that of F5 and F1g neurons. They discharged in strict association with hand movements, regardless of the instrument used. For example, if the maximal discharge was present during hand closure with the normal tool, the same was true with the reverse tool, regardless of the different goals that this movements led to in the two cases. Two examples of F1m neurons are shown in Fig. 3B.
Population Analyses.
In addition to single-neuron analysis, four analyses of population activity were carried out, taking as variable the mean discharge frequency of each neuron in the four epochs, that is: background activity (holding pliers), opening of pliers tips, closing of pliers tips, holding.
A first ANOVA with three factors: Population (three levels: F5, F1g, F1m), Condition (three levels: normal pliers, reverse pliers, hand grasping) and Epoch (four levels) showed a significant interaction among all factors (P < 0.001). Three separate analyses were then carried out for each of the three populations of neurons, with the following main factors: Condition (three levels) and Epoch (four levels).
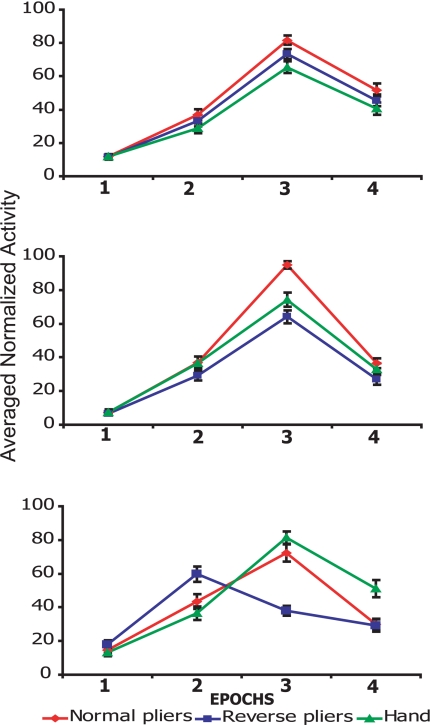
Fig. 4Top shows the average of the normalized mean discharge frequency of F5 neurons during grasping with normal pliers, reverse pliers, and with the hand. ANOVA showed that both factors were significant at P < 0.001, whereas the interaction between them was not significant. In all conditions, Epoch 3 (closing of pliers tips) was the epoch with the highest discharge (P < 0.001).
Fig. 4.
Populations response during grasping with normal pliers, reverse pliers, and hand grasping. Shown are the average of the normalized mean discharge frequency of F5 (Top, n = 55), F1g (Middle, n = 26), and F1m (Bottom, n = 32) neurons, respectively. F5 and F1g neurons showed a statistically significant higher response in Epoch 3 (closure phase) than in the other Epochs in all conditions. For F1m neurons, Epoch 3 (closing of pliers tips) was the epoch with the highest firing rate in hand grasping and normal-pliers grasping conditions. In reverse-pliers condition, the epoch with the highest firing rate was Epoch 2 (hand closure, but opening of pliers tips). Black bars indicate the mean standard error.
The results of the same analysis, performed on F1g population (Fig. 4 Middle) showed that all factors (P < 0.001) and the interaction between them were significant (P < 0.001). A post hoc analysis showed that Epoch 3 was the Epoch with the highest discharge in all conditions (P < 0.001). This analysis also showed that during the use of normal pliers, the activity in Epoch 3 was higher than in the other two conditions (P < 0.001)
Finally, the analysis of F1m neurons showed that the behavior of this category of neurons radically differed from that of F1g and F5 neurons (Fig. 4 Bottom). The ANOVA revealed that the two main factors and the interaction were significant (P < 0.001). The post hoc analysis showed that, for this category of neurons, the activity was maximal in Epoch 3 for hand (hand closure) and normal pliers (hand closure and closure of pliers tips), whereas, for the reverse pliers, it was maximal in Epoch 2 (hand closure, but opening of pliers tips). These findings clearly indicate that F1m neurons discharge in relation to hand movements regardless of the grasping phase in which they are done.
EMG Recording.
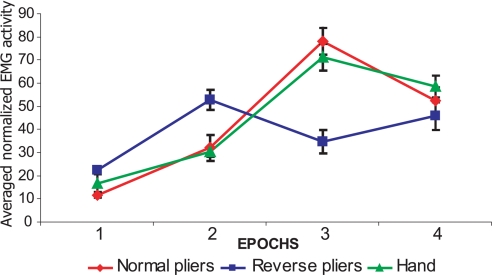
To monitor the hand muscle activity in the three experimental conditions, the activity of an extrinsic hand muscle (flexor digitorum superficialis) was recorded during hand grasping and grasping with the two tools in both monkeys (For details see Methods). The results are shown in Fig. 5. The epochs that showed the highest activity depended on the instrument used. In all conditions, these epochs corresponded to the hand-closure phase. Note the strict similarity between EMG data and the behavior of the F1m neurons.
Fig. 5.
EMG activity. Plots show EMG activity of flexor digitorum superficialis muscle during grasping with the hand and with the two tools. The EMG activity was recorded during the four task Epochs. Data show 20 trials, 10 from each monkey. Rectified EMG was averaged across trials and conditions. Each color plot represents the average activity for a given condition as indicated in the legend at the bottom of the figure (see Methods for details).
Discussion
The use of pliers requires the capacity to separate a proximal goal (grasp the pliers) from a distal goal (grasp an object), a distinction that is not present in natural actions in which the two goals coincide (11, 12). Which transformations had occurred in the motor system once the monkey has learned to use pliers? Our results show that the end effect of training has been the transfer of the temporal discharge pattern that controls hand grasping to the tool use, as if the tool were the hand of the monkey and its tips were the monkey's fingers. This transfer occurs not only when the mechanics of pliers mimics that of the hand (normal pliers), but also when the mechanics is its exact opposite. Also in this case the distal goal, i.e., grasp the object by opening the hand, is the pivotal element around which movements are organized.
This incorporation of the tool in the motor act representation is somehow reminiscent of the finding of Iriki et al. (8), who showed that, with practice, a rake became part of the acting monkey body schema. The present finding shows that, in addition to being incorporated into the body schema, the tool, after learning, is coded in the motor system as if it were an artificial hand able to interact with the external objects, as the natural hand is able to do.
How can this embodiment take place? The most plausible explanation is that this occurs because both F5 and F1 contain neurons that code the goal of the motor act. Some previous evidence suggested that the goal of motor acts, rather than the movements, is also coded in the cortical motor areas that controls reaching movements (16–19). The present data indicate that a goal-coding mechanism is also at the basis of the much more complex motor organization as that of grasping and that it underlies tool embodiment in primate behavior.
What could be the mechanism that allows a transformation of a goal into appropriate movements even when an opposite sequence of movements is necessary to achieve the goal? Our findings show that, after learning, the correct movement selection occurred immediately as soon as the monkey grasped one or the other type of pliers. This correct movement selection may be accounted for if one admits that goal-related F5 and F1g neurons are synaptically connected with two different sets of motor cortex neurons controlling the opening and the closing of the hand, respectively. These movement-related neurons, besides sending their output to the spinal cord (20, 21) (see also ref. 15), would also send a corollary discharge to the goal-related F5 and F1g neurons. In a natural setting, daily interactions with objects reinforce the connections that lead to the desired goal, thus selecting first those neurons that control hand opening and then those that control hand closure. After learning to use the reverse pliers, the opposite connections, reinforced by the success of the tool-mediated motor acts, prevail. As a consequence, the neurons that control hand closure are selected first, and those that control hand opening are selected subsequently. The capacity to learn tool use appears, therefore, to be based on two elements: the goal-centered organization of primate motor cortex and an appropriate interaction with the external world.
Finally, it is well known that in area F5 there is a distinct set of neurons that discharge both during the execution and the observation of actions done by others (mirror neurons; refs. 22 and 23). It has been suggested that the activation of F5 mirror neurons during the observation of motor acts allows the observer to understand the goal of the observed action (24). This suggestion was based on the assumption that neurons in F5 code the goal of motor acts. The evidence, however, in favor of this assumption was rather indirect and based on nonsystematic studies. The present findings, by proving the goal-relatedness of F5 neurons, provide a very strong empirical validation to this proposal. The recording of mirror neurons during tool use confirmed that this distinct set of visuomotor neurons has the same goal-relatedness as the other F5 purely motor neurons. An example of F5 mirror neuron recorded during grasping with tools and during the observation of the same motor act is shown in SI Fig. 7.
Methods
Basic Experimental Procedures.
Two adult macaque monkeys (Macaca nemestrina, one male and one female, weighing 8 and 5 kg, respectively) were used. All experimental protocols were approved by the Veterinarian Animal Care and Use Committee of the University of Parma and complied with the European law on the humane care and use of laboratory animals. Single-unit activity was recorded from areas F5 and F1 in three hemispheres of the two awake monkeys. The monkeys were seated on a primate chair and familiarized with the experimental environment. Before recording, the monkeys were trained to grasp food placed in front of them by using two types of tools (see Fig. 1). The first tool was a common type of pliers (normal pliers), whereas the second one was a type of pliers (reverse pliers) that required the closing of the hand to open the tool tips and the opening of the hand to close them (Fig. 1). Intermixed with tool trials, the monkey grasped food with its hand. After each hand and tool grasping, the monkeys were allowed to bring the food to the mouth and eat it. SI Movies 1–4 illustrate the monkey behavior during grasping with both tools.
The training lasted 6–8 months. After completion of the training, the head-restraint system and a recording titanium chamber were implanted. The surgical procedures for the construction of the head implant were the same as described in previous studies (see ref. 13).
Recording Sites.
The size of the implanted recording chamber provided access to a large cortical area that included the entire ventral premotor cortex, area F1, and the caudal part of the frontal eye fields. After chamber implantation, the accessible cortical area was functionally explored (single-neuron recordings and intracortical microstimulation) to assess the location of areas F1 and F5. The criteria used to characterize functionally the different areas were the following. Area F1: excitable with low-threshold currents (from 40 to 7 μA, average 20 μA), vigorous discharge during active movements, and responses during somatosensory stimulation. At threshold, isolated finger movements were elicited in 90% of the stimulated sites; multiple not dissociable finger movements or wrist movements were observed in the others sites. Area F5: distal movements evoked by microstimulation at higher thresholds (from 50 to 15 μA, average 32 μA) than in F1, neurons discharging in association with hand and mouth motor acts, neurons discharging to the observation of hand and mouth motor acts and to presentation of 3D objects (2, 13).
Neuron Selection.
Clinical testing preceded the study of neurons with the experimental paradigm. Each neuron was tested during active movements and in response to visual and somatosensory stimulation. Active movements consisted of reaching and grasping objects of different size, shape, and orientation, presented in all space sectors. Neurons were classified as grasp-related only when they fired consistently during hand grasping regardless of whether the arm was flexed, extended, adducted, or abducted (for details see refs. 2 and 11). When a neuron was classified as a hand-grasping neuron, it was further tested during grasping with both normal and reverse pliers. Grasping neurons were not subdivided according to their preferred type of grip (e.g., precision grip vs. whole-hand prehension). All neurons that presented motor properties related to hand grasping as well grasping with both reverse and normal tools and that presented stable responses were selected for acquisition. Because the aim of the present study was to investigate the motor properties of the recorded neurons, grasping neurons that showed visual responses (mirror neurons, n = 12) (22, 23) were not included in the main database. The motor behavior of these neurons was, however, indistinguishable from that of purely motor neurons of area F5 (see SI Fig. 7).
Recording and Stimulation Procedures.
Single neurons were recorded by using tungsten microelectrodes (impedance: 0.5–1.5 MΩ measured at 1 kHz) inserted through the dura. Individual action potentials were isolated with a time–amplitude voltage discriminator (BAK Electronics). The output signal from the voltage discriminator was monitored and fed to a PC for analysis. The same microelectrodes were used also for microstimulation. Intracortical microstimulation (ICMS) consisted of trains of cathodal pulses (train duration, 50 ms; pulse width, 0.2 ms; pulse frequency, 330 Hz) generated by a constant-current stimulator. The current intensity used was 3–50 μA. The current intensity was controlled on an oscilloscope by measuring the voltage drop across a 10-kΩ resistor in series with the stimulating electrode. The threshold for each movement evoked by microstimulation was defined as the current intensity at which movements were evoked in 50% of the trials. Recording sites were attributed to areas F5 and F1 based on topographical and physiological properties.
Data Acquisition.
All neurons were recorded during grasping done with hand and with the two types of tools. For each of the three testing (hand, normal pliers, reverse pliers) 10 trials, randomly executed, were acquired. A contact-detecting device, whose signal was fed to a PC, triggered the recording.
A potentiometer (ALPS 16 mm, 50 mW) inserted between the handles of each instrument measured voltage changes (0–2 V), thus giving precise indications on the instantaneous hand position during the opening/closing cycle. These values were fed into the PC used also for spike acquisition. Rasters and histograms could be aligned with the different moments of the opening and closing cycle by means of the signal coming from the potentiometer. The length of normal pliers was 11 cm with an elastic constant of 1.59 Nm. The length of reverse pliers was 14 cm with an elastic constant of 3.35 Nm. The use of the following formula on the data obtained trough the application of known forces to the pliers allowed us to calculate the force exerted by the monkey during grasping performed with both tools: F = Kφ/r, where F is the intensity of the applied force measured in Newtons, K is the torsion constant measured in Newtons per meter, φ is the angle of aperture of the pliers measured in radiants, and r is the distance between the force application point and the rotation axis measured in meters.
Statistical Analysis.
To assess statistically the congruence of the neuronal response in the different experimental conditions (see also SI Text), the discharge of each neuron was subdivided into four epochs: Epoch 1: Background activity (first 300 ms of acquisition time, holding pliers); Epoch 2: Opening phase (the time period −300ms before the beginning of tools closure, opening of pliers tips); Epoch 3: Closing phase (the time period from the beginning to the end of tools closure, closing of pliers tips); Epoch 4: food holding (300 ms after food grasping). As far as the hand grasping without instrument is concerned, the four epochs (Epoch 1, background activity during hand rest; Epoch 2, opening of the hand; Epoch 3, hand closure; Epoch 4, food holding) were calculated by using a digital video camera (25 frames per second) and making a frame-by-frame analysis. This was done off-line, separately for each monkey. The individual grip times were very constant across trials, thus allowing, although not as precisely as by using the potentiometer's measurements, the establishment of the duration of Epochs 2 and 3. Epoch 1 and 4 lasted, as for grasping with instruments, 300 ms. The responses of each recorded neuron were statistically assessed performing an ANOVA (P < 0,05) on the firing rate of each neuron with the following factors: Condition (three levels: hand and two instruments grasping) × Epoch (four levels). All neurons that displayed a significant interaction Condition × Epoch were further tested with a Neuman–Keuls post hoc test to compare the neurons' discharge during background activity with the activity in Epochs 2 and 3. All neurons displaying statistically significant differences (P < 0,05) between Epoch 1 and one of the two subsequent Epochs in all conditions were considered task-related neurons and were included in the database.
Electromyography (EMG) Recording.
The EMG activity of the flexor digitorum superficialis muscle was recorded from both monkeys in the three experimental conditions by using Ag/AgCl surface electrodes (diameter 3 mm). Ten trials for each condition were acquired, and all trials were randomized across all conditions. Continuous EMG recordings were acquired with a CED Micro 1401 analog-to-digital converting unit (Cambridge Electronic Design). The EMG signal was amplified (1,000 times), digitized (sampling rate: 1 kHz), and stored on a PC for off-line analysis. The signal was then rectified and filtered. The normalization procedure and the time-windows subdivision used for the analyses of the neuronal activity were also applied for the analysis of the EMG activity with one entry for each trial. The statistical analysis on the EMG data was done on the normalized EMG activity of each trial in each condition. An ANOVA (P < 0.01) was performed with two factors: Condition (three levels: hand, normal pliers and reverse pliers) and Epochs (four levels). The ANOVA was followed by Neuman–Keuls post hoc comparisons (P < 0.01).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Prof. M. Arbib for most valuable comments on an earlier version of the manuscript. This research was supported by Ministero dell'Università e della Ricerca [Relevant National Interest Projects (PRIN) and the Italian Fund for Basic Research (FIRB)] and by Information Society Technologies (IST)-Future and Emerging technologies (FET) Neurobotics. L.E. was supported by a Marie Curie Fellowship; I.I. was supported by IST-FET Mirrorbot; F.G. was supported by the Fyssen Foundation and the Cognitique program from the French government; M.R. was supported by FIRB; and A.J. was supported by IST-FET Mirror and IST-FET Neurobotics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705985105/DC1.
References
- 1.Jeannerod M, Arbib M-A, Rizzolatti G, Sakata H. Trends Neurosci. 1995;7:314–320. [PubMed] [Google Scholar]
- 2.Rizzolatti G, Camarda R, Fogassi M, Gentilucci M, Luppino G, Matelli M. Exp Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- 3.Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- 4.Rizzolatti G, Luppino G. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 5.Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- 6.Raos V, Umiltà MA, Fogassi L, Gallese V. J Neurophysiol. 2006;95:709–729. doi: 10.1152/jn.00463.2005. [DOI] [PubMed] [Google Scholar]
- 7.Van Lawick-Goodall J. In: Advances in the Study of Behavior. Hinde RA, editor. New York: Academic; 1970. pp. 195–249. [Google Scholar]
- 8.Iriki A, Tanaka M, Iwamura Y. NeuroReport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Berti A, Frassinetti F. J Cognit Neurosci. 2000;12:415–420. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- 10.Maravita A, Iriki A. Trends Cognit Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Johnson SH, Grafton ST. Prog Brain Res Rev. 2003;42:127–139. doi: 10.1016/S0079-6123(03)42010-4. [DOI] [PubMed] [Google Scholar]
- 12.Johnson-Frey SH. Trends Cognit Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Brain Behav Evol. 1989;33:118–121. doi: 10.1159/000115912. [DOI] [PubMed] [Google Scholar]
- 14.Hepp-Reymond M-C, Husler E-J, Maier M-A, Qi H-X. Can J Physiol Pharmacol. 1994;72:571–579. doi: 10.1139/y94-081. [DOI] [PubMed] [Google Scholar]
- 15.Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon; 1993. [Google Scholar]
- 16.Crutcher MD, Alexander GE. J Neurophysiol. 1990;64:151–163. doi: 10.1152/jn.1990.64.1.151. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GE, Crutcher MD. J Neurophysiol. 1990;64:164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- 18.Kakei S, Hoffman DS, Strick PL. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- 19.Kakei S, Hoffman DS, Strick PL. Nat Neurosci. 2001;4:1020–1025. doi: 10.1038/nn726. [DOI] [PubMed] [Google Scholar]
- 20.Dum RP, Strick PL. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dum RP, Strick PL. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 23.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Brain Res Cognit Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 24.Rizzolatti G, Fogassi L, Gallese V. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.