Abstract
A formal synthesis of the Aspidosperm a alkaloids aspidospermidine, aspidospermine, and quebrachamine is reported through an efficient preparation of Stork’s penultimate intermediate. The key step of the sequence involved an intramolecular [3 + 2] cycloaddition of the 2-azapentadienyllithium 21 formed in situ from the corresponding imine 1, which after N-alkylation of the resulting cycloadduct, provided 2 in excellent yield. The synthesis represents a new disconnection of the classical tricyclic ketone used for appendage of the requisite indole.
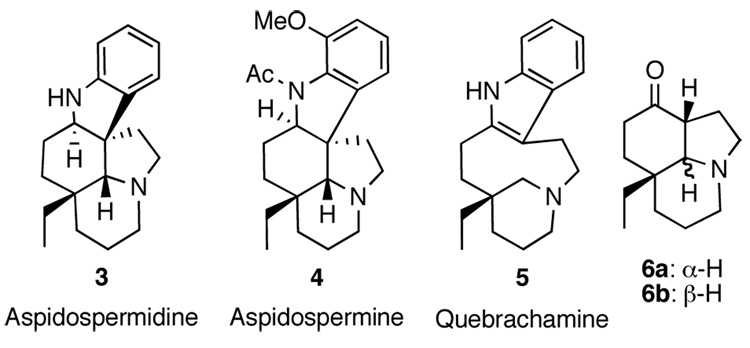
The Aspidosperma alkaloids have long been the target of synthetic studies due to their structural complexity and interesting biological activities. To date, more than 250 alkaloids in this family have been isolated1 and are composed of vastly diverse structural elements with the majority containing the core exemplified by aspidospermidine 3 (Figure 1). Vinblastine has found clinical use for the treatment of various cancers and is comprised of perhaps the most complicated molecular architecture in this family.2 In contrast, aspidospermidine, the most basic alkaloid to retain the characteristic pentacyclic structure, is not of pharmacological interest and is devoid of sensitive functional groups.3 For this reason, aspidospermidine has often been used as a sharpening stone for developing new routes to these natural products, thereby resulting in numerous total syntheses.4 A classic in this area, Stork’s synthesis of aspidospermine 4 and quebrachamine 5, relied on a late stage introduction of the indole moiety onto a tricyclic ketone intermediate (6a) prepared by a sequential annulation strategy.5 Since then, many others have intercepted Stork’s route.6 Aubé6h,i and Banwell6j have demonstrated that ketone 6b (Scheme 1, a diastereomer of Stork's intermediate) could also be used in the preparation of aspidospermidine 3.
Figure 1.
Aspidosperma Alkaloid Synthetic Targets.
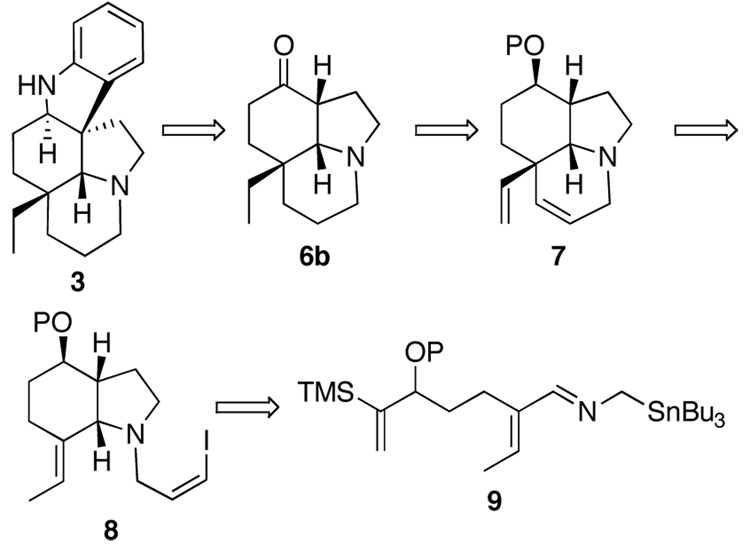
Scheme 1.
As part of an ongoing project aimed at the use of [3 + 2] cycloadditions of 2-azallylstannanes in alkaloid synthesis, 7 we sought to develop a strategy for the efficient preparation of the Stork-type tricycle 6 by a new disconnection. We have previously reported on the preparation of α,β-unsaturated-2-azaallylstannanes and their use in the generation and cycloaddition of 2-azapentadienyl anions and azomethine ylides.8,9 Cycloaddition of the anions gives products that result from the all-trans-2-azapentadienyl anion (the “W” conformation; vide infra). We predicted that the resultant pyrrolidines would be synthetically useful because they contain an alkenyl group in the 2-position that provides a handle for further functionalization.
According to the retrosynthetic analysis shown in Scheme 1, it was recognized that 6b should be attainable using fairly simple precursors. As illustrated, the ketone 6b should be accessible by reduction of the diene in 7 and oxidation of the corresponding alcohol that results after deprotection. The tricyclic diene 7 would be assembled by an intramolecular Heck reaction involving the vinyl iodide 8. This bicyclic amine 8 should result from an intramolecular cycloaddition of the α,β-unsaturated-2-azaallylstannane 9. The vinylsilane functionality was employed because it is a much better anionophile than a simple alkene.10 Further, desilylation should be possible at the stage of the oxidation of the secondary alcohol to an α-silylketone.
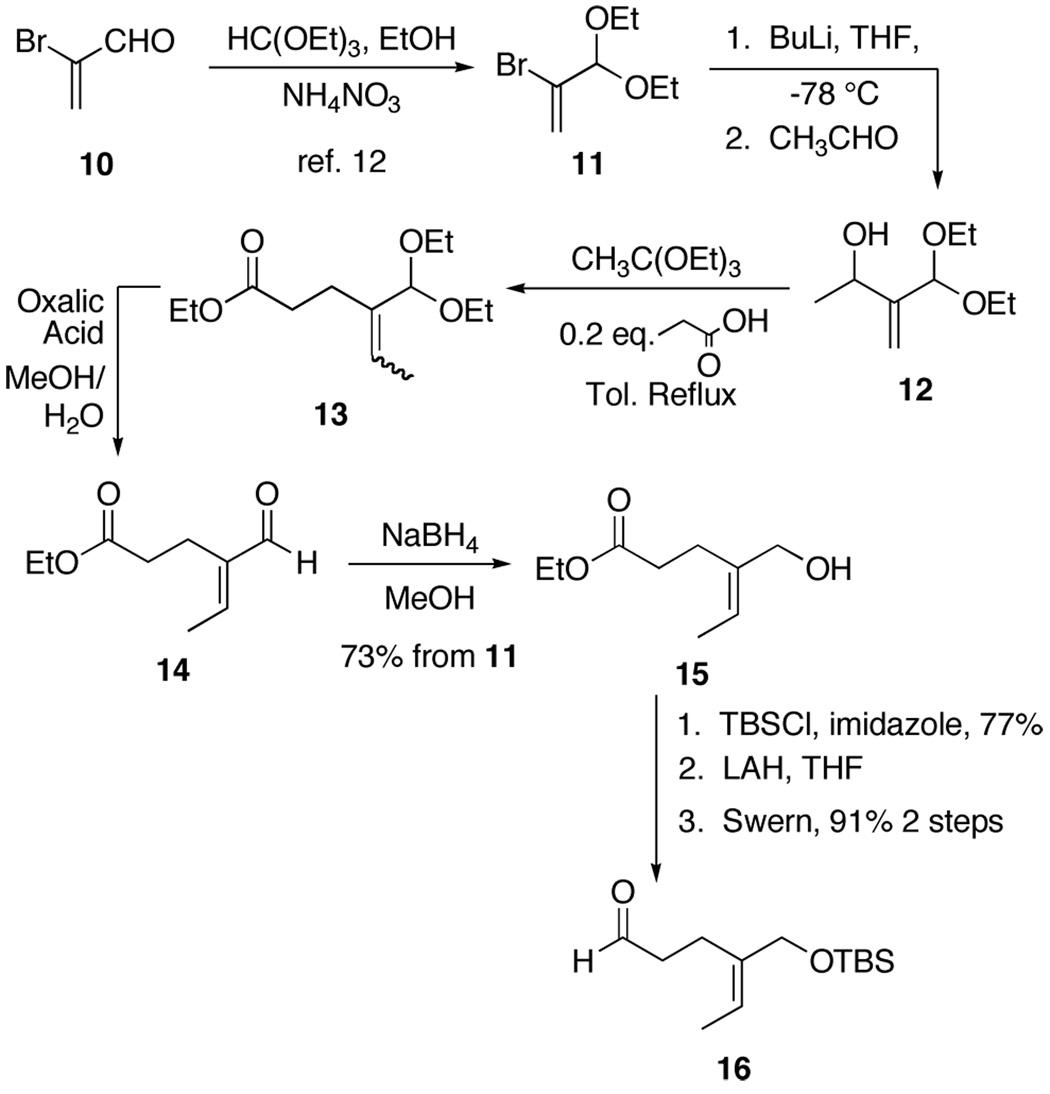
The key acyclic imine 9 should be accessible from the corresponding aldehyde and amine by condensation. Examination of the requisite aldehyde suggested the possibility of installing the carbonyl functionality by a Claisen rearrangment, which would also define the olefin geometry. This strategy had previously been employed in a similar system to give the aldehyde 1411 (Scheme 2) that should easily be transformed into the key intermediate 9 after some functional group manipulation. To this end, the acetal 11 was formed by protecting 2-bromoacrolein 10 using the conditions reported by Smith (Scheme 2).12 Multigram quantities of 11 were easily prepared, and following distillation, found to have an excellent shelf life when stored cold. Lithium-halogen exchange was carried out using n-butyllithium. The resulting organolithium was quenched with excess acetaldehyde to form the alcohol 12. Depezay’s Johnson orthoester Claisen conditions were used to give the ethyl ester 13.11 Although Depezay reported isolating exclusively the Z isomer, in our hands the E/Z ratio was batch dependent. Fortunately, upon deprotection of the acetal, the aldehyde isomerized to the E-α,β-unsaturated aldehyde 14. Reduction with sodium borohydride in methanol then gave the allylic alcohol 15 in 5 steps and 73% yield from 11. After protection of the alcohol as the t-butyldimethylsilyl ether and adjusting the ester oxidation state, the aldehyde 16 was formed in good yield.
Scheme 2.
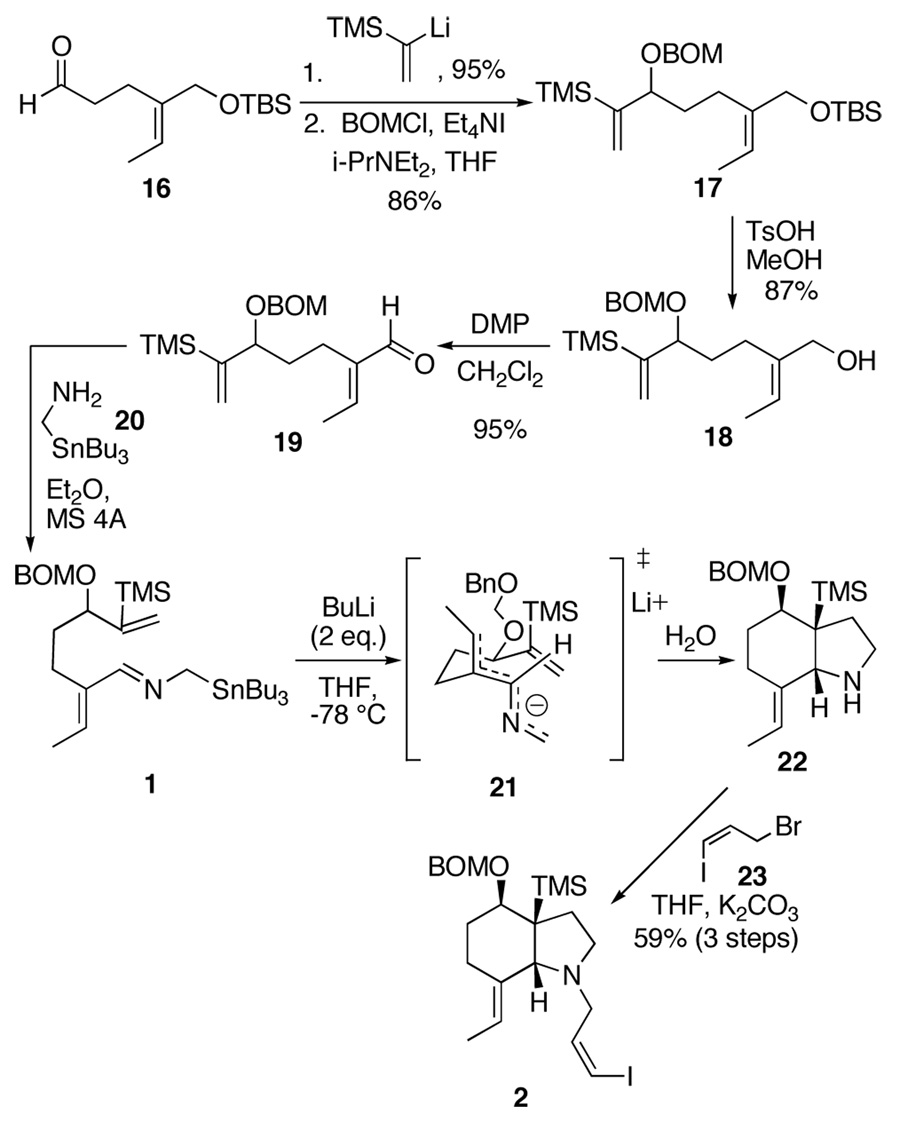
With a good source of 16 in hand, we set out to advance to a suitably protected version of 9 and test the key cycloaddition (Scheme 3). To this end, 1-lithio-1-trimethylsilylethylene was added to the aldehyde 16, and the resultant alcohol was protected as the BOM ether to give 17 in 86% yield. The BOM protecting group was chosen because it should be cleaved at the same time the diene of 7 (Scheme 1) is reduced by hydrogenation. The t-butyldimethylsilyl ether in 17 was removed by treatment with a catalytic amount of p-toluenesulfonic acid in methanol to give the alcohol 18 in 87% yield. The alcohol was oxidized to the aldehyde using Dess-Martin periodinane to give the aldehyde 19, and condensation of the α-stannylamine was effected under standard conditions. After removal of the solvent, the imine 1 was added to a dilute solution of n-butyllithium and the cycloadduct 22 was obtained, presumably through chair transition state 21 in which the anion exists in the extended all “W” conformation. After alkylation of the amine with the allyl bromide 23,13 the vinyl iodide 2 was obtained in 59% yield from 19. The stereochemistry of 2 was assigned through a series of NOE experiments.
Scheme 3.
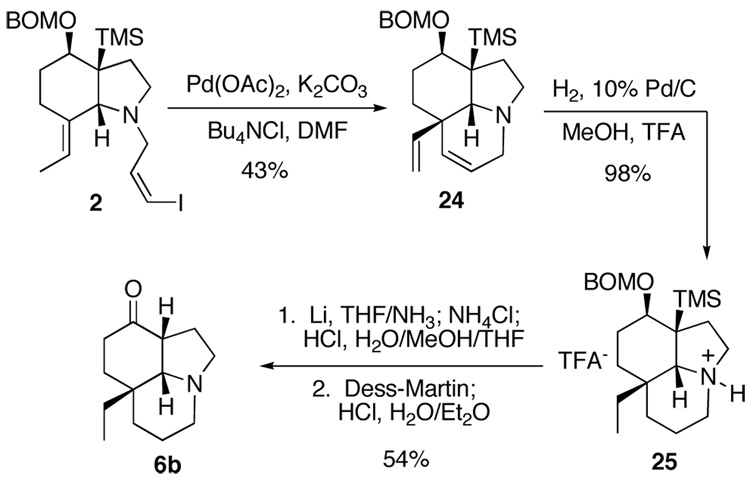
To complete the formal synthesis, the final ring of the tricyclic ketone 6b had to be installed and several functional group manipulations carried out. Heck reaction of the vinyl iodide 2 using the Jeffrey conditions established by Rawal for intramolecular reactions on indole alkaloids,14 gave the tricyclic amine 24 in 43% yield (Scheme 4). Similar yields have been reported on closely related systems.15 The next step was to reduce the diene by hydrogenation, which should also cleave the BOM protecting group. Several sets of conditions were tried. The reactions were carried out at room temperature in a Parr shaker under a hydrogen atmosphere at 45 psi, while the solvent and catalyst were varied. Using 10% palladium on carbon in THF, ethanol, and methanol with 1 equivalent of trifluoroacetic acid as well as Pearlman’s catalyst in ethanol, reduction of both olefins was observed in each case, however BOM deprotected material was never observed. At this point, a two step procedure proved necessary. Under optimized conditions, using 10% palladium on carbon in methanol with 1 molar equivalent of trifluoroacetic acid, the reduction of the olefins was complete in 4 hours, and the TFA salt 25 was obtained in 98% yield.
Scheme 4.
Deprotection of the BOM group needed to be accomplished by other means. This proved to be a challenging task. Since the product of the reaction would be a β-hydroxysilane which might undergo a Peterson elimination16 the choice of reagents seemed crucial. Acyclic β-hydroxysilanes have been studied extensively and it has been found that acid catalyzed elimination proceeds through an antiperiplanar transition state, while the base catalyzed reactions give s y n elimination products.16a Fortunately, given that cyclic structures reduce the degree of conformational mobility, it may be difficult for the system in question to adopt the correct geometry for either acid or base catalyzed olefination to occur.16a
Since after deprotection of 25 the resultant hydroxyl group and the silane are cis, they cannot adopt an antiperiplanar geometry. To a first approximation, it would seem that acidic hydrolysis of the BOM group would be best suited to this system. However, analysis of molecular models also indicates that while not impossible, it is also difficult to obtain the geometry necessary for the syn elimination. Bearing these data in mind, a dissolving metal reduction with lithium in ammonia was first attempted because difficulty was predicted in isolating the product from acidic media. To this end, the salt 25 was exposed to the conditions outlined in Scheme 4.17 The product of the reaction, which had been followed by GCMS and showed the disappearance of the starting material, seemed to be composed of multiple inseparable products. It was eventually surmised that the additional compound was indeed debenzylated, but the hemiacetal remained and unfortunately was not detectable by GCMS.18 This was overcome by treating the reduction product with dilute acid in water to liberate the fully deprotected product. Finally, treatment of the β-hydroxysilane with an excess of Dess-Martin periodinane resulted in a mixture of α-silylketone and the desired product 6b. Once again, exposure of the crude reaction product to dilute aqueous acid gave the ketone 6b in 54% yield from 25 as the sole product. Analysis of this material provided data identical to spectra provided by Professor Aubé, completing a formal synthesis.
Concise formal syntheses of the Aspidosperma alkaloids (±)-aspidospermidine 3, (±)-aspidospermine 4 , and (±)-quebrachamine 5 were completed by intercepting the classic Stork-type intermediate 6b. Inclusion of a trimethylsilyl group in the acyclic precurser allows for a smooth cycloaddition and provides the desired cycloadduct as one diastereomer in excellent yield. The product observed is consistent with the chair transition state 21, demonstrating the predictable nature of the all “W” conformation of this conjugated anion. The synthesis also exemplifies the usefulness of intramolecular cycloaddition reactions of 2-azapentadienyl anions (conjugated 2-azaallyl anions) in complex systems.
Supplementary Material
Full experimental procedures and spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank the National Institutes of Health (GM-52491) for financial support of this work. A.A. gratefully acknowledges the Eastman Kodak Corporation and the American Chemical Society Division of Organic Chemistry sponsored by the Schering Plough Research Institute for graduate fellowships. We thank Professor Jeffrey Aubé (University of Kansas) for providing spectra of ketone 6b for comparison.
References
- 1.Saxton JE. Chapter 1. In: Cordell GA, editor. The Alkaloids. Vol. 51. New York: Academic Press; 1998. [Google Scholar]
- 2.Brossi A, Suffness M, editors. The Alkaloids: Chemistry and Biology. Vol. 37. San Diego, CA: Academic Press; 1990. [Google Scholar]
- 3.Toczko MA, Heathcock CH. J. Org. Chem. 2000;65:2642. doi: 10.1021/jo991599s. [DOI] [PubMed] [Google Scholar]
- 4.For a comprehensive listing of previous syntheses, see ref. 6i
- 5.(a) Stork G, Dolfini JE. J. Am. Chem. Soc. 1963;85:2872. [Google Scholar]; (b) Stork G. Pure Appl. Chem. 1966;9:131. [Google Scholar]
- 6.(a) Ban Y, Sato Y, Inoue I, Nagai M, Oishi T, Terashim M, Yonemits O, Kanaoka Y. Tetrahedron Lett. 1965:2261. doi: 10.1016/s0040-4039(00)70368-6. [DOI] [PubMed] [Google Scholar]; (b) Ban Y, Ikuo I, Inoue I, Akagi M, Oishi T. Tetrahedron Lett. 1969;25:2067. doi: 10.1016/s0040-4039(01)88087-4. [DOI] [PubMed] [Google Scholar]; (c) Kuehne ME, Bayha C. Tetrahedron Lett. 1966:1311. [Google Scholar]; (d) Stevens RV, Fitzpatrick JM, Kaplan M, Zimmerman RL. Chem. Commun. 1971:857. [Google Scholar]; (e) Martin SF, Desai SR, Phillips GW, Miller AC. J. Am. Chem. Soc. 1980;102:3294. [Google Scholar]; (f) Wu PL, Chu M, Fowler FW. J. Org. Chem. 1988;53:963. [Google Scholar]; (g) Meyers AI, Berney D. J. Org. Chem. 1989;54:4673. [Google Scholar]; (h) Iyengar R, Schildknegt K, Aube J. Org. Lett. 2000;2:1625. doi: 10.1021/ol005913c. [DOI] [PubMed] [Google Scholar]; (i) Iyengar R, Schildknegt K, Morton M, Aube J. J. Org. Chem. 2005;70:10645. doi: 10.1021/jo051212n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Banwell MG, Smith JA. J. Chem. Soc., Perkin Trans. 1. 2002:2613. [Google Scholar]; (k) Fukuda Y, Shindo M, Shishido K. Org. Lett. 2003;5:749. doi: 10.1021/ol034020s. [DOI] [PubMed] [Google Scholar]
- 7.For a review see Pearson WH, Stoy P. Synlett. 2003:903.
- 8.(a) Pearson WH, Jacobs VA. Tetrahedron Lett. 1994;35:7001. [Google Scholar]; (b) Pearson WH, Barta NS, Kampf JW. Tetrahedron Lett. 1997;38:3369. [Google Scholar]
- 9.Mi Y. Ph.D. Thesis. Ann Arbor, MI: University of Michigan; 1999. [Google Scholar]
- 10.Pearson WH, Szura DP, Postich MJ. J. Am. Chem. Soc. 1992;114:1329. [Google Scholar]
- 11.Depezay JC, LeMerrer Y. Tetrahedron Lett. 1975;16:3469. [Google Scholar]
- 12.Smith AB, III, Levenberg PA, Jerris PJ, Scarborough RM, Wovkulich PM. J. Am. Chem. Soc. 1981;103:1501–1513. [Google Scholar]
- 13.(a) Marek I, Alexakis A, Normant JF. Tetrahedron Lett. 1991;32:5329. [Google Scholar]; (b) Brasseur D, Marek I, Normant JF. Tetrahedron. 1996;52:7235. [Google Scholar]
- 14.Rawal VH, Michoud C. J. Org. Chem. 1993;58:5583. [Google Scholar]
- 15.(a) Patro B, Murphy JA. Org. Lett. 2000;2:3599. doi: 10.1021/ol006477x. [DOI] [PubMed] [Google Scholar]; (b) Kuehne ME, Wang T, Seaton PJ. J. Org. Chem. 1996;61:6001. [Google Scholar]
- 16.(a) Ager DJ. Org. React. 1990;38:1. [Google Scholar]; (b) Hudrlik PF, Peterson D. J. Am. Chem. Soc. 1975;97:1464. [Google Scholar]; (c) Peterson DJ. J. Org. Chem. 1968;33:780. [Google Scholar]
- 17.Daly JW, McNeal E, Gusovsky F, Ito F, Overman LE. J. Med. Chem. 1988;31:477. doi: 10.1021/jm00397a036. [DOI] [PubMed] [Google Scholar]
- 18.This was discovered after oxidation of the crude reaction mixture gave what appeared to be a mixture of desired product and formate protected alcohol, resulting from oxidation of the hemiacetal.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental procedures and spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.