Abstract
Repetitive transcranial magnetic stimulation (rTMS) has emerged as a promising tool to induce plastic changes that are thought in some cases to reflect N-methyl-d-aspartate-sensitive changes in synaptic efficacy. As in animal experiments, there is some evidence that the sign of rTMS-induced plasticity depends on the prior history of cortical activity, conforming to the Bienenstock–Cooper–Munro (BCM) theory. However, experiments exploring these plastic changes have only examined priming-induced effects on a limited number of rTMS protocols, often using designs in which the priming alone had a larger effect than the principle conditioning protocol. The aim of this study was to introduce a new rTMS protocol that gives a broad range of after-effects from suppression to facilitation and then test how each of these is affected by a priming protocol that on its own has no effect on motor cortical excitability, as indexed by motor-evoked potential (MEP). Repeated trains of four monophasic TMS pulses (quadripulse stimulation: QPS) separated by interstimulus intervals of 1.5–1250 ms produced a range of after-effects that were compatible with changes in synaptic plasticity. Thus, QPS at short intervals facilitated MEPs for more than 75 min, whereas QPS at long intervals suppressed MEPs for more than 75 min. Paired-pulse TMS experiments exploring intracortical inhibition and facilitation after QPS revealed effects on excitatory but not inhibitory circuits of the primary motor cortex. Finally, the effect of priming protocols on QPS-induced plasticity was consistent with a BCM-like model of priming that shifts the crossover point at which synaptic plasticity reverses from depression to potentiation. The broad range of after-effects produced by the new rTMS protocol opens up new possibilities for detailed examination of theories of metaplasticity in humans.
Repetitive transcranial magnetic stimulation (rTMS), which activates cortical-output neurons trans-synaptically (Day et al. 1989), is a promising tool to induce lasting plastic changes in humans that are thought in some cases to reflect N-methyl-d-aspartate (NMDA)-sensitive changes in synaptic efficacy such as long-term potentiation (LTP) and long-term depression (LTD) (Stefan et al. 2002; Huang et al. 2007). As in animal experiments, in which prior history of neuronal activity determines the sign and magnitude of subsequent synaptic plasticity (i.e. metaplasticity; Abraham & Bear, 1996), some evidence indicates that the direction of the rTMS-induced plasticity depends on prior cortical activity (e.g. Iyer et al. 2003; Ziemann et al. 2004). The implication is that non-invasive methods might be able to probe the homeostatic mechanisms which prevent neuronal circuits from becoming destabilized and which maintain synapses within a dynamic range of modifiability (Abbott & Nelson, 2000).
Previous experiments explored the effects of priming stimulation on only a limited number of rTMS protocols, often using designs in which the effects of priming protocols alone were larger than those of the principle conditioning protocol. For example, Lang et al. (2004) and Siebner et al. (2004) used a priming protocol that itself produced lasting increase of motor cortical excitability whereas the primary conditioning alone had no effect on MEP size. More recently, Müller et al. (2007) employed two sessions of paired associative stimulation, both of which could modulate MEP amplitudes alone. Although the results were in accord with the BCM model of synaptic metaplasticity, data from animal studies show that prior induction of LTP or LTD is not essential for changing subsequent synaptic plasticity (Huang et al. 1992; Abraham & Tate, 1997; Abraham, 2008). Even if the priming protocol was below threshold for LTP (i.e. it induced only transient increase of basic synaptic efficacy), subsequent tetanic stimulation, which was able to induce LTP when given alone, failed to elicit LTP (Huang et al. 1992; Abraham & Tate, 1997). In the present experiments we tested whether it was possible to produce similar effects in human primary motor cortex using a priming protocol that itself was unable to evoke any lasting effects on cortical excitability.
The experiments use a new rTMS protocol termed quadripulse stimulation (QPS). In their original description of QPS, Hamada et al. (2007b) applied four equal monophasic TMS pulses at an interstimulus interval (ISI) of 1.5 ms. When repeated at a rate of 0.2 Hz for 30 min, this led to significant increases in MEP amplitude lasting more than an hour. Here we employ QPS with a range of ISIs from 1.5 to 1250 ms and show that increasing the ISI leads to a gradual shift in the sign of the after-effect from facilitation to suppression. We then test how the threshold ISI at which this transition occurs is affected by priming. This allows for a direct comparison with the sliding modification threshold (θM) of the Bienenstock–Cooper–Munro (BCM) theory (Bienenstock et al. 1982).
Methods
Subjects
Subjects were 10 healthy volunteers (three women, seven men; 27–53 years old, mean ±s.d., 38.6 ± 6.9 years) who gave their written informed consent to participate in the experiments. No subjects had neurological, psychiatric, or other medical problems, or had any contra-indication to TMS (Wassermann, 1998). All were right-handed according to the Oldfield handedness inventory (Oldfield, 1971). The protocol was approved by the Ethics Committee of the University of Tokyo and was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Recordings
Subjects were seated on a comfortable chair. Motor-evoked potentials (MEPs) were recorded from the right first dorsal interosseous muscle (FDI). Pairs of Ag/AgCl surface cup electrodes (9 mm diameter) were placed over the muscle belly (active) and the metacarpophalangeal joint of the index finger (reference). We also recorded surface electromyogram (EMG) from the abductor digiti minimi (ADM) and flexor carpi ulnaris (FCU) muscles on the same side in the experiment to study the input specificity and spread of excitation. Responses were input to an amplifier (Biotop; GE Marquette Medical Systems, Japan) through filters set at 100 Hz and 3 kHz; they were then digitized and stored on a computer for later offline analyses (TMS bistim tester; Medical Try System, Japan).
Stimulation
First, TMS was given over the hand area of the motor cortex using a hand-held figure-of-eight coil (9 cm external diameter at each wing; The Magstim Co. Ltd, Whitland, Dyfed, UK) placed tangentially over the scalp with the handle pointing backwards at about 45 deg laterally, which is perpendicular to the central sulcus. Single monophasic TMS pulses were delivered using a magnetic stimulator (Magstim 200; The Magstim Co. Ltd). Quadripulse stimuli were delivered using four magnetic stimulators (Magstim 2002; The Magstim Co. Ltd) connected with a specially designed combining module (The Magstim Co. Ltd). This device combines the outputs from four stimulators to allow a train of four monophasic magnetic pulses to be delivered through a single coil.
The optimal site for eliciting MEPs in the right FDI muscle (i.e. hot spot) was determined before each experiment. In this determination, we stimulated several positions 1 cm distant from each other using the same intensity. The hot spot was defined as the site at which the largest responses were elicited. This position was marked using a red pen on the scalp for repositioning the coil. Placing the coil over this position, the resting motor threshold (RMT) was defined as the lowest intensity that evoked a response of at least 50 μV in the relaxed FDI in at least five of 10 consecutive trials (Rossini et al. 1994). The active motor threshold (AMT) was defined as the lowest intensity that evoked a small response (> 100 μV) when the subjects maintained a slight contraction of the right FDI (5–10% of the maximum voluntary contraction), as observed using an oscilloscope monitor, in more than 5 of 10 consecutive trials. The stimulus intensity was changed in steps of 1% of the maximum stimulator output.
Experiment 1. ISI dependency of QPS-induced plasticity
Conditioning protocols consisted of 360 trains of TMS pulses with an inter-train interval (ITI) of 5 s (i.e. 0.2 Hz) over 30 min (total 1440 magnetic pulses) (Fig. 1A). Each train consisted of four magnetic pulses (i.e. quadripulse stimulation: QPS) separated by a certain interstimulus interval (ISI) 1.5 ms, 5 ms, 10 ms, 30 ms, 50 ms, 100 ms, or 1250 ms. These conditioning types were designated as QPS-1.5 ms, QPS-5 ms, …, QPS-1250 ms. The stimulus intensity of each pulse was set to 90% AMT. The trains were applied over the hot spot for FDI. During the conditioning, no MEPs were observed. The subjects participated in different protocols at least 1 week after the preceding experiment. The numbers of subjects participating in each experimental condition are presented in the Results section. The order of the experiments was randomized and counter-balanced among all subjects.
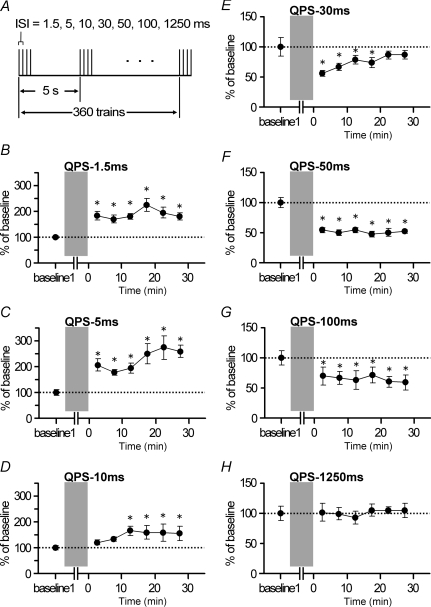
Figure 1. QPS protocol and bidirectional QPS-induced plasticity.
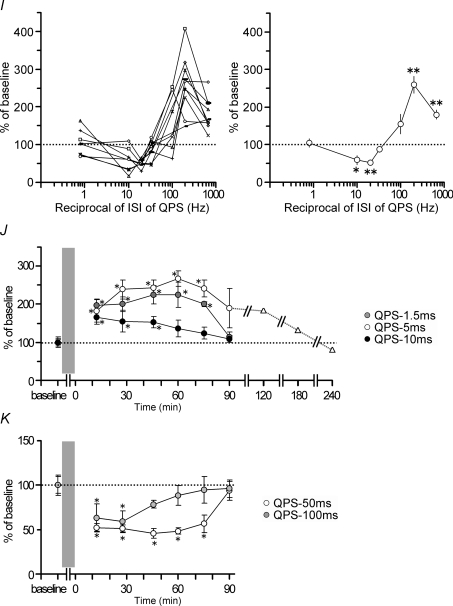
A, the conditioning protocol comprises 360 trains of TMS pulses during 30 min. Each train comprises four monophasic magnetic pulses (i.e. quadripulse stimulation: QPS) delivered at interstimulus intervals of 1.5–1250 ms. One train was given once in 5 s. B–H, normalized amplitudes of MEPs (mean ±s.e.m.) for 30 min after QPS. B–D, QPS at short intervals provided significant and sustained MEP facilitation for at least 30 min compared to the baseline: B, QPS-1.5 ms, n = 10; C, QPS-5 ms, n = 10; D, QPS-10 ms, n = 7. E–H, QPS at long intervals yielded significant MEP suppression compared to the baseline: E, QPS-30 ms, n = 10; F, QPS-50 ms, n = 10; G, QPS-100 ms, n = 7; H, QPS-1250 ms, n = 8. QPS-30 ms produced a transient decrease of MEPs up to 20 min. A sustained MEP suppression (for 30 min) was found after QPS-50 ms and QPS-100 ms. No significant MEP changes were found after QPS-1250 ms. Asterisks denote P < 0.05 by post hoc Dunnett's test. I, normalized amplitudes of MEP measured at 30 min after QPS as a function of the reciprocal of ISI of QPS: left, individual results; right, group result, mean (±s.e.m.) of baseline, n = 7 for each point. Asterisks (*P < 0.05, **P < 0.001) denote significant difference from QPS-1250 ms. J and K, normalized amplitude of MEP for a longer period after QPS. J, QPS-1.5 ms (grey circles, n = 7), QPS-5 ms (open circles, n = 6), and QPS-10 ms (black circles, n = 7). In one subject, MEP measurements were extended beyond 180 min after QPS-5 ms (triangles). The MEP sizes returned to the baseline level at 240 min post QPS-5 ms. Asterisks denote P < 0.05 by post hoc Dunnett's test. K, QPS-50 ms (circles, n = 7) and QPS-100 ms (grey circles, n = 6). Asterisks denote P < 0.05 by post hoc Dunnett's test.
Motor cortical excitability was assessed by measuring the peak-to-peak amplitude of MEPs from the relaxed right FDI muscle for all experiments. During the experiments, the EMG activity of the FDI was monitored using an oscilloscope monitor. Trials contaminated with voluntary EMG activity were discarded from analyses. Before QPS, 20 MEPs were obtained every 14.5–15.5 s using single-pulse TMS at a fixed intensity. The stimulus intensity was adjusted to produce MEPs of about 0.4–0.5 mV in the right FDI muscle at baseline; the intensity was kept constant throughout the same experiment. After QPS conditioning, MEPs were measured every 5 min for 30 min and then every 15 min for 90 min. For one subject, we extended MEP measurements beyond 180 min. At each time point of the measurements, MEPs were collected in the same manner as baseline measurements. For each subject and time point, MEP amplitudes were averaged and normalized to the MEP amplitude measured at the baseline (i.e. baseline 1 in Fig. 1).
Experiment 2. Basic properties of QPS-induced plasticity
Experiment 2a. Motor thresholds and recruitment curves
The AMT and RMT were measured; recruitment curves were constructed before and after QPS-5 ms (n = 5) or QPS-50 ms (n = 5). Stimuli were applied at the hot spot for FDI. After the AMT and RMT determination, eight stimuli were applied every 7.5–8.5 s at an intensity of 10% below RMT. The stimulus intensity was then increased by 5% and another eight stimuli were applied. This process was repeated until the intensity reached 135% RMT. The time of evaluation after QPS was 30 min after conditioning.
Experiment 2b. MEP from FDI, ADM and FCU muscles
The MEPs from the right FDI and FCU were simultaneously recorded before and after QPS-5 ms (n = 5) or QPS-50 ms (n = 5) over the hot spot for FDI, in order to see the topographically specific modulation of QPS. They were collected in separate trials with 20 stimuli every 14.5–15.5 s. The stimulus intensity was set to elicit MEPs of 0.2–0.3 mV in the FCU muscle at the baseline. At 30 min after QPS, MEPs from target muscles were measured in the same manner as that used for baseline measurements. Additionally, we also recorded MEPs from the right FDI and ADM muscles before and after QPS-5 ms or QPS-50 ms in same subjects in separate experiments. The stimulus intensity was set to elicit MEPs of 0.4–0.5 mV in the ADM muscle.
Experiment 2c. Motor cortical excitability accessed by paired-pulse TMS
In 10 subjects, short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were evaluated using the paired-pulse TMS technique described by Kujirai et al. (1993) to clarify the effects of QPS on excitatory and inhibitory circuits of the primary motor cortex.
First, SICI was examined at an interstimulus interval (ISI) of 3 and 4 ms using a conditioning stimulus (CS) intensity of 70%, 80% and 90% AMT and ICF at an ISI of 10 and 15 ms with a CS intensity of 90% AMT. The intensity of the test stimulus (TS) was adjusted to elicit MEPs of 0.4–0.5 mV in relaxed FDI. Second, short-interval intracortical facilitation (SICF) was examined at an ISI of 1.2, 1.3, 1.5, 1.7, 1.9 and 2.1 ms (Tokimura et al. 1996; Ziemann et al. 1998; Hanajima et al. 2002). The intensity of the first stimulus (S1) was adjusted to elicit MEPs of 0.4–0.5 mV and that of second stimulus (S2) was set at 10% below AMT.
In both studies, 15 trials were recorded for each condition and randomly intermixed with 15 trials with the TS alone. Inter-trial intervals were 6.5–7.5 s. Each experiment was performed in one session using the four magnetic stimulators (i.e. three stimulators produced the different CS intensities and one gave the TS). Because AMT did not change significantly after QPS-5 ms or QPS-50 ms (see Results), the CS intensity was kept constant. However, the TS intensity was adjusted to evoke test MEPs with amplitudes of 0.4–0.5 mV after QPS-5 ms or QPS-50 ms.
Measurements of SICI, ICF and SICF were performed in blocks immediately before (baseline) and just after QPS-5 ms or QPS-50 ms to the left primary motor cortex (M1) (post 1) as well as 30–60 min (post 2) after QPS-5 ms or QPS-50 ms. Each block lasted for approximately 30 min (20 min for SICI and ICF, and 10 min for SICF).
Experiment 3. Priming-induced effects on QPS-induced plasticity
To investigate metaplasticity of the human motor cortex, priming stimulation was performed prior to QPS conditioning (Figs 5 and 7).
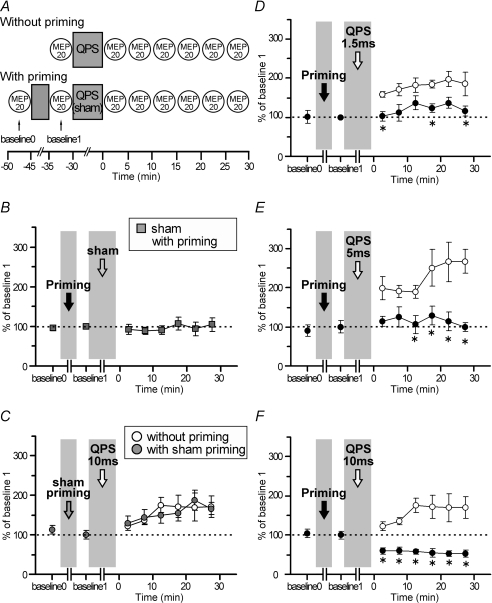
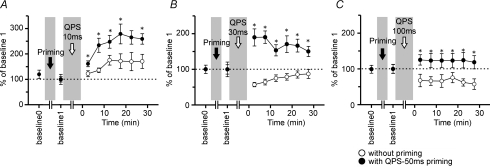
Figure 5. QPS-5 ms priming effects on QPS-induced plasticity (n = 6).
A, timeline of experiments (see Methods). The priming is QPS-5 ms for 10 min. B, sham conditioning with real priming did not modify motor cortical excitability. C, the after-effects of QPS-10 ms without priming (open circles) were not different from those of QPS-10 ms with sham priming (grey circles). D–I, time courses of MEP amplitude following QPS at various intervals with (•) and without priming (^). Asterisks denote significant difference of MEP sizes with priming from those without priming at each time point (P < 0.05 by post hoc paired t tests). D and E, priming occluded MEP facilitation induced by QPS-1.5 ms and QPS-5 ms. F, priming stimulation reversed the QPS-10 ms-induced long-lasting MEP facilitation to the lasting MEP suppression. G, when priming was applied before QPS-30 ms, MEP suppression did not revert to the baseline level at 30 min post conditioning. H and I, QPS-50 ms and QPS-100 ms: priming slightly enhanced MEP suppression after conditioning.
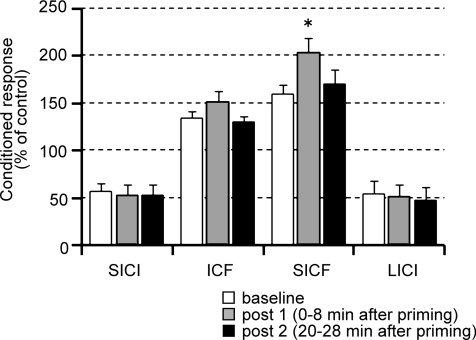
Figure 7. Effects of QPS-5 ms for 10 min on SICI, ICF SICF LICI.
Neither SICI, ICF, nor LICI was altered by 10 min QPS-5 ms alone. SICF was enhanced after QPS-5 ms for only 10 min. Baseline (open bars); post 1 (grey bars), 0–8 min after QPS; post 2 (black bars), 20–28 min after QPS. *P < 0.05 by post hoc Dunnett's test.
Experiment 3a. QPS conditioning with QPS-5 ms priming
Main experiments
Six subjects participated in this study. QPS-5 ms for 10 min (i.e. four pulses at an ISI of 5 ms with an ITI of 5 s for 10 min) was selected as the priming stimulation because LTP is occluded by high-frequency priming, which transiently enhances synaptic efficacy (i.e. using a protocol below the threshold for LTP induction) (Huang et al. 1992). QPS-5 ms (at 90% AMT) for 10 min induces no substantial effects on MEP sizes (see Results). We presumed that even this priming stimulation, which did not induce an LTP-like phenomenon by itself, would have considerable effects on subsequent QPS-induced plasticity according to precedent animal studies, which showed that prior induction of LTP is not a requisite for metaplasticity (Abraham & Tate, 1997).
Before the priming stimulation (baseline 0 in Fig. 5A), 20 MEPs were collected every 14.5–15.5 s using single-pulse TMS at a fixed intensity, which was adjusted to elicit MEPs of about 0.4 mV in the right FDI muscle at baseline 0 and kept constant throughout the experiment. After priming, 20 MEPs were again obtained in the same manner as measurements at baseline 0 (baseline 1 in Fig. 5A). Following this measurement at baseline 1 (i.e. immediately after priming), QPS conditioning of various types (QPS-1.5 ms, QPS-5 ms, QPS-10 ms, QPS-30 ms, QPS-50 ms, and QPS-100 ms) was then performed for 30 min. The stimulus intensity of each pulse of QPS conditioning was set to 90% AMT. After each QPS, MEPs were measured every 5 min for 30 min.
Control experiments
In the first control experiment (Fig. 5B), priming stimulation (i.e. QPS-5 ms) was followed by sham conditioning stimulation (i.e. sham with priming) to examine whether the priming alone affects motor cortical excitability. In the second control experiment (Fig. 5C), sham priming stimulation was followed by QPS-10 ms to confirm that sham priming did not affect QPS-induced plastic changes (QPS-10 ms with sham priming). In fact, QPS-10 ms was chosen because this protocol induced mild facilitatory after-effects (see Results) rendering it more susceptible, if at all, to the effects of sham priming.
The sham stimulation procedure used for these control experiments was identical to those described in a previous report (Okabe et al. 2003). In brief, four electric pulses (each electric pulse was of 0.2 ms duration with an intensity of twice the sensory threshold) were given to the head skin at 0.2 Hz at an ISI of 10 ms for sham conditioning (first control experiment) and of 5 ms for sham priming (second control experiment) with a conventional electric peripheral nerve stimulator to mimic the skin sensation of TMS. Electric pulses were applied through the electrodes placed over the left-hand motor area and the vertex (Cz of international 10–20 system). A coil, which was not connected to the stimulator, was placed over the left-hand motor area to mimic real TMS. Another coil, which was connected to a combining module with four Magstim (2002) stimulators, was held off the scalp but placed near the subject. This coil was discharged simultaneously with the scalp electrical stimulation to produce a similar sound to that associated with real QPS. For each subject and time point (including baseline 0), the MEP amplitudes were averaged and normalized to the MEP amplitude measured at baseline 1 (Fig. 5A).
Supplementary experiments
As shown in Results, QPS-5 ms priming for 10 min followed by 30 min of QPS-5 ms produced no lasting changes in MEP size. To clarify whether a pause between priming and conditioning is necessary to induce metaplastic changes, we performed two additional experiments.
First, we performed an experiment using QPS-5 ms for 40 min with an ITI of 5 s in 5 subjects (Fig. 6A). The stimulus intensity of each pulse was set to 90% AMT. We measured MEPs before and after conditioning in a manner similar to Experiment 1. Second, QPS-5 ms for 20 min with an ITI of 5 s at 90% AMT was applied to the hot spot for FDI in 5 subjects to verify whether QPS-5 ms for 30 min can be regarded as QPS-5 ms for 20 min with 10 min QPS-5 ms priming. MEPs were measured before and after this conditioning in the same manner as Experiment 1.
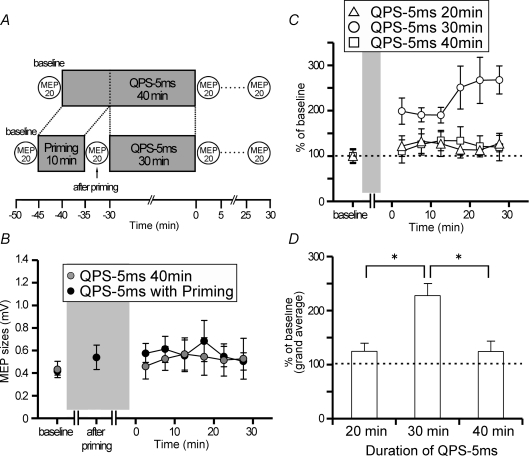
Figure 6.
A, timeline of experiment with QPS-5 ms for 40 min (see Methods). B, the after-effects of QPS-5 ms for 40 min (grey circles) did not differ from those of QPS-5 ms for 30 min with 10 min QPS-5 ms priming (black circles). C, time courses of the after-effects of QPS-5 ms with different conditioning durations. D, the grand average of normalized amplitudes of MEPs for 30 min post conditioning as a function of the conditioning duration of QPS-5 ms. *P < 0.05.
Experiment 3b. Effects of QPS-5 ms priming on SICI, ICF, SICF and LICI
Nine subjects participated in this experiment. To explore the effects of QPS-5 ms priming alone on either excitatory or inhibitory circuits of the primary motor cortex, SICI, ICF, SICF and long-interval intracortical inhibition (LICI) (Valls-Sole et al. 1992; Wassermann et al. 1996) were all measured before and after QPS-5 ms priming (i.e. four pulses at an ISI of 5 ms with an ITI of 5 s for 10 min; the stimulus intensity of each pulse, 90% AMT).
SICI was examined at an ISI of 3 ms using a CS intensity of 80% AMT. ICF was measured at an ISI of 10 ms with a CS intensity of 90% AMT. SICF was measured at an ISI of 1.5 ms with a S2 intensity of 90% AMT. LICI was measured at an ISI of 100 ms with a CS intensity of 110% RMT. The intensity of TS was adjusted to elicit MEPs of 0.4–0.5 mV in relaxed FDI at baseline. Twelve trials were recorded for each condition and randomly intermixed with 12 trials of TS alone with an ITI of 7.5–8.5 s. The SICI, ICF, SICF and LICI were all studied simultaneously in one session (about 8 min). Measurements of these values were performed in blocks immediately before (baseline) and just after QPS-5 ms priming (post 1) as well as 20–28 min (post 2). Conditioning intensities and test intensity were not changed after priming.
Experiment 3c. QPS conditioning with QPS-50 ms priming
Six subjects participated in this study. According to the BCM model, the crossover point can shift horizontally in either direction, so that priming stimulation that reduced neuronal activity might shift the crossover point leftward. Thus, QPS-50 ms for 10 min (i.e. four pulses at an ISI of 50 ms with an ITI of 5 s for 10 min; the stimulus intensity of each pulse, 90% AMT) was selected as another priming stimulation because QPS-50 ms for 30 min produced significant MEP and SICF suppression (see Results). We expected that QPS-50 ms priming might shift the stimulus–response curve of QPS-induced plasticity to the left. Amplitudes of MEPs were measured in the same manner as Experiment 3a before and after QPS-10 ms, QPS-30 ms, or QPS-100 ms with the priming stimulation of QPS-50 ms for 10 min.
Data analyses
Experiment 1
The after-effects of different conditioning protocols were analysed with absolute MEP amplitudes using two-way repeated-measures analysis of variance (ANOVA) (between-subject factor, CONDITION (QPS-1.5 ms, QPS-5 ms, …, QPS-1250 ms); within-subject factor, TIME (baseline 1 and following six time points)) because of the different numbers of subjects among conditions. If the factors CONDITION and TIME showed significant interaction, post hoc paired t tests (two-tailed) with Bonferroni's corrections for multiple comparisons were used for further analyses. The time course of after-effects of each conditioning type on absolute MEP sizes was analysed using one-way repeated measures ANOVA (within-subject factor, TIME). Dunnett's post hoc test was used for further analyses. The Greenhouse–Geisser correction was used if necessary to correct for non-sphericity; P values less than 0.05 were considered significant.
Experiment 2a
Recruitment curves before and after QPS were compared using two-way repeated-measures ANOVA (within-subject factors: TIME and INTENSITY). Bonferroni's post hoc test was used for further analyses. Paired t tests (two-tailed) were also used to compare variables (RMT and AMT) before and after QPS.
Experiment 2b
Absolute MEP amplitudes from FDI, ADM and FCU before and after QPS were pooled and compared using two-way repeated-measures ANOVA (within-subject factors: TIME and MUSCLE). Post hoc paired t tests (two-tailed) were used for further analyses.
Experiment 2c
The ratio of the mean amplitude of the conditioned response to that of the control response was calculated for each condition in each subject. These individual mean ratios were then averaged to give a grand mean ratio. The values of SICI (individual mean ratios) were entered in three-way repeated-measures ANOVA with conditioning INTENSITY (70% AMT, 80% AMT and 90% AMT), ISI (3 ms and 4 ms), and TIME (baseline, post 1, and post 2) as within-subject factors. For ICF and SICF, the ratios were compared using two-way repeated measures ANOVA (within-subject factors; ISI (10 ms and 15 ms for ICF; 1.2 ms, 1.3 ms, …, 2.1 ms for SICF), and TIME (baseline, post 1, and post 2)). Dunnett's post hoc test was used for further analyses.
Experiment 3a
Main experiments
Absolute values of MEPs at baseline 0 and baseline 1 (Fig. 5A) were compared using paired t tests in each experiment. To evaluate priming effects on subsequent QPS-induced plasticity, the absolute amplitudes of MEPs collected in Experiment 1 (i.e. without priming) and Experiment 3a (i.e. with QPS-5 ms priming) were entered in three-way repeated-measures ANOVA with PRIMING (with and without priming), CONDITION (QPS-1.5 ms, QPS-5 ms, …, and QPS-100 ms), and TIME (baseline 1, and following six time points) as within-subject factors to match the measurement time points relative to QPS conditioning between Experiments 1 and 3a. Additionally, it might be valid to evaluate the effect of priming stimulation on subsequent QPS-induced plasticity using these values because the absolute amplitudes obtained at baseline 0 and 1 were not significantly different (see Results).
Control experiments
The time course of after-effects for the first control experiment (i.e. sham conditioning with real priming) on absolute MEP sizes was analysed using one-way repeated measures ANOVA (within-subject factor, TIME (baseline 1 and following six time points)). For the second control experiment, the after-effects of QPS-10 ms with sham priming were compared with those of QPS-10 ms without priming using two-way repeated-measures ANOVA (within-subject factors, CONDITION (QPS-10 ms with sham priming, QPS-10 ms without priming) and TIME (baseline 1, and following six time points)). Post hoc paired t tests (two-tailed) with Bonferroni's corrections for multiple comparisons were used for further analyses, if the factors PRIMING, CONDITION and TIME or PRIMING and CONDITION showed significant interaction.
Supplementary experiments
First, the after-effects of QPS-5 ms for 30 min with 10 min QPS-5 ms priming and QPS-5 ms for 40 min on absolute MEP sizes were compared using two-way repeated-measures ANOVA (within-subject factors, CONDITION (QPS-5 ms with priming, QPS-5 ms for 40 min) and TIME (baseline 1, and following six time points)). Second, the after-effects of QPS-5 ms with different durations (20 min, 30 min and 40 min) on absolute MEP sizes were compared using two-way repeated-measures ANOVA (within-subject factors, DURATION (QPS-5 ms for 20 min, 30 min and 40 min) and TIME (baseline, and following six time points)). Post hoc Bonferroni's method was used for further analysis. The time course of after-effects of each condition (QPS-5 ms for 20 min, 30 min and 40 min) on absolute MEP sizes was analysed using one-way repeated measures ANOVA (within-subject factor, TIME (baseline 1 and following six time points)).
Experiment 3b
The time course of after-effects on each value of SICI, ICF, SICF and LICI was analysed using one-way repeated measures ANOVA (within-subject factor, TIME). Dunnett's post hoc test was used for further analyses.
Experiment 3c
Absolute values of MEPs at baseline 0 and 1 were compared using paired t tests in each experiment. To evaluate priming effects on subsequent QPS-induced plasticity, the absolute amplitudes of MEPs collected in Experiment 1 (i.e. without priming) and Experiment 3c (i.e. with QPS-50 ms priming) were entered in three-way repeated-measures ANOVA with PRIMING (with and without priming), CONDITION (QPS-10 ms, QPS-30 ms and QPS-100 ms), and TIME (baseline 1, and following six time points) as within-subject factors. Post hoc paired t tests (two-tailed) with Bonferroni's corrections for multiple comparisons were used for further analyses, if the factors PRIMING, CONDITION and TIME showed significant interaction.
Data were analysed using software (SPSS vers.13.0 for Windows; SPSS Inc.). All figures depict group data.
Results
No subject reported any adverse effect during or after any experiment. Baseline physiological data (Table 1) did not differ significantly among different experiments.
Table 1.
Physiological parameters (mean ±s.d.)
| RMT | AMT | MEP size (FDI) | Test MEP size (for Expts 2c and 3b) | ||||
|---|---|---|---|---|---|---|---|
| Experiment 1 | Baseline 1 | Baseline 1 | Baseline 1 | Baseline | Post 1 | Post 2 | |
| QPS-1.5 ms | 52.3 ± 11.7% | 35.0 ± 8.6% | 0.39 ± 0.08 | — | — | — | |
| QPS-5 ms | 53.6 ± 12.7% | 37.5 ± 8.8% | 0.43 ± 0.10 | — | — | — | |
| QPS-10 ms | 53.5 ± 10.7% | 37.6 ± 8.0% | 0.48 ± 0.11 | — | — | — | |
| QPS-30 ms | 56.2 ± 19.0% | 36.6 ± 7.9% | 0.42 ± 0.16 | — | — | — | |
| QPS-50 ms | 52.5 ± 10.3% | 36.7 ± 7.9% | 0.47 ± 0.12 | — | — | — | |
| QPS-100 ms | 54.9 ± 12.1% | 35.8 ± 9.2% | 0.45 ± 0.14 | — | — | — | |
| QPS-1250 ms | 52.5 ± 16.1% | 34.9 ± 16.1% | 0.44 ± 0.16 | — | — | — | |
| Experiments 2a & 2b | |||||||
| QPS-5 ms | 51.8 ± 12.2% | 37.4 ± 6.3% | — | — | — | ||
| QPS-50 ms | 50.3 ± 6.1% | 38.8 ± 2.1% | — | — | — | ||
| Experiment 2c | |||||||
| QPS-5 ms | 59.6 ± 8.6% | 39.0 ± 3.6% | 0.45 ± 0.12 | 0.44 ± 0.13 | 0.47 ± 0.14 | ||
| QPS-50 ms | 56.2 ± 9.2% | 39.0 ± 5.5% | 0.46 ± 0.13 | 0.48 ± 0.12 | 0.43 ± 0.10 | ||
| Experiment 3a | Baseline 0 | Baseline 0 | Baseline 0 | Baseline 1 | |||
| Main experiments; with QPS-5 ms priming | |||||||
| QPS-1.5 ms | 55.5 ± 14.8% | 37.2 ± 11.1% | 0.45 ± 0.13 | 0.48 ± 0.25 | — | — | — |
| QPS-5 ms | 56.0 ± 7.3% | 37.1 ± 5.1% | 0.42 ± 0.08 | 0.45 ± 0.17 | — | — | — |
| QPS-10 ms | 58.0 ± 6.0% | 39.7 ± 6.2% | 0.46 ± 0.17 | 0.46 ± 0.17 | — | — | — |
| QPS-30 ms | 55.5 ± 12.6% | 39.7 ± 11.7% | 0.48 ± 0.11 | 0.43 ± 0.16 | — | — | — |
| QPS-50 ms | 53.2 ± 12.2% | 37.8 ± 4.9% | 0.49 ± 0.11 | 0.45 ± 0.15 | — | — | — |
| QPS-100 ms | 54.7 ± 13.0% | 36.0 ± 7.6% | 0.47 ± 0.10 | 0.48 ± 0.16 | — | — | — |
| Control experiments | |||||||
| Sham with priming | 53.6 ± 9.2% | 34.6 ± 6.2% | 0.46 ± 0.06 | 0.51 ± 0.14 | — | — | — |
| QPS-10 ms with sham priming | 54.5 ± 12.1% | 37.4 ± 10.1% | 0.47 ± 0.12 | 0.48 ± 0.18 | — | — | — |
| Supplementary experiments | |||||||
| QPS-5 ms for 20 min | 55.5 ± 12.5% | 37.7 ± 7.7% | — | 0.43 ± 0.15 | — | — | — |
| QPS-5 ms for 40 min | 55.8 ± 12.5% | 35.8 ± 7.9% | — | 0.47 ± 0.16 | — | — | — |
| Experiment 3b | |||||||
| QPS-5 ms priming | 52.5 ± 16.1% | 34.9 ± 16.1% | — | — | 0.46 ± 0.12 | 0.48 ± 0.21 | 0.48 ± 0.22 |
| Experiment 3c | |||||||
| with QPS-50 ms priming | |||||||
| QPS-10 ms | 50.0 ± 10.0% | 36.8 ± 9.3% | 0.44 ± 0.11 | 0.42 ± 0.18 | — | — | — |
| QPS-30 ms | 52.8 ± 12.6% | 36.6 ± 11.6% | 0.40 ± 0.12 | 0.41 ± 0.15 | — | — | — |
| QPS-100 ms | 51.7 ± 12.2% | 36.3 ± 7.5% | 0.46 ± 0.07 | 0.49 ± 0.14 | — | — | — |
ISI-dependency of QPS-induced plasticity (Experiment 1)
Figure 1 shows time courses of MEP size following QPS conditioning. QPS at short ISIs produced an increase in the MEP amplitude (Fig. 1B–D), whereas QPS at long ISIs suppressed MEPs (Fig. 1E–H). Two-way repeated measures ANOVA revealed a significant CONDITION (QPS-1.5 ms, QPS-5 ms, …, and QPS-1250 ms) × TIME interaction (F21.090,193.321= 3.794, P < 0.001). The MEPs to single-pulse TMS were facilitated for at least 30 min after QPS at short ISIs (one-way repeated measures ANOVA: QPS-1.5 ms, effect of TIME, F6,54= 4.680, P < 0.001; QPS-5 ms, effect of TIME, F6,54= 4.512, P < 0.001; QPS-10 ms, effect of TIME, F6,36= 3.286, P = 0.011) (Fig. 1B–D). By contrast, QPS at long ISIs induced MEP suppression (one-way repeated measures ANOVA: QPS-30 ms, effect of TIME, F6,54= 4.509, P < 0.001; QPS-50 ms, effect of TIME, F6,54= 27.073, P < 0.001; QPS-100 ms, effect of TIME, F6,36= 3.987, P = 0.004). The duration of suppression depended strongly on the ISI. In fact, QPS-30 ms induced significant MEP suppression up to 20 min (Fig. 1E). Marked MEP suppression was elicited for 30 min after QPS-50 ms and QPS-100 ms (Fig. 1F and G). No significant MEP changes were found after QPS-1250 ms (one-way repeated measures ANOVA: effect of TIME, F6,42= 0.717, P = 0.638) (Fig. 1H).
Figure 1I presents the MEP amplitude normalized to the baseline MEP 30 min after QPS as a function of the reciprocal of the ISI used in each QPS burst. There was a non-linear relation between MEP excitability and ISI, which was similar to the BCM-like sigmoid curve (Bienenstock et al. 1982; Dudek & Bear, 1992). Post hoc analysis revealed that QPS-1.5 ms, QPS-5 ms and QPS-50 ms were significantly different from QPS-1250 ms (QPS-1.5 ms versus QPS-1250 ms, P < 0.001; QPS-5 ms versus QPS-1250 ms, P < 0.001; QPS-50 ms versus QPS-1250 ms, P < 0.001, QPS-100 ms versus QPS-1250 ms, P = 0.021).
We extended the period of MEP measurement to 90 min or longer to examine when the after-effects of QPS revert to the baseline level (Fig. 1J). Significant MEP facilitation was found for 75 min after QPS-1.5 ms and QPS-5 ms (QPS-1.5 ms, effect of TIME, F6,36= 7.807, P < 0.001; QPS-5 ms, effect of TIME, F6,30= 5.887, P < 0.001) (Fig. 1J). The mean MEPs remained larger than the baseline level (189.9 ± 57%) at 90 min after QPS-5 ms, although the difference was no longer statistically significant (P = 0.085). For one subject, we extended MEP measurements beyond 180 min and confirmed that MEPs had returned to the baseline at 240 min post QPS-5 ms (Fig. 1J). Transient MEP facilitation was found after QPS-10 ms (effect of TIME, F6,36= 3.552, P = 0.007), which lasted up to 45 min (P < 0.05), but not to 60 min (P > 0.05). For QPS at long intervals, MEPs returned to the baseline level at 90 min after QPS-50 ms (effect of TIME, F6,36= 13.965, P < 0.001) (Fig. 1K). The after-effect did not last beyond 45 min after QPS-100 ms (effect of TIME, F6,30= 4.147, P = 0.004) (Fig. 1K).
Basic properties of QPS-induced plasticity (Experiment 2)
Motor thresholds and recruitment curves (Experiment 2a)
Motor thresholds and recruitment curves were compared before and after QPS-5 ms (n = 5; Fig. 2A and B) and QPS-50 ms (n = 5; Fig. 2D and E) to elucidate the basic properties of QPS-induced plasticity. These two conditioning types were chosen because they induced the strongest facilitatory or suppressive after-effects. Neither the resting motor threshold (RMT) nor the active motor threshold (AMT) was altered (Fig. 2A and D), although the recruitment curves were modulated by QPSs (Fig. 2B and E). After QPS-5 ms, the curve became steeper than before (two-way repeated-measures ANOVA: TIME × INTENSITY interaction, F9,36= 5.862, P < 0.001; effect of TIME, F1,36= 12.728, P < 0.023; effect of INTENSITY, F9,36= 9.026, P < 0.001). Post hoc analysis with Bonferroni's adjustment showed significantly larger MEPs elicited at intensities greater than 125% RMT after QPS-5 ms than before (Fig. 2B). On the other hand, for QPS-50 ms (Fig. 2E), the recruitment curve became shallower after conditioning (two-way repeated-measures ANOVA: TIME × INTENSITY interaction, F1,36= 6.141, P < 0.001; effect of TIME, F1,36= 33.939, P = 0.004; effect of INTENSITY, F9,36= 30.388, P < 0.001). Post hoc analysis using Bonferroni's adjustment showed significantly smaller MEPs elicited at intensities greater than 115% RMT after QPS-50 ms than before (Fig. 2E). These changes cannot be explained by the variability of measurements because the baseline recruitment curve showed good reproducibility. The curves before QPS-5 ms and QPS-50 ms did not differ significantly from each other (two-way repeated-measures ANOVA: CONDITION × INTENSITY interaction, F9,72= 0.301, P = 0.972; effect of CONDITION, F1,8= 0.025, P = 0.879; effect of INTENSITY, F9,72= 20.322, P < 0.001).
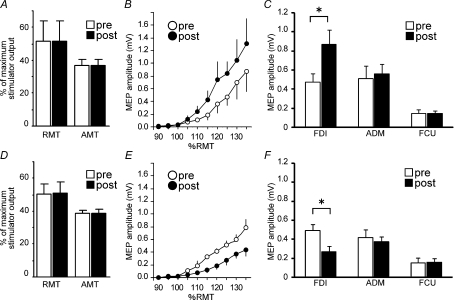
Figure 2. Basic properties of QPS-induced plasticity.
A–C, effects of QPS-5 ms on variables. A, motor thresholds (mean ±s.d.). QPS-5 ms affected neither RMT nor AMT (P > 0.5). Pre-conditioning (open bars); post conditioning (30 min post conditioning) (filled bars). B, the recruitment curve (mean ±s.e.m.). The ordinate gives the MEP size in millivolts; the abscissa shows the stimulus intensity relative to RMT. Pre-conditioning (^); post conditioning (•). Significantly larger MEPs were elicited at intensities greater than 125% RMT after QPS-5 ms. C, MEPs from the FDI, ADM and FCU (mV, mean ±s.e.m., n = 5). D–F, effects of QPS-50 ms on variables. D, motor thresholds (mean ±s.d.). Neither RMT nor AMT was altered by QPS-50 ms. E, the recruitment curve (mean ±s.e.m.). Smaller MEPs were elicited at intensities greater than 115% RMT after QPS-50 ms. F, MEPs from the FDI, ADM and FCU (mV, mean ±s.e.m., n = 5). *P < 0.05.
MEPs from FDI, ADM and FCU muscle (Experiment 2b)
The MEPs to single-pulse TMS from the right ADM and FCU were measured to confirm the topographical specificity of modulation by QPS. For QPS-5 ms (Fig. 2C), two-way repeated measures ANOVA showed a significant TIME × MUSCLE interaction (F2,8= 5.195, P = 0.036). Post hoc paired t test results revealed that only MEPs from the FDI were facilitated after QPS-5 ms. For QPS-50 ms (Fig. 2F), similar results were obtained (two-way repeated measures ANOVA: TIME × MUSCLE interaction, F2,8= 6.579, P = 0.019). Therefore, only MEPs from the FDI were suppressed after QPS-50 ms.
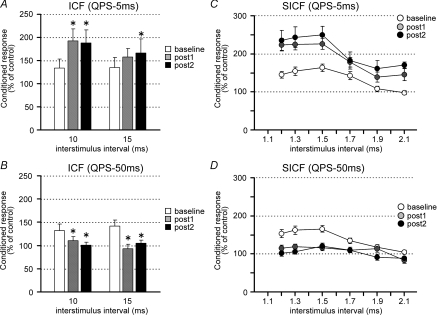
Motor cortical excitability accessed by paired-pulse TMS (Experiment 2c)
To clarify the effects of QPS on excitatory and inhibitory circuits of the primary motor cortex, SICI, ICF and SICF were measured before and after QPS.
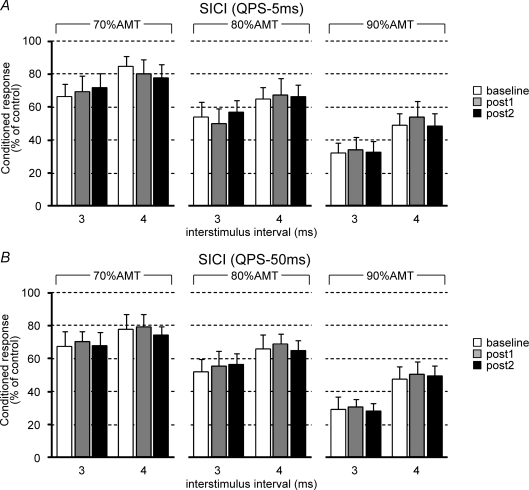
Figure 3 shows the effects of QPS-5 ms (Fig. 3A) or QPS-50 ms (Fig. 3B) on SICI. The test MEP sizes were adjusted before and after QPS conditioning (Table 1). For QPS-5 ms, three-way repeated measures ANOVA revealed a significant main effects of ISI (F1,9= 22.444, P = 0.001) and INTENSITY (F2,18= 28.731, P < 0.001), but no significant effects of TIME (F2,18= 0.003, P = 0.997), ISI × TIME interaction (F2,18= 0.012, P = 0.642), INTENSITY × TIME interaction (F4,36= 0.328, P = 0.857), nor TIME × ISI × INTENSITY interaction (F4,36= 0.679, P = 0.611). Similar results were obtained for QPS-50 ms (Fig. 3B) (three-way repeated measures ANOVA: a main effect of ISI, F1,9= 16.616, P = 0.003; effect of INTENSITY, F2,18= 28.936, P < 0.001; effect of TIME, F2,18= 0.367, P = 0.698; ISI × TIME interaction, F2,18= 0.181, P = 0.836; INTENSITY × TIME interaction, F4,36= 0.095, P = 0.983; TIME × ISI × INTENSITY interaction, F4,36= 0.286, P = 0.885), indicating that QPS did not modulate inhibitory circuits within the primary motor cortex as evaluated by SICI.
Figure 3. Effects of QPS on short-interval intracortical inhibition (SICI).
A and B, SICI before and after QPS-5 ms (A) or QPS-50 ms (B). Conditioning intensities were 70% active motor threshold (AMT; left), 80% AMT (middle), and 90% AMT (right). The abscissa shows conditioning–test intervals (3 ms and 4 ms). Baseline (open bars); post 1 (grey bars), 0–30 min after QPS; post 2 (black bars), 30–60 min after QPS.
In contrast, QPS significantly affected intracortical excitatory circuits. Figure 4A and B shows that ICF was significantly enhanced by QPS-5 ms (two-way repeated measures ANOVA: effect of TIME, F2,18= 4.641, P = 0.024; ISI × TIME interaction, F2,18= 2.299, P = 0.129) and suppressed by QPS-50 ms (two-way repeated measures ANOVA: effect of TIME, F2,18= 6.728, P = 0.007; ISI × TIME interaction, F2,18= 1.358, P = 0.282). Post hoc analysis revealed significant modulation of ICF at post 1 and post 2 compared with those at baseline, suggesting that the effects on ICF lasted longer than 60 min (Fig. 4A and B).
Figure 4. Effects of QPS on intracortical facilitation (ICF) and short-interval ICF (SICF).
A and B, ICF before and after QPS-5 ms (A) or QPS-50 ms (B). Conditioning intensity was 90% AMT. Conditioning–test intervals were 10 and 15 ms. C and D, SICF before and after QPS-5 ms (C) or QPS-50 ms (D). Conditioning intensity was 90% AMT. The abscissa shows conditioning–test intervals.
SICF was also modulated by QPS (Fig. 4C and D). After QPS-5 ms, SICF was enhanced (two-way repeated measures ANOVA: effect of TIME, F2,18= 20.828, P < 0.001; ISI × TIME interaction, F10,90= 1.173, P = 0.319). Post hoc analysis showed significant enhancement of SICF at post 1 and 2 (P < 0.001). By contrast, QPS-50 ms suppressed SICF (Fig. 4D). Two-way repeated measures ANOVA revealed significant ISI × TIME interaction (F10,90= 2.175, P = 0.026). Post hoc analysis revealed significant suppression of SICF with 1.5 ms at post 1 and 2 (P < 0.005).
Priming-induced effects on QPS-induced plasticity (Experiment 3)
QPS conditioning with QPS-5 ms priming (Experiment 3a)
Control experiments
First, the after-effects of sham conditioning with real priming were monitored to examine whether priming alone (i.e. QPS-5 ms for 10 min) affects motor cortical excitability. Figure 5B shows the time course of the normalized MEP amplitude following sham conditioning with real priming. No difference was found in MEP amplitudes at baseline 0 and 1 (paired t test, P > 0.5). Sham conditioning with real priming did not change the MEP amplitude for at least 30 min after conditioning (one-way repeated measures ANOVA: effect of TIME, F6,30= 0.548, P = 0.767).
Second, the after-effect of real conditioning (QPS-10 ms) with sham priming was compared to that of real conditioning without priming to confirm that sham priming does not affect motor cortical plasticity induced by real conditioning. Figure 5C shows the time courses of normalized amplitude of MEPs following real conditioning (QPS-10 ms) without priming and also with sham priming. No difference was found between MEPs of baseline 0 and 1 (paired t test, P > 0.5). Furthermore, MEP amplitudes following QPS-10 ms with sham priming were not different from those without priming (two-way repeated measures ANOVA: effect of CONDITION (QPS-10 ms with sham priming, QPS-10 ms without priming), F1,5= 0.046, P = 0.839; CONDITION × TIME interaction, F6,30= 0.354, P = 0.902).
Main experiments
Figure 5D–I shows the time courses of MEP amplitude following QPS at various intervals with and without priming. No difference in MEP amplitudes at baselines 0 and 1 was found in any condition (paired t test, P > 0.5).
Priming stimulation occluded MEP facilitation induced by QPS-1.5 ms and QPS-5 ms without priming (Fig. 5D and E). In fact, QPS-10 ms with priming induced lasting MEP suppression, whereas that without priming elicited MEP facilitation (Fig. 5F). Priming stimulation enhanced the suppression observed after QPS-30 ms (Fig. 5G). Both QPS-50 ms and QPS-100 ms with priming induced slightly stronger MEP suppression than that induced by QPS without priming (Fig. 5H and I). Three-way repeated measures ANOVA revealed a significant PRIMING × CONDITION interaction (F2.764,13.813= 9.624, P = 0.001), but revealed no significant main effect of TIME (F2.548,12.738= 1.662, P = 0.227) nor a significant PRIMING × CONDITION × TIME interaction (F3.384,16.920= 2.466, P = 0.092). The results reveal that priming stimulation affected subsequent QPS-induced plasticity, irrespective of the time after QPS conditioning. Post hoc paired t tests revealed a significant effect of QPS-5 ms priming on the after-effects of QPS-1.5 ms, QPS-5 ms, QPS-10 ms and QPS-30 ms (Fig. 5D–G).
Supplementary experiments
Figure 5E shows that QPS-5 ms priming for 10 min followed by 30 min of QPS-5 ms produced no lasting changes in MEP sizes. To clarify whether the gap between priming and primary conditioning is necessary for inducing a metaplastic change, we performed an additional experiment using QPS-5 ms for 40 min (Fig. 6A and B). No significant difference was found between the after-effects of QPS-5 ms for 40 min and QPS-5 ms for 30 min with 10 min QPS-5 ms priming, indicating that the after-effects of QPS-5 ms for 30 min were altered by priming stimulation irrespective of the presence or absence of a gap (two-way repeated measures ANOVA: effect of CONDITION (QPS-5 ms with priming, QPS-5 ms for 40 min), F1,4= 0.040, P = 0.851; CONDITION × TIME interaction, F6,24= 0.299, P = 0.931).
Since QPS-5 ms for 30 min can be regarded as QPS-5 ms for 20 min with 10 min QPS-5 ms priming, we also performed an experiment using QPS-5 ms for 20 min with an ITI of 5 s at 90% AMT to identify whether the first part of QPS-5 ms for 30 min conditioning ‘primes’ subsequent 20 min QPS-5 ms conditioning (Fig. 6C). The results showed that QPS-5 ms for 20 min did not produce any plastic changes. In terms of the duration of QPS-5 ms conditioning, two-way repeated measures ANOVA revealed a significant main effect of DURATION (F2,8= 5.923, P = 0.026), but no significant effect of DURATION × TIME interaction (F12,48= 1.126, P = 0.370), indicating that the effect of DURATION did not depend on the measurement time points. Separate one-way repeated measures ANOVA on the time course of each conditioning revealed no significant facilitation of MEPs after QPS-5 ms for 20 min or 40 min compared with baseline MEP sizes (effect of TIME, QPS-5 ms for 20 min, F6,24= 0.655, P = 0.686; QPS-5 ms for 40 min, F6,24= 0.460, P = 0.831). Figure 6D shows the grand averages for 30 min post conditioning as a function of duration of QPS-5 ms. QPS-5 ms for 30 min significantly facilitated MEPs compared with those for 20 min and 40 min. Post hoc analysis of these data using Bonferroni's method revealed a significant difference in DURATION of QPS-5 ms (20 min versus 30 min, P < 0.05; 30 min versus 40 min, P < 0.05).
Effects of QPS-5 ms priming on SICI, ICF, SICF and LICI (Experiment 3b)
Although the QPS-5 ms priming did not produce any lasting changes as indexed by MEP sizes (Table 1), Fig. 7 shows that SICF (but no other measures) was significantly enhanced after QPS-5 ms for 10 min (one-way repeated measures ANOVA: SICI, effect of TIME, F2,14= 0.916, P = 0.824; ICF, effect of TIME, F2,14= 1.193, P = 0.332; SICF, effect of TIME, F2,14= 8.434, P = 0.004; LICI, effect of TIME, F2,14= 0.236, P = 0.793). However, the effects were only transient, being significant only at post 1 (Fig. 7).
QPS conditioning with QPS-50 ms priming (Experiment 3c)
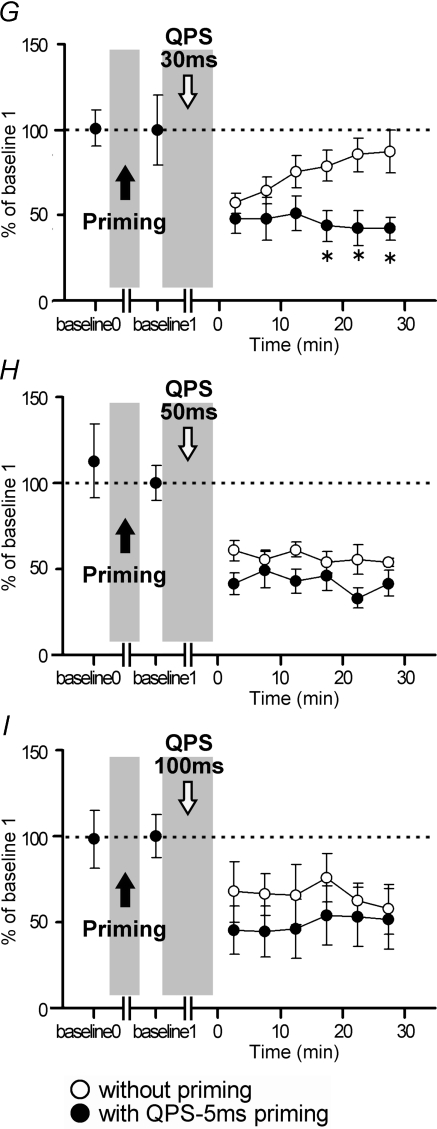
Figure 8 shows significant facilitation of MEP sizes following QPS-50 ms priming in comparison to those without priming (three-way repeated measures ANOVA: PRIMING × CONDITION × TIME interaction, F12,60= 2.226, P = 0.021). Post hoc paired t tests revealed a significant effect of QPS-50 ms priming on the after-effects of QPS-10 ms, QPS-30 ms and QPS-100 ms (Fig. 8A–C).
Figure 8. QPS-50 ms priming effects on QPS-induced plasticity (n = 6).
A–C, time courses of MEP amplitude following QPS at various intervals with (•) and without QPS-50 ms priming (^). Asterisks denote significant difference of MEP sizes with priming from those without priming (P < 0.05 by post hoc paired t tests). A, QPS-10 ms; B, QPS-30 ms; C, QPS-100 ms.
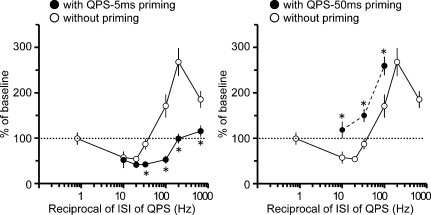
Stimulus–response function with priming
Figure 9 shows the normalized MEP amplitudes at 30 min post conditioning as a function of the reciprocal of the ISI used in each QPS with and without priming. It appears as if the stimulus–response function can be shifted in either direction along the x-axis according to which priming stimulation was employed. Post hoc analysis with these data revealed that QPS-5 ms priming stimulation significantly reduced MEP sizes after QPS-1.5 ms, QPS-5 ms, QPS-10 ms and QPS-30 ms, whereas QPS-50 ms priming facilitated MEP sizes after QPS-10 ms, QPS-30 ms and QPS-100 ms (Fig. 9).
Figure 9. Priming-induced shifts in the stimulus–response function.
Left, the normalized amplitudes of MEP at 30 min post conditioning as a function of the reciprocal of ISI of QPS with (•) and without QPS-5 ms priming (^) (n = 6). Right, the normalized amplitudes of MEP at 30 min post conditioning as a function of the reciprocal of ISI of QPS with (•) and without QPS-50 ms priming (^) (n = 6). *P < 0.05 by post hoc paired t tests.
Discussion
Our study had three major findings. First, there was a non-linear relationship between the sign and duration of plastic changes induced by QPS and the ISI between each of the four pulses in the QPS burst. Second, QPS modulated intracortical excitatory circuits of the primary motor cortex, whereas inhibitory circuits remained unchanged. Third, QPS-induced plasticity was altered by priming stimulation in a manner consistent with the BCM rule.
Relationship to conventional rTMS studies
QPS at short intervals induced facilitatory after-effects with prolonged duration (Fig. 1). We hypothesized previously that I-wave periodicity (i.e. 1.5 ms), a basic property of the human motor cortex (Hanajima et al. 2002), is suitable for LTP-like plasticity induction (Thickbroom et al. 2006; Hamada et al. 2007a,b). However, QPS-5 ms, which does not correspond to I-wave periodicity, also yielded MEP facilitation lasting longer than 75 min. The fact that it is not necessary to use ISIs at an I-wave periodicity to induce prolonged changes in motor cortical excitability raises the intriguing possibility that I-wave periodicity is not the critical factor in the QPS protocol.
The sign and duration of the after-effects depended on a complex function of the stimulus parameters. Prolonged suppression was induced by QPS-50 ms and QPS-100 ms, whose frequency corresponds to 20 Hz and 10 Hz; regular rTMS at those frequencies usually gives rise to transient, short-lasting facilitation (less than a few minutes) (Pascual-Leone et al. 1994; Maeda et al. 2000). The difference in effects is presumably attributable to the number of pulses per train because the stimulus intensity and the total number of pulses used for our study were comparable to those in previous studies (Maeda et al. 2000; Arai et al. 2007). Consequently, the ISI and the number of pulses per train are critical determinants for the sign and duration of the after-effects in the present experiments. Although it would be of value to investigate other parameters of the QPS protocol such as the ITI or the number of pulses per train, this is not possible at the present time. There are no devices which can combine more than four monophasic stimulators and the ITI cannot be shortened to less than a few seconds because of the re-charging time for a monophasic stimulator.
Stimulus–response function of QPS-induced plasticity
The principal finding of this study was that changing the ISIs of QPS induced various levels of plastic changes. As the ISIs of QPS were shortened, the duration of MEP suppression (i.e. produced by QPS-100 ms) first increased (i.e. QPS-50 ms, to more than 75 min) and then decreased (i.e. QPS-30 ms). Further reductions in ISI altered the sign and duration of the plastic changes; transient facilitation (i.e. QPS-10 ms) became stronger and longer-lasting as the ISIs were further shortened (i.e. QPS-5 ms and QPS-1.5 ms). The stimulus–response function using the values of plastic changes obtained at 30 min after QPS exhibited a smooth transition from net suppression to net facilitation. Although the stimulation frequency (i.e. reciprocal of ISI) within one train generally determines the direction of rTMS-induced plasticity (Hallett, 2007), results of our study demonstrate that the ISI within a train not only defines the sign of the plastic changes, but also determines its duration in a non-linear form.
At first sight, the non-linear stimulus–response function appears very similar to those obtained in animal studies. Dudek & Bear (1992) demonstrated a frequency–response function with a smooth transition from net LTD to net LTP as the stimulation frequency was increased systematically, conforming to the proposal of the BCM theory, which states that the strength of synapses depends on postsynaptic activity in a particular non-linear form (Bienenstock et al. 1982). A similar non-linear function has been demonstrated in the visual cortex (Kirkwood et al. 1996) and the hippocampus (Wang & Wagner, 1999; Zhang et al. 2005). These findings indicate that a BCM-like non-linear relation of synaptic plasticity to stimulation frequency is a fundamental characteristic of synaptic plasticity, although the critical factors for inducing synaptic plasticity are probably the integrated postsynaptic depolarization and Ca2+ entry, and not the stimulation frequency per se (Bear, 1996).
Possible mechanism of QPS-induced plasticity
Despite the general concordance of the non-linear property of the stimulus–response function of QPS with those of animal studies of synaptic plasticity, the lack of direct recording of synaptic response in conscious humans renders any hypothesis explaining precise neuronal mechanisms underlying rTMS-induced or QPS-induced plasticity speculative (Cooke & Bliss, 2006). Nevertheless, results obtained through the present study might provide some evidence that favours the long-term alteration of synaptic efficacy as the mechanism of QPS-induced plasticity.
First, motor thresholds which were not altered by QPS are considered to reflect the membrane excitability of postsynaptic neurons (Mavroudakis et al. 1994, 1997; Ziemann et al. 1996b; Chen et al. 1997). Consequently, general changes in membrane excitability, which play an important role in motor learning (Woody et al. 1991; Aou et al. 1992), might not be the main mechanism for QPS-induced plasticity, which is consistent with the results of our previous work (Hamada et al. 2007b). Second, QPS caused bidirectional modulation of the recruitment curves. The slope of the curve depends on the distribution of cortical neurons' excitability; its synaptic connectivity is a possible factor causing changes of this curve. Third, QPS-induced plasticity was topographically specific to the stimulation site, indicating one basic property of synaptic plasticity: input specificity (Bliss & Collingridge, 1993). Fourth, the plastic changes of QPS lasted for about 75 min. This persistence of plasticity might be comparable to that of LTP, rather than that of post-tetanic or short-term potentiation (Bliss & Collingridge, 1993).
Fifth, the results of paired-pulse measurements to study intracortical excitability (Experiment 2c) revealed that QPS mainly modulated excitatory circuits within the primary motor cortex. Because SICI, which is considered to reflect γ-aminobutyric acid (GABA)-ergic inhibitory function of the human motor cortex (Kujirai et al. 1993; Ziemann et al. 1996a, b; Hanajima et al. 1998), remained unchanged (Fig. 3), modulation of the GABA-ergic system is probably not responsible for the MEP changes after QPS. Several reports have demonstrated that SICI is differentially modulated by various rTMS protocols which induce LTP or LTD-like plasticity. For example, SICI was altered by the theta burst stimulation (TBS) protocol (Huang et al. 2005), whereas SICI did not show any changes after the paired associative stimulation (PAS) (Stefan et al. 2002). These data provide corroborating evidence that lasting MEP modulation induced by several interventions in humans is not always conferred by some alteration of the GABA-ergic system contributing to SICI, even though GABA-ergic blockers are frequently required to induce LTP in neocortex in animal studies (Kirkwood & Bear, 1994; Hess et al. 1996).
By contrast, both ICF and SICF were altered by QPS protocols. Although ICF has been considered to be produced at the motor cortex (Kujirai et al. 1993; Ziemann et al. 1996c), the origin of ICF is still unclear since there were no changes in amplitude or number of descending volleys by ICF (Di Lazzaro et al. 2006). Therefore, we cannot draw any firm conclusion about the mechanism for ICF modulation by QPS. Firmer conclusions can perhaps be drawn from the studies of SICF.
SICF has been proposed to be caused by an interaction of I-wave inputs (Tokimura et al. 1996; Ziemann et al. 1998; Hanajima et al. 2002), probably by summation of excitatory postsynaptic potentials (EPSPs) elicited by the first suprathreshold TMS with subliminal depolarization of interneurons elicited by the second subthreshold TMS at cortical interneurons (Hanajima et al. 2002). Modulation of SICF after QPS would therefore be consistent with the idea that QPS alters the efficiency with which I-waves summate during paired-pulse TMS and would support the view that the effects of QPS involve changes at intracortical synapses. However, at some ISIs (e.g. QPS-5 ms), the trough of SICF was also substantially modulated as well as its peak (Fig. 4). In such cases, as at QPS-5 ms, it may still be possible that QPS did not specifically enhance intracortical excitatory circuits reflected by SICF, but changed the excitability of cortical interneurons and/or cortical output neurons undetectable by MT measurements and which in turn modulated both the peak and the trough of SICF. It should be noted that in these measurements of intracortical excitability, the intensity of the test stimulus after QPS was adjusted to match the amplitudes of test responses before QPS conditioning, so that the difference in test MEP sizes after QPS cannot contribute to the after-effects on ICF and SICF or the lack of changes in SICI.
Taken together, the points described above lead us to surmise that QPS mainly modulates the excitatory circuits of the primary motor cortex. We consider that the mechanism of QPS-induced plasticity involves long-term synaptic plasticity in those circuits with features of non-linear dependence on ISI of QPS, which is reminiscent of previous findings of synaptic plasticity in animal studies (Dudek & Bear, 1992). Further studies with pharmacological interventions are necessary to address synaptic mechanisms in more detail.
Metaplasticity of QPS-induced plasticity
The second main finding of this study was that priming stimulation that itself had no effect on MEP amplitude led to a shift in the stimulus–response function of QPS. QPS-5 ms priming shifted the function to the right along the x-axis, such that suppressive plastic changes were promoted over a wider range of ISI than before priming. By contrast, QPS-50 ms priming shifted the curve to the left, with the effect that facilitatory plastic changes were favoured over suppressive ones.
Effects of QPS-5 ms priming alone on excitatory and inhibitory circuits
Two control experiments revealed that neither priming alone nor cutaneous stimulation produced by the TMS pulses had any lasting effect on MEPs. Furthermore, QPS-5 ms priming did not change SICI, ICF or LICI. Its only effect was a transient increase in SICF, which did not persist as long as the priming effects on QPS. We cannot exclude the possibility that subtle changes in inhibitory circuits were missed because paired-pulse measurements addressing intracortical excitability were only assessed at a single ISI with a single conditioning intensity. In addition, determination of SICI and LICI resulted in about 50% inhibition at baseline measurements (Fig. 6), which is submaximal for SICI and LICI according to previous paired-pulse studies (Valls-Sole et al. 1992; Kujirai et al. 1993; Wassermann et al. 1996), thus excluding a possible floor effect in the results. Another possibility to be ruled out is that the repeated application of suprathreshold TMS pulses during MEP measurements might have influenced priming and conditioning effects. However, such measurements do not alter SICF so that its effects, if present at all, should be negligible.
Priming-induced effects on subsequent QPS-induced plasticity
A homeostatic relationship between the prior history of neuronal activity and subsequent QPS-induced plasticity is consistent with the BCM theory, which states that θM, the point of crossover from LTD to LTP, is not fixed, but varies as a function of the activation history of postsynaptic neurons (Bienenstock et al. 1982). Compelling evidence now supports slidingθM, which is exemplified by the shift in crossover point of the frequency–response function of synaptic plasticity in conditioning frequency–response experiments (Kirkwood et al. 1996; Wang & Wagner, 1999; Zhang et al. 2005). According to the BCM model of sliding θM in animal experiments, we propose that priming stimulation transiently modulates neuronal activity of the human primary motor cortex and this prior history of cortical activity determines the sign of subsequent QPS-induced plasticity: QPS-5 ms priming transiently enhanced cortical activity, which led the following QPS conditioning to favour suppressive plastic changes. By contrast, QPS conditioning favoured facilitatory plastic changes after QPS-50 ms priming which might transiently reduce cortical activity.
Theoretically, the stimulus–response function should move only to the left or right along the horizontal axis as a function of postsynaptic activity (Bienenstock et al. 1982), but our stimulus–response function also seems to shift vertically (Fig. 9). Until further ISIs are tested we cannot comment in detail on this effect. However, a vertical shift in frequency–response function of synaptic plasticity has also been found in several animal studies and it has been proposed that its mechanism differs from that of the horizontal shift (Philpot et al. 2003; Zhang et al. 2005). For example, the priming-induced effects in the present study might be related to the substantial modulation of α-Ca2+/calmodulin-dependent kinase II (αCaMKII) in activity-dependent form, which has been revealed to be a pivotal component for the vertical shifts in hippocampal slices (Zhang et al. 2005).
Duration of QPS conditioning: inverted-U relationship
We have additionally shown that QPS-5 ms for 20 min or 40 min did not produce any MEP size changes, whereas QPS-5 ms for 30 min induced LTP-like plasticity. The amount of facilitation and the duration of QPS conditioning showed an inverted U-shaped relationship (Fig. 6D), indicating that there is a threshold for inducing LTP-like plasticity, in line with our previous reports (Hamada et al. 2007b). These findings are consistent with previous animal studies. Christie et al. (1995) revealed that LTP induction depended on the number of TBS trains: 2 trains of TBS produced no plasticity and 8 TBS trains induced maximal LTP, while 16 TBS trains produced less LTP in the hippocampal slices. Another study showed that peak amounts of LTP occurred after 8–16 TBS trains, but 24 or 32 TBS trains produced no LTP (Abraham & Huggett, 1997). These lines of evidence suggest an inverted U-shaped relation between the amount of TBS and the degree of LTP (Christie et al. 1995). Such a time-dependent LTP reversal process (or the over-stimulation effect) was probably attributable to a depotentiation mechanism during the massed presentation of tetanic stimulation (Abraham & Huggett, 1997). Likewise, the after-effects of QPS-5 ms for 40 min might entail a similar mechanism.
The gap between priming and primary conditioning
The fact that the effects of QPS-5 ms for 40 min were comparable to those of QPS-5 ms for 30 min with 10 min QPS-5 ms priming suggests that the time gap between priming and subsequent primary conditioning is unnecessary. This finding also raises the intriguing possibility that the first part of the QPS conditioning might ‘prime’ subsequent conditioning. However, without data at different ISIs, it is difficult to comment further on this point.
Relationship to previous human studies of metaplasticity
The present findings with regard to the shifts in stimulus–response function of QPS-induced plasticity are in harmony with those of previous human studies which have revealed a similar effect of priming stimulation on subsequent rTMS-induced after-effects, suggesting metaplasticity of the human primary motor cortex. High-frequency rTMS priming enhanced the transient suppressive effect of 1 Hz rTMS (Iyer et al. 2003). The polarity of transcranial direct current stimulation (Nitsche & Paulus, 2001) determines the direction of subsequent after-effects of 1 or 5 Hz rTMS, which imparted no effect on motor cortical excitability when applied alone (Lang et al. 2004; Siebner et al. 2004). Reportedly, priming using PAS affected subsequent PAS-induced plasticity (Müller et al. 2007). Although they have explored only a limited number of rTMS protocols for primary conditioning (Iyer et al. 2003; Lang et al. 2004; Siebner et al. 2004; Müller et al. 2007), they have all used priming which itself induced LTP or LTD-like phenomena.
Based on the experimental evidence of metaplasticity, however, priming which does not itself change the basic synaptic efficacy can also alter the subsequent synaptic plasticity (Huang et al. 1992; Abraham & Tate, 1997; Wang & Wagner, 1999). It has also been suggested that the investigation of metaplasticity is facilitated when the priming stimulation does not alter the strength of synaptic transmission, because if LTP is produced by the priming stimulation, it is difficult to preclude the possibility that a lack of further LTP induction by tetanic stimulation is attributable to the ceiling effect of LTP (i.e. saturation of LTP) or a homeostatic mechanism that entails active inhibition of LTP (Abraham, 2008). Consistent with these results in animals, the critical new finding of the present investigation is that priming stimulation over the primary motor cortex that did not itself change MEP sizes (but altered SICF for a short period), has a large impact on subsequent QPS-induced plasticity. Thus, prior induction of LTP- or LTD-like phenomena is unnecessary to induce metaplastic changes in humans. Our findings are, at least partly, consistent with recent studies which have shown that voluntary muscle contraction (which may be a consequence of cortical activity enhancement of various motor-related areas) that is not enough to induce lasting effects on motor cortical excitability influenced the subsequent after-effects induced by TBS (Gentner et al. 2007; Huang et al. 2008). Although a potential weakness of the study is that the interpretation of the present data is speculative and not based upon experimental data on human motor cortical function, our new protocol, giving bidirectional after-effects with prolonged duration, enables us to test the effect of priming over a wide range. In fact, we showed that priming stimulation induced bidirectional shifts of the non-linear stimulus–response function of motor cortical plasticity in agreement with those revealed in animal studies of metaplasticity.
Safety issues
No subject reported any adverse effects during or after any intervention. Moreover, the spread of excitation to proximal muscles was not observed. We have previously shown that QPS at 1.5 ms with higher intensity than in the present study (using 130 ± 24% AMT, that is 82 ± 7% RMT: 74–98% RMT) can safely induce motor cortical plasticity in normal subjects with regard to seizure induction, because (1) no spread of excitation was observed, and (2) the occurrence rate of post-TMS EMG activity, which was thought to be a possible correlate of after-discharge, was not different from those during sham stimulation (Hamada et al. 2007b). These findings provide evidence that QPS can safely induce motor cortical plasticity. Obviously, adequate EMG monitoring is absolutely imperative to recognize early signs of seizure during future rTMS studies. Recent advances in rTMS devices heighten the need for establishing new safety guidelines for complex rTMS protocols such as QPS (Hamada et al. 2007b), paired pulse stimulation (PPS) (Thickbroom et al. 2006; Hamada et al. 2007a) and TBS (Huang et al. 2005).
Conclusions
The mechanism of QPS-induced plasticity favours long-term synaptic plasticity with features of non-linear dependence on stimulation frequency. Priming elicited bidirectional shifts in the stimulus–response function of motor cortical plasticity. The data support a BCM-like model of priming that shifts the crossover point at which the synaptic plasticity reverses from LTD to LTP. Such a broad range of after-effects produced by the new rTMS protocol opens up new possibilities for examining the details of metaplasticity theories in humans.
Acknowledgments
We are grateful to Professor John C. Rothwell for his constructive comments and English editing. We also thank Mr Isao Furuya and Mr Eisaku Ohyama for technical assistance. Part of this work was supported by Research Project Grants-in-aid for Scientific Research no. 18590928 (Y.T.), no. 17590865 (R.H.), and no. 16500194 (Y.U.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, grants for the Research Committee on rTMS Treatment of Movement Disorders from the Ministry of Health, Labour and Welfare of Japan (17231401), the Research Committee on Dystonia, the Ministry of Health, Labour and Welfare of Japan, and a grant from the Committee of the Study of Human Exposure to EMF, Ministry of Internal Affairs and Communications. The author (M. H.) is supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Aou S, Woody CD, Birt D. Increase in excitability of neurons of the motor cortex of cats after rapid acquisition of eye blink conditioning. J Neurosci. 1992;12:560–569. doi: 10.1523/JNEUROSCI.12-02-00560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Okabe S, Furubayashi T, Mochizuki H, Iwata NK, Hanajima R, Terao Y, Ugawa Y. Differences in after-effect between monophasic and biphasic high-frequency rTMS of the human motor cortex. Clin Neurophysiol. 2007;118:2227–2233. doi: 10.1016/j.clinph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. Hippocampus. 1995;5:52–59. doi: 10.1002/hipo.450050107. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss VP. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and the effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depresson of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm239. DOI 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. Origin of facilitation in repetitive, 1.5 ms interval, paired pulse transcranial magnetic stimulation (rPPS) of the human motor cortex. Clin Neurophysiol. 2007a;118:1596–1601. doi: 10.1016/j.clinph.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin Neurophysiol. 2007b;118:2672–2682. doi: 10.1016/j.clinph.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I-waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MG, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Woe O, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of vigabatrin on motor potentials with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:124–127. doi: 10.1016/s0924-980x(96)96607-2. [DOI] [PubMed] [Google Scholar]
- Müller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci. 2007;25:3461–3468. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Okabe S, Ugawa Y, Kanazawa I, Effectiveness of rTMS on Parkinson's Disease Study Group 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson's disease. Mov Disord. 2003;18:382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis of metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol. 2006;117:61–66. doi: 10.1016/j.clinph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wang H, Wagner JJ. Priming-induced shift in synaptic plasticity in the rat hippocampus. J Neurophysiol. 1999;82:2024–2028. doi: 10.1152/jn.1999.82.4.2024. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Woody CD, Gruen E, Birt D. Changes in membrane currents during Pavlovian conditioning of single cortical neurons. Brain Res. 1991;539:76–84. doi: 10.1016/0006-8993(91)90688-r. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kirschstein T, Sommerberg B, Merkens M, Manahan-Vaughan D, Elpersma Y, Beck H. Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. J Neurosci. 2005;25:7697–7707. doi: 10.1523/JNEUROSCI.2086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between cortical inhibition and facilitation in human motor cortex. J Physiol. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I-wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]