Abstract
The production of adult-born neurons is an ongoing process accounting for > 10 000 immature neurons migrating to the olfactory bulb every day. This high turnover rate necessitates profound control mechanisms converging onto neural stem cells and neuroblasts to achieve adequate adult-born neuron production. Here, we elaborate on a novel epigenetic control of adult neurogenesis via highly coordinated, non-synaptic, intercellular signalling. This communication engages the neurotransmitters GABA and glutamate, whose extracellular concentrations depend on neuroblast number and high affinity uptake systems in stem cells. Previous studies show that neuroblasts release GABA providing a negative feedback control of stem cell proliferation. Recent findings show an unexpected mosaic expression of glutamate receptors leading to calcium elevations in migrating neuroblasts. We speculate that stem cells release glutamate that activates glutamate receptors on migrating neuroblasts providing them with migratory and survival cues. In addition, we propose that the timing of neurotransmitter release and their spatial diffusion will determine the convergent coactivation of neuroblasts and stem cells, and provide a steady-state level of neuroblast production. Upon external impact or injury this signalling may adjust to a new steady-state level, thus providing non-synaptic scaling of neuroblast production.
The production of adult-born neurons persists in two brain regions, the subventricular zone (SVZ, Fig. 1A) and the dentate gyrus subgranular zone (SGZ) in the hippocampus. The SVZ contains the largest pool of dividing neural stem cells in the adult mammalian brain, including in humans (Sanai et al. 2004; Curtis et al. 2007). The division of stem cells generates intermediate progenitors (called transit-amplifying cells), which in turn divide to give rise to neuroblasts (Doetsch et al. 1999a) (Fig. 2). Neural stem cells have several properties of mature astrocytes and will be called stem cells or astrocytes interchangeably throughout this text. Neuroblasts migrate along the rostral migratory stream (RMS) to the olfactory bulb where they differentiate into interneurons (Bryans, 1959; Altman, 1969; Luskin, 1993; Lois & Alvarez-Buylla, 1994). Here, we discuss data obtained in the SVZ and RMS. We do not discuss data on GABAergic signalling in the SGZ that can be found in other reviews (Bordey, 2006, 2007; Ge et al. 2007).
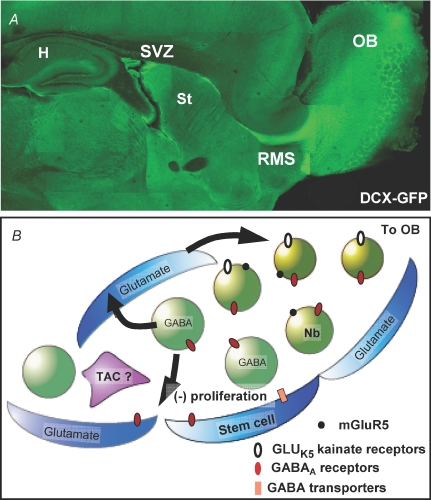
Figure 1.
A, montage of mid-sagittal sections from a transgenic mouse expressing green fluorescent protein (GFP) under the doublecortin (DCX) promoter. Chains of DCX-expressing neuroblasts from the subventricular zone (SVZ) converge to form a bright green rostral migratory stream (RMS), which terminates in the olfactory bulb. H, hippocampus; St, striatum; OB, olfactory bulb. B, simplified diagram illustrating the expression of GABA and glutamate signalling molecules in the SVZ. Neuroblasts (green, Nb) express both GABAA receptors and release GABA into the extracellular space. This GABA release results in autocrine activation of neuroblasts and paracrine activation of the astrocyte-like stem cells, decreasing their rate of proliferation (blue) through GABAA receptors. Stem cells (blue) are able to regulate the amount of GABA in the extracellular space through uptake mechanisms. Stem cells also contain glutamate that may serve as a feedback signal to neuroblasts through either or both GLUK5 kainate receptors and mGluR5 metabotropic glutamate receptor activation. The role of transit-amplifying cells (purple) in GABA and glutamate signalling has yet to be discovered.
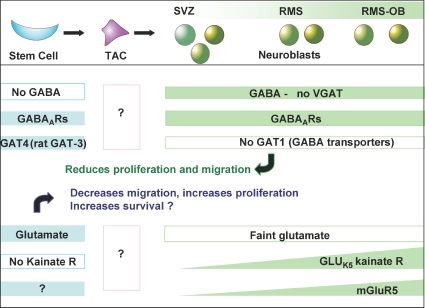
Figure 2. Chart summarizing known GABA and glutamate signalling molecules along the SVZ cell lineage.
Top panel: schematic diagram depicting the lineage of major cell types in the SVZ. Stem cells (blue) divide asymmetrically to both self-renew and give rise to a population of transit-amplifying cells (TACs), which undergo an unknown number of asymmetrical divisions, renewing themselves and generating neuroblasts (green). Neuroblasts are born in the SVZ or RMS, where they migrate, and are fated to become interneurons in the olfactory bulb. Middle panel: GABAergic signalling molecules are summarized here. The neuroblasts (green) are the source of GABA in the SVZ and RMS. The stem cells (blue) do not contain any GABA, while both cell types express GABAA receptors. Stem cells regulate the extracellular concentration of GABA via uptake through GAT4 GABA transporters. GABA decreases the speed of neuroblast migration and the number of proliferative stem cells. Bottom panel: glutamatergic molecules are summarized here. Stem cells appear to be the major source of glutamate in the SVZ and RMS. Neuroblasts express both mGluR5 and GLUK5-containing kainate receptors.
Adult neuron production is an ongoing process. Estimates suggest that 10 000–30 000 neurons migrate to the olfactory bulb every day (Lois & Alvarez-Buylla, 1994). This high turnover rate necessitates profound homeostatic control mechanisms. The rate of neuroblast production and the number that ultimately integrate into the olfactory bulb is determined by the combined outcome of cell proliferation, migration and survival, each of which is regulated by genetic and epigenetic mechanisms. For example, it is thought that each cell has an internal clock determining the number of divisions it can undergo or the length of time it can remain in the cell cycle. Epigenetic factors, which include mitogenic or antiproliferative factors in the local environment, have been shown to control the length of the cell cycle or the number of cells cycling and the speed of neuroblast migration prior to their integration into the olfactory bulb circuitry (for reviews see Hagg, 2005; Bordey, 2006).
Here, we will discuss the function of the local signalling molecules GABA and glutamate on cell proliferation, migration and survival in the SVZ and RMS prior to the acquisition of synaptic inputs. While we start to understand how these signals operate at the single cell level, we do not clearly understand how these signals are integrated at a population level to deliver the appropriate number of neuroblasts. We propose that cell number controls the concentration and supply of local GABA and glutamate that determines the level of receptor activation thus providing a feedback control on cell production.
GABA: a stop signal of neuroblast production
Many components of GABAergic signalling have been identified in the SVZ (Figs 1 and 2). GABA is synthesized and released by neuroblasts (Stewart et al. 2002; Bolteus & Bordey, 2004; Liu et al. 2005). Both neuroblasts and stem cells express GABAA receptors that are activated by ambient GABA (Stewart et al. 2002; Wang et al. 2003; Bolteus & Bordey, 2004; Liu et al. 2005). GABA levels are tightly regulated by high affinity GABA transporters in stem cells (mouse GAT4) but not in neuroblasts that lack GAT1 (a neuronal GAT) (Bolteus & Bordey, 2004; Platel et al. 2007). It remains unknown whether transit-amplifying progenitors contain GABA and express GABAergic signalling molecules. Functionally, tonic GABAA receptor activation reduces the number of proliferative neuroblasts (Nguyen et al. 2003) and stem cells (Liu et al. 2005). It also reduces the speed of neuroblast migration (Bolteus & Bordey, 2004) perhaps as a result of its enhancement of dendritic growth (Gascon et al. 2006). The intracellular mechanisms leading to these effects on cell development as well as the mode of Ca2+-dependent GABA release are not clearly understood. This is true particularly for the antiproliferative effect of GABA on stem cell proliferation. A recent study reported that GABA blocks embryonic stem cell proliferation through S-phase checkpoint kinases and the histone variant H2AX, independently of Ca2+ increases (Andang et al. 2008). This mechanism remains to be explored in SVZ stem cells.
Mosaic expression of glutamate receptors in migrating neuroblasts
Glutamatergic signals are conveyed by different glutamate receptor subtypes, namely ionotropic NMDA and non-NMDA or AMPA/kainate receptors (Sommer & Seeburg, 1992; Hollmann & Heinemann, 1994) and metabotropic glutamate receptors (mGluRs; groups I–III subtypes; Cartmell & Schoepp, 2000). The mGluR5 metabotropic glutamate receptors are expressed in SVZ cells (Di Giorgi Gerevini et al. 2004), including neuroblasts (Platel et al. 2008 in this issue of The Journal of Physiology) (Fig. 2). Our recent data suggest that mGluR5 activation leads to Ca2+ increases in neuroblasts. Stem cells do not express functional AMPA or NMDA receptors (Liu et al. 2006). In addition, it was thought that neuroblasts did not express AMPA/kainate and NMDA receptors until they entered the granule cell layer of the olfactory bulb (for review see Lledo et al. 2006). However, recent findings from our group suggest the expression of functional AMPA/kainate receptors in neuroblasts in the SVZ and RMS (Platel et al. 2007). More precisely, we found that neuroblasts express Ca2+-permeable GLUK5-containing kainate receptors (Platel et al. 2008). This finding is exciting as GLUK5 receptors provide an additional pharmacological target for novel therapeutics aimed at controlling neuroblast production. In addition, unpublished observations from our laboratory (J.-C. Platel) suggest that neuroblasts acquire functional NMDA receptors during their migration along the RMS.
Here are two important remarks regarding glutamate receptor expression in neuroblasts. First, there is a mosaic expression of GLUK5, mGluR5 and GABAA receptors. About 40% of rostral SVZ cells express all three receptors, ∼30% express either GLUK5 or mGluR5 with GABAA receptors while ∼90% of neuroblasts express GABAA receptors either alone (∼30%) or with these other receptors. Second, there is an increase in the number of neuroblasts expressing GluK5 and mGluR5 along the rostral–caudal axis. This suggests that neuroblasts mature during their tangential migration to the olfactory bulb (Fig. 2).
Glutamate: a mitogenic and survival signal for neuroblasts?
It was recently reported that adult mice lacking mGluR5 or treated with mGluR5 antagonists showed a dramatic reduction in the number of proliferating cells in the SVZ (Giorgi-Gerevini et al. 2005). These findings suggest that mGluR5s are tonically activated by ambient glutamate presumably originating from a local source of glutamate in the SVZ consistent with our strong immunoreactivity for glutamate in SVZ stem cells (Platel et al. 2007). It was also shown that astrocytes release a molecule promoting neuroblast migration from SVZ explants (Mason et al. 2001). This molecule was not identified, but glutamate is a good candidate to promote neuroblast migration as both AMPA and NMDA receptors have been shown to promote the migration of neuronal precursors in other developmental systems (for review see Schlett, 2006). The role of GluK5 kainate receptors in cell development prior to synapse formation has remained unexplored. In our recent study, we found that inhibition of GLUK5 kainate receptors using a selective antagonist resulted in a significant increase in the speed of neuroblast migration (Platel et al. 2008). This finding suggests that GLUK5 kainate receptors are tonically activated in migrating neuroblasts and that their activation decreases the speed of neuroblast migration. This finding was surprising at first because we expected glutamate to counteract GABA's effect on cell migration. However, a mosaic expression of glutamate receptors in neuroblasts may allow glutamate to exert differential effects on neuroblast production and migration. Glutamate acting at GLUK5 kainate receptors may cooperate with GABA and act as ‘a backup mechanism’ to limit neuroblast migration. Glutamate tonically acting at mGluR5 did not affect the speed of neuroblast migration. The effect of glutamate acting at AMPA receptors on neuroblasts remains to be determined.
Collectively, we propose that using distinct Ca2+-dependent intracellular pathways each glutamate receptor type, GLUK5 kainate receptor and mGluR5, differentially affects the development of neuroblasts. As in other developing systems (Schlett, 2006), we propose that glutamate acting at both ionotropic and metabotropic receptors has a positive effect overall on the production of adult-born neurons, but activation of some glutamate receptors may cooperate with GABA or mimic GABA's effect as part of a backup mechanism.
Integration of GABA and glutamate signals at the population level
At the population level, the ambient GABA concentration provides information to stem cells about the size of the neuroblast pool (i.e. number of neuroblasts). As more neuroblasts are generated, more GABA is expected to be released into the extracellular space, resulting in increased GABAA receptor activation in stem cells. Thus, GABA carries information on the number of neuroblasts produced and provides a negative feedback control of stem cell proliferation and therefore neuroblast production. This negative feedback reconciles well with the constant migration of neuroblasts to the olfactory bulb, which would limit ambient GABA accumulation in the SVZ, and with increased proliferation of astrocytes following elimination of neuroblasts (Doetsch et al. 1999b). Assuming that stem cells release glutamate, glutamate has to diffuse variable distances to activate glutamate receptors on migrating neuroblasts. The glutamate concentration and receptor location may determine which receptors are activated resulting in a selective effect on neuroblast development. It remains unclear how GABA and glutamate signals are integrated in neuroblasts. Both lead to Ca2+ increases and may also shunt their respective depolarization of neuroblasts.
Based on our recent finding that stem cells (i.e. SVZ astrocytes) do not migrate, we propose that stem cells act as ‘lighthouses’ in the SVZ where neuroblasts sail by. During their passage, neuroblasts receive signals from stem cells that are expected to positively affect their development. We propose that one of these signals is carried by glutamate.
Conclusion
Tumours are rarely observed in the SVZ, suggesting very strong regulatory mechanisms of neuroblast production. We propose that glutamate and GABA act as homeostatic signals to regulate neuroblast production. These signals, in turn, are subject to regulation by other factors. For example, growth factors may up- or down-regulate the expression of transporter and/or receptors leading to new steady-state levels of ambient neurotransmitter levels and receptor activation. The production of neuroblasts may thus be scaled up or down following drug injection or injuries modulating non-synaptic GABA and glutamate signalling. Such a homeostatic non-synaptic scaling of neurogenesis may explain that under various treatments or disorders, cell proliferation in the SVZ is altered but reaches a new steady-state of neuroblast production.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS048256 and DC007681, A.B.), Yale Brown-Coxe fellowship (J-C.P.), and National Science Foundation Graduate Research Fellowship (K.A.D.).
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibanez CF, Ernfors P. Histone H2AX-dependent GABAA receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A. Adult neurogenesis: basic concepts of signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res Brain Res Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Bryans WA. Mitotic activity in the brain of the adult rat. Anat Rec. 1959;133:65–73. [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Di Giorgi Gerevini V, Caruso A, Cappuccio I, Ricci VL, Romeo S, Della RC, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Mason HA, Ito S, Corfas G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: inducers, inhibitors, and repellents. J Neurosci. 2001;21:7654–7663. doi: 10.1523/JNEUROSCI.21-19-07654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABAA receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J-C, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 Kainate receptors decreases neuroblast migration in whole mounts of the subventricular zone. J Physiol. 2008;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38:602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- Sommer B, Seeburg PH. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992;13:291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABAA receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]