Abstract
Chronic exercise improves endurance and skeletal muscle oxidative capacity. Despite the potential importance of reactive oxygen species (ROS) generated during exercise as regulators of these adaptations, the effect of repeated bouts of aerobic exercise on ROS generation by skeletal muscles during contractions has not been examined. Our aim was to establish the impact of repeated treadmill running exercise on muscle ROS generation and activation of redox-sensitive transcription factors. Following 8 weeks of treadmill running, mice displayed an improvement in running speed that was associated with an enhanced ability of gastrocnemius (GTN) muscles to maintain force during a protocol of isometric contractions. In contrast to GTN muscles of cage-sedentary (Sed) mice, muscles from exercised (Exer) mice did not release superoxide or nitric oxide during the isometric contractions. For male mice, basal levels of nuclear factor κB (NFκB) and activator protein-1 (AP-1) DNA binding were increased by treadmill running, and the contraction-induced activation of NFκB and AP-1 observed in muscles of Sed mice was absent in Exer muscles. Also in contrast to Sed muscles, Exer muscles displayed no reductions in glutathione or protein thiol levels in response to contraction. Our observations of decreases for Exer compared with Sed muscles in contraction-induced (i) ROS generation, (ii) activation of redox-sensitive signalling pathways, and (iii) ROS stress suggest that exercise conditioning enhances the ability of skeletal muscle to readily and rapidly detoxify ROS and/or reduces ROS generation, providing protection from ROS-induced damage and reducing signals that might act to mediate further unnecessary adaptations.
Effects in skeletal muscle of aerobic exercise on both oxidative (Holloszy, 1967; Baldwin et al. 1972; Holloszy & Coyle, 1984; Booth & Thomason, 1991) and antioxidant (Sen et al. 1992; Leeuwenburgh et al. 1994; Ji, 1996; Leeuwenburgh et al. 1997; Clanton et al. 1999; Powers et al. 1999) capabilities have been well described, but the regulatory mechanisms underlying this wide range of adaptations are complex and incompletely understood (reviewed in Flück, 2006; Hood et al. 2006). Reactive oxygen species (ROS) generated by contracting muscles have for many years been viewed as inevitable but unwanted effects of aerobic exercise, but important roles for ROS as signalling molecules that contribute to normal cell function are also recognized (Pahl, 1999; Zhou et al. 2001; Jackson et al. 2002). Based on a growing appreciation of the influence of redox-sensitive signalling pathways on normal cellular processes (Rhee, 2006), a reasonable hypothesis is that an important regulator of the adaptations in skeletal muscle in response to aerobic exercise may be ROS generated during the exercise. This hypothesis was explored recently using a myoblast cell line treated in culture with lactate anion (Hashimoto et al. 2007). Treatment with lactate not only increased hydrogen peroxide production, but also activated redox-sensitive signalling pathways and increased expression of antioxidant enzymes and proteins associated with mitochondrial biogenesis, responses typically seen as a result of aerobic exercise (Hashimoto et al. 2007).
Specific ROS generated by contracting muscles include nitric oxide, superoxide, hydrogen peroxide and hydroxyl radicals (Powers et al. 1999). Despite the potential importance of ROS generated during skeletal muscle contractions as regulators of the adaptations that occur in response to aerobic exercise, no studies have examined the effect of regular exercise on the generation of ROS by skeletal muscle during subsequent contractile activity. Furthermore, the effect of repeated aerobic exercise on the ability of acute contractile activity to stimulate activation of redox-sensitive transcription factors has not been explored. We previously developed a demanding but non-damaging protocol of isometric contractions that results in the production of ROS (McArdle et al. 2001; Vasilaki et al. 2006a) and the activation of the redox-sensitive transcription factors NFκB and AP-1 (Vasilaki et al. 2006b). The aim of the present study was therefore to determine the effect of aerobic exercise on ROS generation and the associated activation of NFκB and AP-1 DNA binding by contracting skeletal muscles. Toward this aim, both male and female mice were exposed to 8 weeks of 5 days per week treadmill running exercise and subsequently muscles of these mice, as well as age-matched sedentary controls, were administered the aforementioned protocol of isometric contractions. The production of ROS in response to the contraction protocol was measured and muscles were subsequently analysed for NFκB and AP-1 activation as well as other corroborating measures of ROS stress, and oxidative and antioxidant enzyme activities.
Methods
Animals
These studies were carried out on specific pathogen-free (SPF) adult (6–8 months old) male and female C57BL/6 mice. Mice were randomly separated into two groups, those that underwent 8 weeks of treadmill running (exercised mice; Exer group) and those that served as unexercised controls (cage sedentary mice; Sed group). Throughout, all mice were housed in an SPF barrier facility in microisolated shoe box cages at the University of Michigan. All experimental procedures were approved by the University Committee for the Use and Care of Animals at the University of Michigan.
Treadmill running exercise
In order to exercise all mice at the same relative intensity, initial fitness levels were evaluated by a graded exercise test that established the maximum running capacities of the mice (Mazzeo et al. 1984). For this test, mice were placed individually on a motorized treadmill (Columbus Instruments, Eco 3/6, Columbus, OH, USA) at a 0% grade and an initial speed of 10 m min−1, a speed that we expected all mice to be capable of tolerating (Pagala et al. 1998). Treadmill speed was increased by 2 m min−1 every 2 min until the mouse could no longer keep pace with the treadmill. Based on the initial graded test, mice were grouped with other mice of the same gender with similar running capabilities for the 8 weeks of treadmill exercise.
Treadmill running was performed using programs similar to those previously described for studies of cardiovascular, skeletal muscle oxidative capacity and antioxidant adaptations in rats (Fitts et al. 1975; Mazzeo et al. 1984; Gore et al. 1998; Hollander et al. 1999). The exercise program consisted of running 5 days per week at an intensity of ∼75% of the maximum running speed. At the outset, mice were run for 15 min at a speed 70–80% of the maximum determined in the graded exercise test. After 2 to 3 days, running times were increased 15 min each day until the animals could run continuously for 1 h per day. Mice were manually encouraged to run, as well as being exposed to brief periods of electric shock if they failed to keep up with the speed of the treadmill. If, at any time, a mouse absolutely refused to run, choosing instead to sit on the shock bar, the animal was removed from the treadmill for the day. Attempts were made each week to progressively increase running speed to maintain the exercise intensity throughout the 8 weeks. If running performance showed evidence of decline, mice were exercised at a lower treadmill speed with the goal of keeping the maximum number of mice running for as close to 60 min as possible. Overall, for the entire 8 week training period, female mice ran an average of 49 ± 3 min per day and male mice ran an average of 40 ± 6 min per day.
At the end of the 8 weeks of treadmill running, mice were once again tested for maximum running speed using the graded exercise test. Within 24 h of the final exposure to treadmill running, the Exer mice were again separated into two groups. One group of Exer mice was killed by intraperitoneal injections of an overdose of the anaesthetic Avertin (tribromoethanol; 400 mg kg−1) and limb muscles were removed, quickly weighed and rapidly frozen in liquid nitrogen and stored at −70°C for subsequent analysis as quiescent muscles. The second group of Exer mice was anaesthetized and the hind limb muscles were subjected to a 15 min protocol of demanding but non-damaging isometric contractions (McArdle et al. 2001; Vasilaki et al. 2006a,b) prior to removing the muscles and killing the animals. Sed mice, that had not undergone treadmill running, were also separated into groups whose hind limb muscles either remained quiescent or were exposed to the isometric contraction protocol and were treated in the same fashion as muscles from Exer animals.
Isometric contraction protocol to assess ROS generation during contractile activity
Mice were anaesthetized with intraperitoneal injections of Avertin (tribromoethanol; 250 mg kg−1), with supplemental doses administered as needed to maintain a depth of anaesthesia sufficient to prevent response to tactile stimuli. Incisions were made at both ankles to expose the gastrocnemius (GTN) muscles. The right Achilles tendon was clamped to the lever arm of a servomotor (Aurora Scientific, Model 305) to monitor the force produced by the right GTN muscle during the contraction protocol. The right knee was immobilized between two sharpened screws, and surface electrodes were placed in a position as proximal along the limbs as possible as well as around the ankles to activate the muscles of both hind limbs (McArdle et al. 2001). Maximum isometric tetanic contractions were produced by square wave pulses of 0.2 ms duration, a voltage slightly greater than that required to produce a maximum twitch (usually ∼70 V), and a frequency of 100 Hz (McArdle et al. 2001). GTN muscle length was set at the optimum length for force production. Isometric contractions were held for 500 ms, with one contraction every 5 s for a total of 180 contractions during the 15 min of contractions.
Levels of ROS generated and released into the interstitial fluid were monitored in the left GTN muscle during the contraction protocol (McArdle et al. 2001). Four microdialysis probes (MAB 3.8.4, Metalant, Stockholm, Sweden) with a molecular mass cut-off of 35 kDa were placed into the muscle with a 22-gauge plastic introducer to permit analysis of extracellular ROS activities. The microdialysis probes consist of a 10 mm tubular dialysis membrane that has a diameter of 0.5 mm with concentric inlet and outlet tubes. Perfusion of the probes was performed at a very slow flow rate so small molecules in the perfusate and extracellular space reached equilibrium (McArdle et al. 2001). Probes were perfused at 4 μl min−1 with normal saline for analysis of hydrogen peroxide and total nitric oxide (NO) content (Vasilaki et al. 2006a) or with 50 μm cytochrome c in normal saline for analysis of superoxide levels (McArdle et al. 2001). Perfusate samples were collected from the probes via the outlet tubes every 15 min, for the 60 min before the contractions, during the 15 min of isometric contractions, and for 15 min after the period of contractions. Mice remained under anaesthesia until the end of the experiment and were then killed with an intraperitoneal injection of an overdose of Avertin.
Biochemical analyses
Muscle masses
At the time of kill, the extensor digitorum longus (EDL), soleus (SOL), tibialis anterior (ATB), gastrocnemius (GTN), plantaris (PLN), flexor digitorum longus (FDL), biceps femoris (BFM), vastus lateralis (VLT) and vastus medialis (VMD) muscles were dissected, trimmed of tendons, and weighed on a microbalance to the nearest 0.1 mg.
Analyses of extracellular ROS activities in microdialysates
Analyses of the microdialysates were undertaken as described by Vasilaki et al. (2006a). Briefly, reduction of cytochrome c was used as an index of superoxide anion radical concentration previously described (McArdle et al. 2001). The total nitrate and nitrite content was measured as an index of total NO generation using a commercial fluorometric assay (Cayman Chemical Co. USA) based on the method of Miles et al. (1995), and hydrogen peroxide content was measured using a modification of the method of Lei et al. (1997).
Analyses of muscle enzyme activities
Frozen GTN muscle samples were analysed as previously described (Larkin et al. 1997) for citrate synthase activity. Briefly, muscle homogenates (from a 700 g supernatant) were used to determine citrate synthase activity (μmol (μg protein)−1 min−1) using the method of Srere (1969). GTN muscle homogenates were also analysed for catalase activity by following the kinetic decomposition of hydrogen peroxide spectrophotometrically at 240 nm with the method described by Claiborne (1985). Total muscle superoxide dismutase (SOD) activity was measured according to the method of Crapo et al. (1978).
Analysis of muscle total glutathione and protein thiol content
The automated glutathione recycling method described by Anderson (1996) was used to assess the total glutathione content of samples with a 96-well plate reader (Benchmark, Bio-Rad). The protein thiol content of samples was analysed by the method of Di Monte et al. (1984), adapted for use on a 96-well plate reader.
Western blotting analysis of muscle SOD content
MnSOD and CuZnSOD contents of GTN muscles were analysed by Western blotting techniques. Muscle samples were homogenized in 1% SDS containing 1 mm iodoacetimide, 1 mm benzithonium chloride, 5.7 mm phenylmethylsulphonyl fluoride and 5 mm EGTA (Sigma Co., Dorset, UK). Cellular debris was removed by centrifugation and samples stored at −70°C until analysis. Protein content of samples was determined using the bicinchoninic acid method (Sigma). One hundred micrograms of total cellular protein were separated on SDS-PAGE followed by Western blotting. MnSOD and CuZnSOD were identified using antibodies obtained from Stressgen Inc. (MnSOD, Cat #SOD-110; CuZnSOD, Cat #SOD-101). Bands were visualized using a Biorad Chemi-Doc System (Biorad).
Gel shift analysis of NFκB and AP-1 DNA binding
Electrophoretic mobility shift assays (EMSA) of DNA biding by nuclear factor κB (NFκB) and activator protein-1 (AP-1) were carried out using previously reported techniques (Broome et al. 2006; Vasilaki et al. 2006b).
Statistical analyses
Changes throughout the treadmill running program in body mass and maximum running speed as determined by graded exercise tests were assessed by two-way repeated measures analysis of variance (gender × repetition). Two-way repeated measures anovas (treadmill running × sample) were also used to determine the effect of treadmill running on changes in levels of extracellular ROS for samples taken before, during, and after the isometric contraction protocol. The effects of gender and treadmill running on the remainder of the variables described above were determined by standard two factor analyses of variance. When significance was detected, Tukey's post hoc comparison was used to assess the individual differences. In all cases, the level of significance was set a priori at P = 0.05.
Results
All data are reported as means ± standard error of the mean (s.e.m.). In cases where the responses of male and female mice differed significantly, data are reported separately. In contrast, in instances when statistical analysis showed no gender effects, data were pooled and the combined results are presented for brevity.
Body mass and running ability
The initial mean body mass of 28.0 ± 0.7 g for the eight male Exer mice was significantly larger than the initial mass of 21.0 ± 0.5 g for the eight female Exer mice. The body mass for the male mice at the time of kill (Table 1) was not different from the initial mass. In contrast, female mice demonstrated a small, but significant, weight gain throughout the 8 weeks of exercise. The lack of weight gain for male mice throughout the 8 weeks running program resulted in body masses for male Exer mice that were smaller than the masses of the 18 age-matched Sed males (Table 1). The Exer females were also slightly smaller than the 23 Sed female mice (Table 1), but the difference was not significant (P = 0.06).
Table 1.
Body mass and hind limb muscle masses
| Body mass | Muscle mass (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g) | EDL | SOL | ATB | GTN | PLN | FDL | BFM | VLT | VMD | |
| Male mice | ||||||||||
| Cage sedentary | 31.9 | 11.6 | 10.2 | 53.3 | 153.2 | 20.2 | 32.8 | 170.7 | 102.3 | 94.7 |
| (Sed) | ± 0.7 | ± 0.2 | ± 0.2 | ± 0.7 | ± 2.4 | ± 0.4 | ± 0.4 | ± 2.7 | ± 1.4 | ± 1.3 |
| Exercised | 28.5 | 10.9 | 10.4 | 50.1 | 135.8 | 18.4 | 30.4 | 151.0 | 94.9 | 88.1 |
| (Exer) | ± 0.7* | ± 0.3* | ± 0.4 | ± 1.2* | ± 2.5* | ± 0.4* | ± 0.8* | ± 4.3* | ± 2.3* | ± 2.3* |
| Female mice | ||||||||||
| Cage sedentary | 24.1 | 8.7 | 7.9 | 39.3 | 112.0 | 13.6 | 23.7 | 128.7 | 77.3 | 68.8 |
| (Sed) | ± 0.4 | ± 0.2 | ± 0.1 | ± 0.5 | ± 1.6 | ± 0.3 | ± 0.4 | ± 1.8 | ± 1.0 | ± 0.9 |
| Exercised | 22.3 | 8.1 | 7.8 | 36.2 | 99.2 | 12.1 | 22.0 | 114.4 | 72.5 | 62.9 |
| (Exer) | ± 0.6 | ± 0.2* | ± 0.3 | ± 1.0* | ± 2.6* | ± 0.4* | ± 0.7* | ± 3.3* | ± 2.4* | ± 1.8* |
Body masses in grams and muscle masses in milligrams are given as means ±s.e.m. for cage sedentary (Sed) mice and for mice that completed 8 weeks of 5 days per week treadmill running exercise (Exer). Muscles are the extensor digitorum longus (EDL), soleus (SOL), tibialis anterior (ATB), gastrocnemius (GTN), plantaris (PLN), flexor digitorum longus (FDL), biceps femoris (BFM), vastus lateralis (VLT) and vastus medialis (VMD). In all cases, values were significantly (P < 0.05) larger for male compared with female mice within an experimental group.
indicates significant difference from cage sedentary mice of the same gender by Tukey post hoc pair-wise multiple comparison.
The maximum running speeds during the initial graded exercise test were lower for male than for female mice, with mean maximum speeds of 20.8 ± 0.4 m min−1 and 24.9 ± 0.7 m min−1, respectively. Both males and females improved their running speed by ∼35% following 8 weeks of treadmill running exercise. The similar increase in running speed for male and female mice was observed despite a 20–25% longer time on average that female mice actually ran. Furthermore, no correlation was found for individual mice between the average time spent on the treadmill and the improvement in maximum running capability suggesting that 40–50 min per day of running elicited similar improvements in function.
Muscle mass and force
The smaller body masses for Exer compared with Sed mice were reflected in smaller muscle masses that were on average 8% smaller for Exer mice than for gender matched Sed mice (Table 1), with no differences between Exer and Sed mice when muscle masses were normalized for body mass. One exception was no difference between Exer and Sed mice for SOL muscle mass (Table 1) and, thus, greater SOL muscle mass/body mass ratios for Exer mice, indicating that SOL muscles hypertrophied in response to the treadmill running exercise. A second exception was a smaller muscle mass to body mass ratio for the GTN muscles of female Exer compared with Sed mice, indicating atrophy of GTN muscles in female mice.
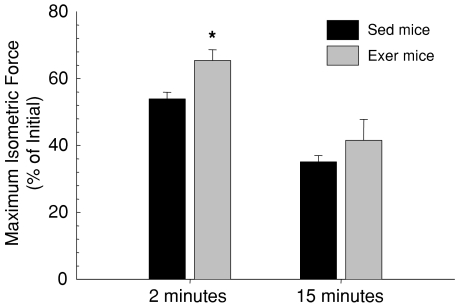
Despite slightly larger masses for GTN muscles of male than female mice, the maximum isometric forces (Po) were not significantly different between males and females. Mean Po for GTN muscles of Sed mice was 3254 ± 127 mN. Consistent with the 11% smaller muscle masses for GTN muscles of Exer compared with Sed mice, the mean Po of 2858 ± 109 mN was 12% lower, although the decrease was not statistically significant. For GTN muscles of Sed mice of both genders, the force dropped during the 15 min isometric contraction protocol by nearly one half within the first 2 min (Fig. 1). Subsequently, force dropped more slowly to a final level that was 35% of the initial Po. The treadmill running program resulted in an increased ability of the GTN muscles to sustain force during the isometric contraction protocol, as evidenced by the maintenance by muscles of Exer mice of greater than 65% of initial Po after 2 min (Fig. 1). Higher variability in the response of muscles, of Exer mice in particular, during the latter part of the contraction protocol precluded a statistically significant improvement in isometric force after the full 15 min of contractions, although the magnitude of the difference between Exer and Sed mice was similar at 15 min to that observed after 2 min.
Figure 1. The drop in maximum isometric force during a demanding protocol of repeated isometric contractions.
Values are shown for force generated by gastrocnemius (GTN) muscles following 2 (left bars) and 15 (right bars) minutes of repeated isometric contractions expressed as a percentage of the initial force levels. Data are for GTN muscles of mice that had been subjected to 8 weeks of treadmill running exercise (grey bars, Exer) and age-matched cage-sedentary controls (black bars, Sed). All bars are means with standard error bars. Significant differences (P < 0.05) between Exer and Sed groups are indicated by asterisks.
Extracellular ROS levels resulting from the isometric contraction protocol
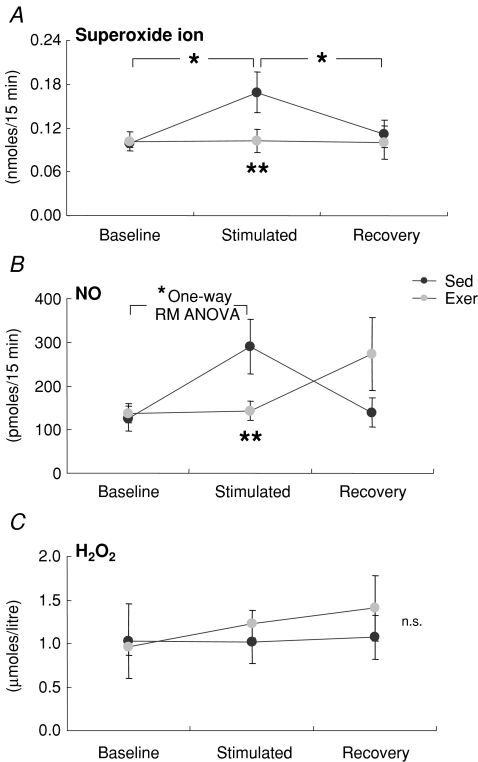
Microdialysates were analysed for superoxide, hydrogen peroxide (H2O2) and nitric oxide (NO) activities in the extracellular fluid of the GTN muscle resulting from the 15 min isometric contraction protocol. For all microdialysis experiments, data from male and female mice were pooled. Baseline levels of all three ROS analysed were not different between muscles of Exer and Sed mice (Fig. 2). For muscle of Sed mice, superoxide activities increased 70% relative to baseline values during the isometric contractions followed by a complete recovery back to baseline levels within 15 min (Fig. 2A). This increase in superoxide was completely absent for muscles of Exer mice resulting in superoxide levels during contractions that were 70% greater for muscles of Sed compared with Exer mice (Fig. 2A). Similarly, NO levels in Exer mice did not change with the onset of the contraction protocol, whereas increased NO levels in Sed muscles during contractions resulted in over 2-fold higher NO levels compared with the values for muscles of Exer mice (Fig. 2B). H2O2 levels remained essentially unchanged from baseline during and after the contraction protocol for muscles of both Exer and Sed mice with no difference between the groups (Fig. 2C).
Figure 2. Release of free radicals into the extracellular space during a demanding protocol of repeated isometric contractions.
Values are shown for: A, superoxide activity (determined by measurement of the reduction in cytochrome c); B. nitric oxide (NO) release (determined by measurement of total nitrate and nitrate content); and C. hydrogen peroxide (H2O2) content in microdialysates collected from gastrocnemius muscles of mice that either had (grey circles, Exer) or had not (black circles, Sed) been subjected to 8 weeks of treadmill running exercise. Data are from samples collected for 15 min periods just prior to (Baseline), during (Stimulated) and following (Recovery) the isometric contraction protocol. Values are means with standard error bars. * indicates significant effects of stimulation (P < 0.05), ** indicates significant differences between Exer and Sed groups.
Activation of transcription factor DNA binding
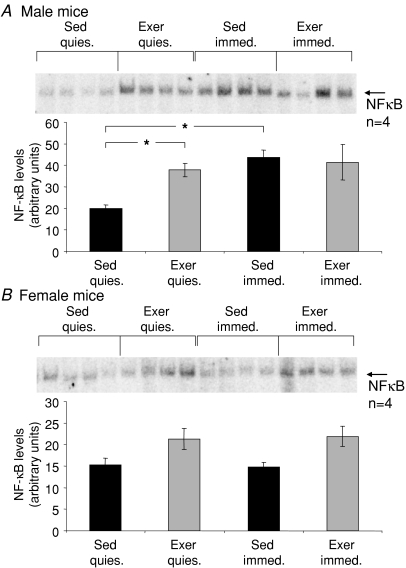
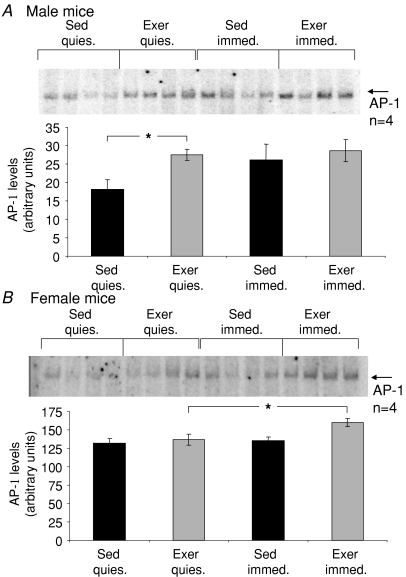
Figures 3 and 4 show representative gel shift analyses of DNA binding by NFκB and AP-1, respectively, along with the average data for each experimental group. Compared with Sed mice, an ∼2-fold increase was observed following the 8 weeks of treadmill running (Exer mice) for males in the basal level of NFκB DNA binding in quiescent muscles (Fig. 3A), and the basal level of AP-1 DNA binding was more than 50% higher in muscles of male Exer compared with Sed mice (Fig. 4A). In addition, the 15 min protocol of isometric contractions elicited a greater than 2-fold increase in NFκB activation (Fig. 3A) and a trend for an increase of ∼50% in DNA binding by AP-1 (Fig. 4A) in muscles of Sed male mice, whereas for both NFκB and AP-1 the increased basal levels of DNA binding in quiescent muscles of Exer mice were coupled with an abolition of the ability of the contraction protocol to induce any further activation of either transcription factor.
Figure 3. Levels of NFκB binding to DNA in response to a protocol of repeated isometric contractions.
Data are shown for levels of NFκB activation in quiescent (quies.) gastrocnemius (GTN) muscles and muscles immediately following exposure to the isometric contraction protocol (immed.) for: A, male and B, female mice that either had (grey bars, Exer) or had not (black bars, Sed) been subjected to 8 weeks of treadmill running exercise. All bars are means with standard error bars. Significant differences (P < 0.05) are indicated by asterisks.
Figure 4. Levels of AP-1 binding to DNA in response to a protocol of repeated isometric contractions.
Data are shown for levels of AP-1 activation in quiescent (quies.) gastrocnemius (GTN) muscles and muscles immediately following exposure to the isometric contraction protocol (immed.) for: A, male and B, female mice that either had (grey bars, Exer) or had not (black bars, Sed) been subjected to 8 weeks of treadmill running exercise. All bars are means with standard error bars. Significant differences (P < 0.05) are indicated by asterisks.
For female mice, basal levels of NFκB binding of DNA showed a trend for an increase in quiescent muscles in response to the 8 weeks of exercise (Fig. 3B), although the effect was not significant, and basal AP-1 activation was not altered appreciably in female mice following the treadmill running (Fig. 4B). The contraction-induced increases in DNA binding of NFκB and AP-1 observed in muscles of Sed male mice were not observed for Sed females (Figs 3B and 4B), nor was any increase in NFκB activation observed following the isometric contraction protocol for muscles of Exer female mice (Fig. 3B). Interestingly, the levels of DNA binding by AP-1 in muscles of female mice that had exercised on the treadmill for 8 weeks increased slightly in response to the 15 min isometric contraction protocol (Fig. 4B).
Adaptations in oxidative and antioxidant systems
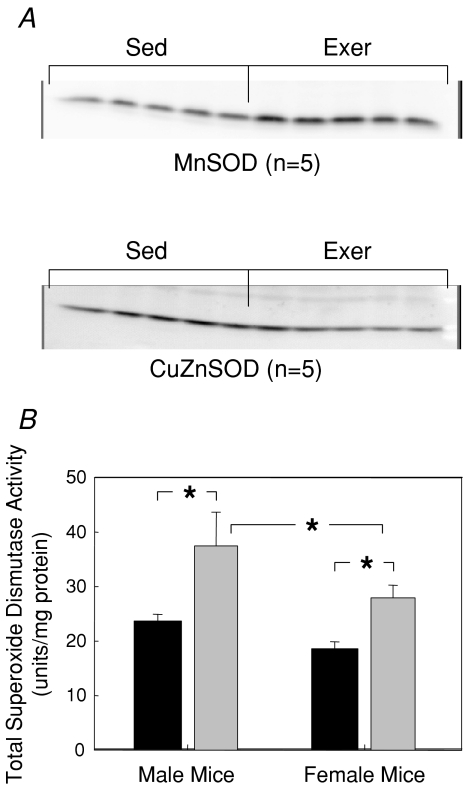
No differences were observed between muscles of male and female Sed mice for citrate synthase, catalase, or SOD activities. Compared with Sed mice, muscles of Exer mice displayed dramatic increases of 50% to 60% in total SOD activity (Fig. 5). The increase in SOD activity in response to the 8 weeks of treadmill running was similar for both male and female mice. The increased SOD activity appeared to be due primarily to an increase in MnSOD protein content, whereas CuZnSOD protein levels were not dramatically affected by the 8 weeks of treadmill running (Fig. 5A). Previous reports of only an 18% increase in citrate synthase activity for quadriceps muscles of mice following 8 weeks of treadmill running for 2 h per day (Chow et al. 2007) as well as no effect in mice of 4 weeks of treadmill running on cytochrome c protein expression in either PLN or GTN muscles (Massett & Berk, 2005) suggest that the classic adaptation of a robust increased in muscle oxidative capacity typical in studies of treadmill running in rats (Fitts et al. 1975; Holloszy, 1967) is much more modest in mice. Consistent with previous studies of mice, 8 weeks of treadmill running exercise resulted in a trend in the present study for a moderate 10% to 15% increase in citrate synthase activity in GTN muscles, but the increase was not significant. Catalase activity was unaltered by exposure to treadmill running for either males or females. The overall mean catalase activity was 6.3 ± 0.4 units (mg protein)−1 when values were pooled for all muscles in all experimental groups.
Figure 5. Effects of treadmill running on superoxide dismutase in gastrocnemius (GTN) muscles of female and male mice.
A, representative Western blots of MnSOD (upper panel) and CuZnSOD (lower panel) protein levels for quiescent gastrocnemius (GTN) muscles of male mice subjected to 8 weeks of treadmill running (Exer) and GTN muscles of age-matched sedentary control (Sed) mice. B, total superoxide dismutase (SOD) activities of GTN muscles of male (left bars) and female (right bars) mice that either had (grey bars, Exer) or had not (black bars, Sed) been subjected to 8 weeks of treadmill running exercise. All bars are means with standard error bars. Significant differences (P < 0.05) are indicated by asterisks.
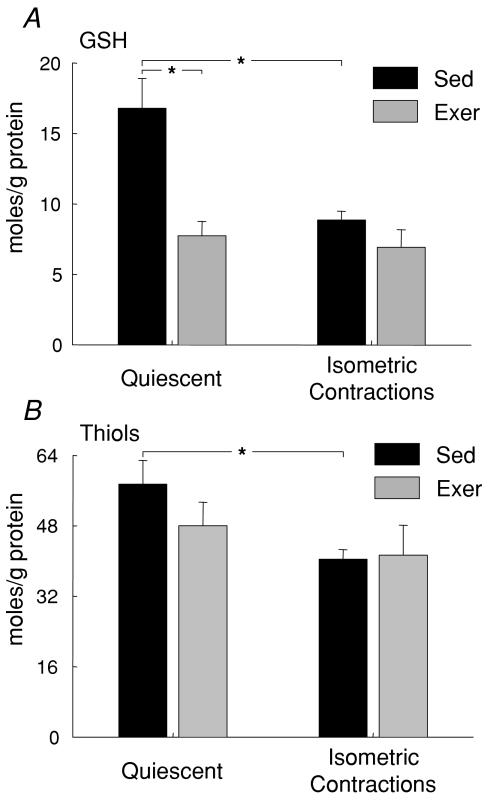
Additional dramatic alterations were observed in response to the treadmill running in non-enzymatic antioxidant systems. Total glutathione and protein thiol contents were not different between GTN muscles of male and female Sed mice, but glutathione content decreased by 50% following the 8 weeks of exercise (Table 2). The effect of the treadmill running program to reduce glutathione levels was similar for muscles of male and female mice. Similarly, a trend was observed for a decrease in response to treadmill running in resting protein thiol levels, but the effect did not reach statistical significance (Table 2). The protocol of isometric contractions elicited a decrease for muscles of Sed mice in glutathione and protein thiol levels of approximately 50% and 30%, respectively, for both males and females (Fig. 6). The significant reduction in response to the treadmill running program in resting levels of glutathione in muscles of Exer mice was coupled with the abolition of the effect of the isometric contraction protocol to induce any further depletion of glutathione (Fig. 6A). Furthermore, although the trend observed for a decrease in response to treadmill running in resting protein thiol levels did not reach statistical significance, the decrease was sufficient to also abolish for muscles of Exer mice the effect of the isometric contraction protocol to diminish protein thiol level (Fig. 6B).
Table 2.
Glutathione and protein thiol levels
| Male mice | Female mice | P (gender) | P (exercise) | |||
|---|---|---|---|---|---|---|
| Sed | Exer | Sed | Exer | |||
| Total glutathione content (μmol (g protein)−1) | 15.8 ± 2.7 | 9.7 ± 1.0 | 18.0 ± 3.6 | 6.2 ± 1.3* | 0.82 | 0.003 |
| Protein thiol content (μmol (g protein)−1) | 52.1 ± 5.6 | 39.0 ± 4.0 | 63.9 ± 9.7 | 55.3 ± 8.0 | 0.08 | 0.16 |
Total glutathione and protein thiol content are given as means ± standard error of the mean for quiescent gastrocnemius muscles from cage sedentary (Sed) male and female mice and male and female mice that completed 8 weeks of 5 days per week treadmill running exercise (Exer). Overall P values are also given for the effects of gender and exercise on glutathione and protein thiol levels by two-way analysis of variance.
indicates significant difference from muscles of Sed mice of the same gender by Tukey post hoc pair-wise multiple comparison.
Figure 6. Glutathione and protein thiol levels in response to a demanding protocol of repeated isometric contractions.
Data are shown for: A, glutathione and B, protein thiol levels in quiescent gastrocnemius (GTN) muscles and GTN muscles immediately following exposure to isometric contractions for mice that either had (grey bars, Exer) or had not (black bars, Sed) been subjected to 8 weeks of treadmill running exercise. All bars are means with standard error bars. Significant differences (P < 0.05) are indicated by asterisks.
Discussion
Our present finding of ROS release by GTN muscles of Sed mice in response to a demanding protocol of isometric contractions is consistent with numerous reports of ROS generation by skeletal muscles during contractile activity (Balon & Nadler, 1994; O'Neill et al. 1996; McArdle et al. 2001; Vasilaki et al. 2006a). In addition, activation by contractile activity of NFκB and AP-1 has previously been associated with ROS generation in mice (Vasilaki et al. 2006b). Thus, our observation of increased DNA binding of these redox-sensitive transcription factors in muscles of Sed mice immediately following the contraction protocol appears to be in response to the ROS generation displayed by these muscles, although alternate mechanisms for activation of skeletal muscle NFκB are also known (Ji et al. 2004). Despite extensive documentation of the effects of repeated bouts of aerobic exercise on the oxidative and antioxidant capacities of skeletal muscle (Holloszy, 1967; Fitts et al. 1975; Davies et al. 1981; Leeuwenburgh et al. 1994; Powers et al. 1994; Oh-ishi et al. 1997; Tonkonogi et al. 2000; Liu et al. 2000; Navarro et al. 2004; Massett & Berk, 2005), whether the generation of ROS or the activation of NFκB or AP-1 during subsequent contractile activity are affected by such exercise had not been studied previously. Our findings of a substantial reduction, or outright elimination, following 8 weeks of treadmill running, of the release of superoxide and NO from contracting muscles and of the activation by the contraction protocol of NFκB or AP-1 are highly significant and could not have been predicted by previous studies of antioxidant enzyme activities or indirect measures of oxidant stress or damage (Leeuwenburgh et al. 1994; Powers et al. 1994; Oh-ishi et al. 1997; Liu et al. 2000; Tonkonogi et al. 2000; Navarro et al. 2004). While our data show strong associations between contraction-induced ROS release and activation of NFκB/AP-1, data on males and females were pooled for our experiments on ROS generation, whereas the data of NF-κB and AP-1 are reported separately. Thus, although a direct effect of ROS to activate NF-κB/AP-1 during contractions has not been definitively demonstrated, these data strongly support the growing view (McArdle et al. 2004; Hashimoto et al. 2007; Gomez-Cabrera et al. 2008) that skeletal muscle not only tolerates without damage the contraction-induced ROS derived from a bout of exercise but the ROS elicit the initiation of pathways leading to adaptations that protect from future stresses.
Our observations that total glutathione and protein thiol contents in GTN muscles of Sed mice fell significantly in response to the isometric contractions also agree with previous data illustrating transient declines in muscle glutathione and protein thiols following a similar contraction protocol (McArdle et al. 2001; Vasilaki et al. 2006a). The mechanisms underlying the decreases in muscle glutathione and protein thiols that accompany contractile activity are still unclear, but our data demonstrating an increase in ROS generation during the isometric contractions by muscles of Sed mice are consistent with the hypothesis proposed by several authors that the thiol groups are oxidized by reaction with ROS generated during activity (Ji et al. 1992; Sastre et al. 1992). This mechanism is further supported by the abolition of both ROS generation and falls in glutathione and protein thiol levels in response to the contraction protocol by muscles of Exer mice that had completed 8 weeks of treadmill running. Collectively, our observations of reductions in response to the demanding protocol of isometric contractions for muscles of Exer compared with Sed mice in (i) ROS generation, (ii) the activation of redox-sensitive signalling pathways, and (iii) evidence for elevated ROS stress suggest that exercise conditioning may afford the muscles the ability to readily and rapidly detoxify ROS. This ability to detoxify ROS is compatible with both protection against ROS-induced damage and prevention of the formation of additional ROS that may act to mediate further unnecessary adaptive responses (Khassaf et al. 2003; Gomez-Cabrera et al. 2008).
Our observation of a dramatic increase in total SOD activity in response to treadmill running is highly consistent with previous findings (Higuchi et al. 1985; Jenkins, 1988; Leeuwenburgh et al. 1994, 1997; Powers et al. 1994; Oh-ishi et al. 1997). NFκB and AP-1 are both thought to be involved in the up-regulation of antioxidant enzymes (Pahl, 1999; Zhou et al. 2001; Jackson et al. 2002). The substantially higher SOD activity in GTN muscles of Exer compared with Sed mice is consistent with the hypothesis that NFκB and/or AP-1 activation by ROS generated in response to the treadmill running may mediate this adaptation. Although explored in a cell culture system, Hashimoto et al. (2007) suggested a similar hypothesis based on their finding that elevated lactate increased hydrogen peroxide production as well as glutathione peroxidase activity. In the present study, muscles from Exer mice also showed NFκB activation at rest. These data, along with the lack of any decrease in glutathione or protein thiol levels in response to the isometric contraction protocol by muscles of Exer mice may be indicative of a chronic oxidizing environment in these muscles and this shift may modify the ability of the muscles to activate NFκB and AP-1. The failure in the present study of Exer muscles to activate NFκB following the demanding contraction protocol represents a possible mechanism whereby signals for inducing adaptation are not transduced. Further adaptation in response to contractile activity is not necessary since the muscle is already adapted to the stress of contractions associated with regular repeated treadmill running. Both the release of superoxide during contractions (Vasilaki et al. 2006a) and the contraction-induced activation of NFκB (Vasilaki et al. 2006b) are also blunted for muscles of old compared with adult mice, but whether the cellular mechanisms are similar for the reductions in superoxide release and NFκB activation for Exer mice and old mice is unclear.
The marked decrease in resting levels of glutathione in the muscles of Exer compared with Sed mice in the present study is clearly contrary to the generally accepted response to endurance training to promote an increase in skeletal muscle glutathione content (Sen et al. 1992; Marin et al. 1993; Leeuwenburgh et al. 1994, 1997; Leeuwenburgh & Ji, 1996; Liu et al. 2000). The observation in the present and previous (McArdle et al. 2001; Vasilaki et al. 2006a) studies that that in mice a single episode of demanding contractile activity leads to a fall in muscle glutathione content, suggests that the net response of muscle glutathione content to an exercise program is likely to depend upon the duration of each bout of exercise and the recovery period between bouts. Thus, altering the frequency and/or duration of each bout of exercise may influence the pattern of changes observed, but no data are currently available to support this possibility. Our finding of no effect of treadmill running on catalase activity is consistent with the majority of previous studies on rats that provide little evidence of an effect of endurance exercise to increase catalase activity in skeletal muscle (Higuchi et al. 1985; Leeuwenburgh et al. 1994; Powers et al. 1994). In fact, several investigators report reduced catalase activity resulting from exercise training (Alessio & Goldfarb, 1988; Laughlin et al. 1990; Leeuwenburgh et al. 1997).
While the gender differences observed in the present study are currently unexplained, they are consistent with other data examining oxidative responses to contraction in mice (Vasilaki et al. 2006b). The most evident gender-specific response was the substantially blunted levels of activation in muscles of female mice of NFκB and AP-1 transcription factors. The discrepancy between males and females with respect to the effects of the treadmill running exercise on NFκB and AP-1 activation was previously unknown and existed despite the observation that male and female mice displayed comparable functional improvements in response to the exercise program, as assessed by running speed and ability to maintain force during the isometric contractions protocol. Biochemical adaptations, such as increased SOD activity and decreased glutathione levels, were also similar between the genders. The clear adaptations observed in female mice with little evidence for activation of NFκB or AP-1 transcription factors indicate that alternate or more likely additional factors are critical to changes in muscle gene expression that mediate many of the adaptations that occur in response to chronic aerobic exercise.
Acknowledgments
The authors would like to thank Cheryl Hassett, Daisy Mothersbaugh, Neil Ray and Jack Van der Meulen for their contributions to the data collection. We also acknowledge John Faulkner, Arlan Richardson and Holly Van Remmen for helpful discussions. Financial support was provided by National Institute on Ageing grant AG-20591.
References
- Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64:1333–1336. doi: 10.1152/jappl.1988.64.4.1333. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Measurement of antioxidants: glutathione. In: Punchard NA, Kelly FJ, editors. Free Radicals, a Practical Approach. Oxford: IRL Press at Oxford University Press UK; 1996. pp. 213–226. [Google Scholar]
- Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972;222:373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- Chow LS, Greenlund LJ, Asmann YW, Short KR, McCrady SK, Levine JA, Nair KS. Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J Appl Physiol. 2007;102:1078–1089. doi: 10.1152/japplphysiol.00791.2006. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press, Inc. FL; 1985. pp. 283–284. [Google Scholar]
- Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutases. Meth Enzym. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- Di Monte D, Bellomo G, Thor H, Nicotera P, Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984;235:343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol. 1975;228:1029–1033. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- Flück M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol. 2006;209:2239–2248. doi: 10.1242/jeb.02149. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gore M, Fiebig R, Hollander J, Leeuwenburgh C, Ohno H, Ji LL. Endurance training alters antioxidant enzyme gene expression in rat skeletal muscle. Can J Physiol Pharmacol. 1998;76:1139–1145. doi: 10.1139/cjpp-76-12-1139. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. Am J Physiol Regul Integr Comp Physiol. 1999;277:R856–R862. doi: 10.1152/ajpregu.1999.277.3.R856. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Jenkins RR. Free radical chemistry. Relationship to exercise. Sports Med. 1988;5:156–170. doi: 10.2165/00007256-198805030-00003. [DOI] [PubMed] [Google Scholar]
- Ji LL. Exercise, oxidative stress, and antioxidants. Am J Sports Med. 1996;24:S20–S24. [PubMed] [Google Scholar]
- Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera M-C, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Faulkner JA, Hinkle RT, Hassett CA, Supiano MA, Halter JB. Functional deficits in medial gastrocnemius grafts in rats: relation to muscle metabolism and beta-AR regulation. J Appl Physiol. 1997;83:67–73. doi: 10.1152/jappl.1997.83.1.67. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol. 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: Responses of glutathione and antioxidant enzyme systems. Am J Physiol Regul Integr Comp Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol Regul Integr Comp Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Ji LL. Alteration of glutathione and antioxidant status with exercise in unfed and refed rats. J Nutr. 1996;126:1833–1843. doi: 10.1093/jn/126.7.1833. [DOI] [PubMed] [Google Scholar]
- Lei B, Adachi N, Arai T. The effect of hypothermia on H2O2 production during ischemia and reperfusion: a microdialysis study in the gerbil hippocampus. Neurosci Lett. 1997;222:91–94. doi: 10.1016/s0304-3940(97)13349-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chu DW, Brooks GA, Ames BN. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol. 2000;89:21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin E, Kretzschmar M, Arokoski J, Hanninen O, Klinger W. Enzymes of glutathione synthesis in dog skeletal muscles and their response to training. Acta Physiol Scand. 1993;147:369–373. doi: 10.1111/j.1748-1716.1993.tb09513.x. [DOI] [PubMed] [Google Scholar]
- Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1006–R1013. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA, Horvath SM. Effects of age on metabolic responses to endurance training in rats. J Appl Physiol. 1984;57:1369–1374. doi: 10.1152/jappl.1984.57.5.1369. [DOI] [PubMed] [Google Scholar]
- Miles AM, Chen Y, Owens MW, Grisham MB. Fluorimetric determination of nitric oxide. Methods. 1995;7:40–47. [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Oh-ishi S, Kizaki T, Nagasawa J, Izawa T, Komabayashi T, Nagata N, Suzuki K, Taniguchi N, Ohno H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin Exp Pharmacol Physiol. 1997;24:326–332. doi: 10.1111/j.1440-1681.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol. 1996;81:1197–1206. doi: 10.1152/jappl.1996.81.3.1197. [DOI] [PubMed] [Google Scholar]
- Pagala MK, Ravindran K, Namba T, Grob D. Skeletal muscle fatigue and physical endurance of young and old mice. Muscle Nerve. 1998;21:1729–1739. doi: 10.1002/(sici)1097-4598(199812)21:12<1729::aid-mus16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31:987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, Vina J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol Regul Integr Comp Physiol. 1992;263:R992–R995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- Sen CK, Marin E, Kretzschmar M, Hanninen O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol. 1992;73:1265–1272. doi: 10.1152/jappl.1992.73.4.1265. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. In: Lowenstein JM, editor. Methods in Enzymology. New York: Academic Press NY; 1969. pp. 3–5. [Google Scholar]
- Tonkonogi M, Walsh B, Svensson M, Sahlin MS. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528:379–388. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki A, Mansouri A, Van Remmen H, Van Der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006a;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006b;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Zhou LZ, Johnson AP, Rando TA. NFkB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]