Abstract
The theory that natural selection has conserved mechanisms by which women subjected to environmental stressors abort frail male fetuses implies that climate change may affect sex ratio at birth and male longevity. Using time series methods, we find that cold ambient temperatures during gestation predict lower secondary sex ratios and longer life span of males in annual birth cohorts composed of Danes, Finns, Norwegians, and Swedes born between 1878 (earliest year with complete life tables) and 1914 (last birth cohort for which male life span can be estimated). We conclude that ambient temperature affects the characteristics of human populations by influencing who survives gestation, a heretofore unrecognized effect of climate on humanity.
Keywords: climate change, global warming, natural selection, Scandinavia, time series
Curiosity about the effect of ambient temperature on human well being has not only endured (1) but, because of concern over climate change, grown to unprecedented levels (2). No research, however, appears to have focused on how temperature affects population health through natural selection in utero. This finding remains true despite three widely disseminated epidemiologic findings that together suggest such a connection. First, only 30–50% of conceptions yield live births, making gestation as much an opportunity for natural selection as for maturation (3, 4). Second, population stressors increase fetal deaths, particularly among males (5). Third, extreme ambient temperature demonstrably stresses human populations (6).
Natural selection has conserved mechanisms by which women subjected to environmental stressors abort male fetuses least likely to yield grandchildren (7). These mechanisms purportedly cull males because sons more likely die before reproductive age than do daughters despite receiving greater maternal investment (8, 9). A woman without such mechanisms would have fewer grandchildren because environmental stressors would weaken both her and her son, thereby reducing his already low, relative to a daughter's, odds of survival and her capacity to invest in him. A stressed woman with these mechanisms would, conversely, abort a frail male, making her available to bear a daughter or more robust son.
Environmental stressors reported to affect the gender of birth cohorts include cold ambient temperature (10). Cold temperature induces the stress response in those not sheltered against it (11). In addition, low temperature can indirectly stress populations by, for example, disrupting the economies that support them (12). Research in Scandinavia, moreover, reports that stressors of all sorts may be more virulent when unusually cold summer weather constrains restorative behaviors (13). Any of these direct or indirect mechanisms could cause gravid women to lower their tolerance for frail male fetuses. Epidemiologic research reports, for example, that preterm delivery, an outcome of gestation that affects more male than female fetuses, appears elevated among cohorts whose second and/or third trimester in utero coincides approximately with cold winter months (14).
The above arguments imply that males from cold-stressed cohorts, which have been “culled” in utero of their least-fit members (15), should, on average, live longer than males from other cohorts. Because of the importance of this implication for evolutionary theory, climate studies, and public health, we test the hypotheses that temperature moves positively with the secondary sex ratio (ratio of male to female live births) but inversely with the life span of men, controlling for life span of women, surviving to age 1 year. We test our hypotheses among annual cohorts born in Denmark, Finland, Norway, and Sweden between 1878 (the earliest year with complete Scandinavian life tables) and 1914 (the last birth cohort for which male life span can be estimated) [University of California Berkeley and Max Planck Institute for Demographic Research (2007) Human Mortality Database, www.mortality.org]. We use data from these four countries because they provide a unique combination of high-quality vital statistics as well as continuous instrument-based measurements, kept since the mid-19th century, of surface temperature. Empirical research, moreover, confirms the importance of ambient temperature to the health and well being of populations in this region (16, 17).
We use temperature data that climate scientists have validated (18, 19) through well described procedures and that gauge temperature in northern as well as southern latitudes in our test region. The data, the longest available time series of instrument-based measurements of surface air temperature in the world, come from the Tornedalen region of Sweden (i.e., ≈68° north) (18) and from instruments near Stockholm (i.e., ≈59° north) (19). Climate scientists have calculated daily, monthly, and annual average temperatures from these instrument-based data. We use the average of the two annual series as our independent variable.
We specified our temperature variable such that we could detect an association between temperature in year t and cohorts born in that year as well as those born in year t + 1 because fetuses in gestation in one year could have been born in the next. The effect of cold temperature on gestation in a given year can, in other words, “persist” into the next, and our tests capture such “persistence.”
Results
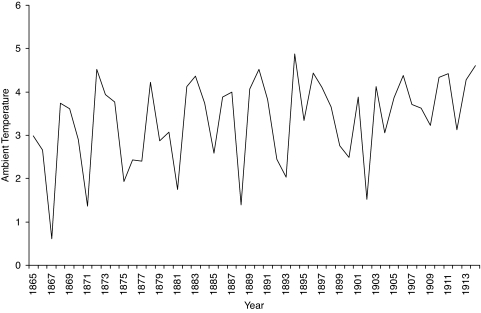
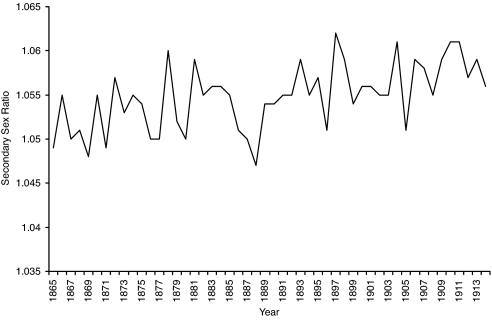
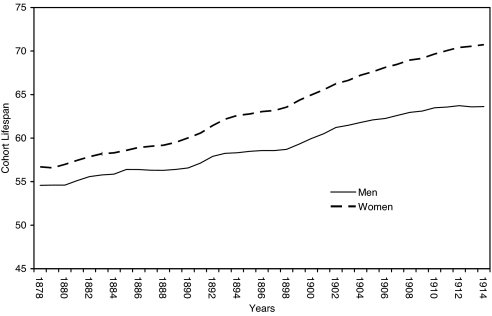
Mean annual temperature ranged from 0.6125°C to 4.8792°C over the years 1865 through 1914 [3.3520 ± 1.0006 (mean ± SD)] (see Fig. 1). The sex ratio variable ranged from 1.0471 to 1.0617 over the test period (Fig. 2). Cohort life span varied from 54.57 to 63.72 years for the males and 56.59 to 70.73 for the females (Fig. 3).
Fig. 1.
Mean annual ambient temperature in degrees Celsius.
Fig. 2.
Secondary sex ratio (i.e., live male to live female births) for the sum of Danes, Finns, Norwegians, and Swedes.
Fig. 3.
Cohort life span at age 1 year for the sum of the Danes, Finns, Norwegians, and Swedes.
Consistent with the culled cohort argument, the annual secondary sex ratio in Scandinavia rose with warmer, and declined with colder, ambient temperatures (P < 0.0001; see Table 1). The association persisted, although attenuated, into the following year. The sex ratio exhibited autocorrelation in that “echoes” in the opposite direction of high or low values appeared 5 years later.
Table 1.
Final models of the secondary sex ratio and male cohort life span as a function of temperature in year of birth and of autocorrelation
| Variables | Secondary sex ratio | Male cohort life span |
|---|---|---|
| Mean | 1.0167* (0.0122) | None |
| Female cohort life span | Not applicable | 0.6616* (0.0380) |
| Change in dependent variable associated with increase of 1°C in ambient temperature | 0.0010* (0.0002) | −0.0377* (0.0155) |
| Proportion of coefficients from above row persisting into subsequent year | 0.9168* (0.0406) | None |
| Parameters needed to express autocorrelation in the dependent variable | Moving average parameter at 5 years 0.5633* (0.1217) | First differencing Autoregressive parameter at 5 years −0.3838* (0.1588) |
*, P < 0.05; two-tailed test. Standard errors are in parentheses.
Given the relatively short time series available for our test, we assessed whether our result could have been induced either by outliers in the sex ratio or variability in its variation over time. We used the methods devised by Chang et al. (20) to detect and adjust outliers; none was detected. We then transformed the sex ratio to its natural logarithms and estimated our final equation again to assess any effect of variability in variation. The results of the test, with the obvious exception of the metric of the coefficients, remained the same.
The temperature coefficients (Table 1) suggest that a 1°C increase in annual temperature predicts one more male than expected for every 1,000 females born in a year. The median annual number of female births over the test period was 167,046. A rise of 1°C would, therefore, imply preservation of ≈167 males in the median year.
As evident in Fig. 3, life span for both men and women rose significantly during our test period, requiring us to use the first difference of both variables in our tests. Like the sex ratio, the differenced life expectancy variable exhibited autocorrelation in that echoes of high or low values appeared 5 years later.
Consistent with the culled cohort argument, the cohort life span of Scandinavian males declined with warmer, and increased with colder, ambient temperatures during their gestation (P = 0.02; see Table 1). This association survived controlling for variance shared between male and female cohort life span. As with the sex ratio test, we repeated our life span test, controlling for outliers and, separately, with the dependent variable transformed to natural logarithms. The results remained the same as from the original test.
The trends inherent in longevity required us to take the first differences of the life span series to arrive at their expected values. We, therefore, also differenced the temperature variable to ensure that our tests detected associations that persisted into subsequent years. To determine the sensitivity of our test to differencing of the temperature variable, we reestimated the life span equation with the temperature variable in its original rather than differenced metric. The resulting coefficient [i.e., −0.0324 ± 0.0113 (SE)] changed very little from the original estimation [i.e., −0.0377 ± 0.0155 (SE)].
The temperature coefficient, shown in Table 1, implies that an increase of 1°C from one year to the next predicts a decrease in male life span of, on average, 14 days among those who survived to age 1. Combining the sex ratio and life span results suggests that in a year with the median number of live female births (i.e., ≈167,000), a 1°C rise in annual temperature from the previous year would preserve ≈167 male gestations that would otherwise have ended without live births. The unexpectedly early deaths of these males, however, would reduce the average life span of males who survived to age 1 year in their birth cohort by 2 weeks.
We combined the northern and southern temperature measurements, but population in the region has historically been greater in the south. We estimated our final equations for both outcomes separately with only the northern and then only the southern temperature series. Results did not differ significantly from those yielded by the average of the two temperature series. The point estimates for the southern series, however, were larger, although not statistically different from, those of the northern series.
Very warm temperature may also induce maternal stress through direct and indirect mechanisms. Temperatures in our study area unlikely reached levels that would induce heat stress in humans, but we repeated both of our tests with the square of the temperature variable added to the test equations. This addition tests whether the “dose–response” of our dependent variables to temperature resembled hyperbola more than straight lines. Neither squared term yielded significant associations.
Discussion
Data from Denmark, Finland, Norway, and Sweden suggest that at least one ubiquitous stressor, unexpectedly cold ambient temperature, influences the characteristics of populations by affecting selection in utero. Mean temperature varies positively with the sex ratio but inversely with male cohort life span at age 1 year. Low temperatures, therefore, may cull males in utero and leave a more robust cohort compared with males born in years with warmer mean temperature.
Our findings may not describe other regions, particularly those with warmer climates. In addition, changes in interrelated climate parameters, particularly temperature and humidity, may mask or amplify associations between temperature and human adaptation and do so differently in different regions. Cold might stress pregnant women by several mechanisms, including direct thermal impact, lower nutrition because of poorer crop yields, changes in cloudiness/sunlight, and more exposure to indoor pollutants. Additional work must disentangle these effects and test for dose–response differences by latitude before we can make more global inferences regarding climate change, selection in utero, and life span.
Although these northern European datasets provide an opportunity to gauge the association of population health with cold temperature, they do not allow a symmetrically satisfying test for heat. Such a test awaits the discovery of life table and temperature data from warmer regions.
Our findings do not directly contribute to estimates of the health effects of long-term global climate change because we studied annual variations from long-term trends in longevity. The results, however, suggest a heretofore unrecognized impact of variation in global climate. Changes in ambient temperature affect the characteristics of human populations by influencing who survives gestation.
Methods
Variables and Data.
We acquired annual birth counts of males and females as well as birth cohort life span for Sweden, Norway, Finland, and Denmark from the Human Mortality Database (HMD) (www.mortality.org). Birth registry data for all four countries allowed calculating a sex ratio time series dating to 1865. We computed the secondary sex ratio by summing all male births across the four countries in each year and dividing this value by the sum of all female births.
Complete cohort life table data in all four countries begins in 1878 and ends in 1914 with the birth of the last cohort in which sufficient deaths have occurred to estimate life span. Consistent with the earlier literature (15), we chose male life span at age 1 year rather than life span at birth as our other dependent variable because culling induced by unexpectedly cold temperature could presumably act on newborn males as well as on male fetuses.
We calculated cohort life span at age 1 year through the following steps. We retrieved, from the HMD cohort life tables, the standardized number of individuals surviving to age 1 year (i.e., l1 in life table labeling conventions) as well as the standardized total person-years remaining for individuals that survived to age one (i.e., T1). We also retrieved the HMD estimates of annual counts of individuals who survived to age 1 year. We calculated the total person-years remaining for individuals that survived to age 1 by applying the standardized values to the annual population counts. We then summed these person-years across the four countries and divided this value by the sum, also across four countries, of the population counts of individuals aged 1.
Growing concern over climate change has led to the validation and publishing of historic climate data. Much of this work has focused on Scandinavia, where scientists maintained continuous instrument-based measurements of surface temperature from as early as the mid-18th century. We used two criteria in selecting among several available temperature series for our tests. First, we wanted series that climate scientists had validated through well described procedures and made widely available to researchers like us and those who might replicate our work. Second, we wanted series that gauged temperature in northern as well as southern latitudes. As noted above, we chose a series based on instruments in the Tornedalen region of Sweden (18) (i.e., ≈68° north) and another derived from instruments near Stockholm (19) (i.e., ≈59° north).
Statistical Analyses.
Our correlational tests turn on whether the observed sex ratios and male cohort life span vary, in the direction predicted by theory, from their statistically expected values in the same years that temperature varies from its expected values. Such tests typically use the mean of a dependent variable as its expected value. Cohort sex ratios and life span, however, often exhibit autocorrelation, including trends, cycles, and the tendency to remain elevated or depressed after high or low values. Autocorrelation complicates correlational tests because the expected value of an autocorrelated series is rarely its mean.
Researchers typically respond to this problem by identifying autocorrelation and expressing it as an effect of earlier values of the dependent variable itself. The residuals from these equations exhibit no autocorrelation. The analyst can, therefore, add the independent variables to the equation to determine whether their coefficients differ from zero in the hypothesized direction. Removing autocorrelation from the dependent variable has the added benefit of precluding type I errors that could otherwise result from third variables that exhibit trends, seasonality, or other patterns.
Our test of the hypothesized relationship between temperature and the sex ratio proceeded through the following steps.
We used strategies attributed to Box and Jenkins (21) to identify and model autocorrelation in the sex ratio. The strategy, autoregressive, integrated, moving average (i.e., ARIMA) modeling, draws from a very large family of models to empirically specify autocorrelation in time series. Autoregressive parameters typically express long-term memory in a series such that high or low values persist, although diminish, over at least several time periods. Moving average parameters gauge short-term memory of outliers. Integration induces secular trends and strong cycles that must be removed by differencing before modeling autoregressive and moving average behavior.
We specified the test equation by adding the annual temperature variable to the model resulting from Step 1. We specified the weather variable such that we could detect an association between temperature in year t and cohorts born in that year as well as those born in year t + 1 because fetuses in gestation during year t could have been born early in the next year. The effect of cold temperature on gestation in a given year can, in other words, persist into the next, and our tests capture such persistence.
- We estimated the equation resulting from Step 2 and inspected the error terms for autocorrelation. When needed, we included additional ARIMA parameters and estimated the resulting equation,
where ∇d is the difference operator that indicates differencing of a series (i.e., values at t subtracted from values at t + d) to remove any secular trends or cycles; Yt is the sex ratio for the cohort born during year t; c is the mean, if significantly (P < 0.05; two-tailed test) different from 0, of the observations; Xt is the temperature variable, defined above, for year t; ω is the effect parameter for the temperature variable; δ measures the proportion of ω that carries into year t + 1 because a fraction of infants in gestation during year t were born in year t + 1; θ is the moving average parameter; φ is the autoregressive parameter; B is the “backshift operator” that yields the value of the series it conditions at time t − p for the autoregressive parameter or t − q for the moving average parameter; and at is the error term for month t.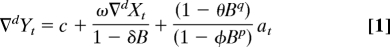
We specified a final model by removing any variables with coefficients that failed to reach significance at Step 4. We then estimated the final equation.
Our test of the hypothesized inverse association between temperature and male cohort life span proceeded as above except female cohort life span was included as a predictor in the model. Including female cohort life span controlled for the effect of unmeasured confounders that affected the both males and females over the test period.
ACKNOWLEDGMENTS.
We appreciate comments by Ross Anderson and Sari Kovatz. This Robert Wood Johnson Foundation supported the analyses described in this article.
Footnotes
The authors declare no conflict of interest.
References
- 1.Guy WA. On temperature and its relation to mortality: An illustration of the application of the numerical method to the discovery of truth. J Stat Soc London. 1881;44:235–268. [Google Scholar]
- 2.Parry M, Canziani O, Palutikof J, van der Linden P, Hanson C, editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 3.Boklage CE. The survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35:75–94. [PubMed] [Google Scholar]
- 4.Wilcox AJ, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 5.Catalano R, Bruckner T, Anderson E, Gould JB. Fetal death sex ratios: A test of the economic stress hypothesis. Int J Epidemiol. 2005;34:944–948. doi: 10.1093/ije/dyi081. [DOI] [PubMed] [Google Scholar]
- 6.Vandentorren S, et al. Mortality in 13 French cities during the August 2003 heat wave. Am J Public Health. 2004;94:1518–1520. doi: 10.2105/ajph.94.9.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 8.Wells J. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- 9.Mace R, Sear R. Birth interval and the sex of children in a traditional African population: An evolutionary analysis. J Biosoc Sci. 1997;29:499–507. doi: 10.1017/s0021932097004999. [DOI] [PubMed] [Google Scholar]
- 10.Lerchl A. Sex ratios at birth and environmental temperatures. Naturwissenschaften. 1999;86:340–342. doi: 10.1007/s001140050630. [DOI] [PubMed] [Google Scholar]
- 11.Kelsey RM, Alpert BS, Patterson SM, Barnard M. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;101:2284–2289. doi: 10.1161/01.cir.101.19.2284. [DOI] [PubMed] [Google Scholar]
- 12.de Vries J. Measuring the impact of climate on history: The search for appropriate methodologies. J Interdisc History. 1980;10:599–630. [Google Scholar]
- 13.Hartig T, Catalano R, Ong M. Cold summer weather, constrained restoration, and the use of antidepressants in Sweden. J Env Psychol. 2007;27:107–116. [Google Scholar]
- 14.Lawlor DA, Leon DA, Davey Smith G. The association of ambient outdoor temperature throughout pregnancy and offspring birthweight: Findings from the Aberdeen Children of the 1950s cohort. BJOG. 2005;112:647–657. doi: 10.1111/j.1471-0528.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 15.Catalano R, Bruckner T. Secondary sex ratios and male lifespan: Damaged or culled cohorts. Proc Natl Acad Sci USA. 2006;103:1639–1643. doi: 10.1073/pnas.0510567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyllerup S, Lanke J, Lindholm LH, Schersten B. Cold climate is an important factor in explaining regional differences in coronary mortality even if serum cholesterol and other established risk factors are taken into account. Scott Med J. 1993;38:169–172. doi: 10.1177/003693309303800604. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Eskild A. Seasonal variation in the occurrence of pre-eclampsia. BJOG. 2001;108:1116–1119. doi: 10.1111/j.1471-0528.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 18.Klingbjer P, Moberg A. A composite monthly temperature record from Tornedalen in northern Sweden, 1802–2002. Int J Climatol. 2003;23:1465–1494. [Google Scholar]
- 19.Moberg A, Bergstrom H. Homogenization of Swedish temperature data: The long temperature records from Uppsala and Stockholm. Int J Climatol. 1997;17:667–699. [Google Scholar]
- 20.Chang I, Tiao G, Chen C. Estimation of time series parameters in the presence of outliers. Technometrics. 1988;30:193–204. [Google Scholar]
- 21.Box G, Jenkins G, Reinsel G. Time Series Analysis: Forecasting and Control. London: Prentice–Hall; 1994. [Google Scholar]