Abstract
Theta burst stimulation (TBS) is a form of repetitive transcranial magnetic stimulation (TMS). When applied to motor cortex it leads to after-effects on corticospinal and corticocortical excitability that may reflect LTP/LTD-like synaptic effects. An inhibitory form of TBS (continuous, cTBS) suppresses MEPs, and spinal epidural recordings show this is due to suppression of the I1 volley evoked by TMS. Here we investigate whether the excitatory form of TBS (intermittent, iTBS) affects the same I-wave circuitry. We recorded corticospinal volleys evoked by single pulse TMS of the motor cortex before and after iTBS in three conscious patients who had an electrode implanted in the cervical epidural space for the control of pain. As in healthy subjects, iTBS increased MEPs, and this was accompanied by a significant increase in the amplitude of later I-waves, but not the I1 wave. In two of the patients we tested the excitability of the contralateral cortex and found a significant suppression of the late I-waves. The extent of the changes varied between the three patients, as did their age. To investigate whether age might be a significant contributor to the variability we examined the effect of iTBS on MEPs in 18 healthy subjects. iTBS facilitated MEPs evoked by TMS of the conditioned hemisphere and suppressed MEPs evoked by stimulation of the contralateral hemisphere. There was a slight but non-significant decline in MEP facilitation with age, suggesting that interindividual variability was more important than age in explaining our data. In a subgroup of 10 subjects we found that iTBS had no effect on the duration of the ipsilateral silent period suggesting that the reduction in contralateral MEPs was not due to an increase in ongoing transcallosal inhibition. In conclusion, iTBS affects the excitability of excitatory synaptic inputs to pyramidal tract neurones that are recruited by a TMS pulse, both in the stimulated hemisphere and in the contralateral hemisphere. However the circuits affected differ from those influenced by the inhibitory, cTBS, protocol. The implication is that cTBS and iTBS may have different therapeutic targets.
The phenomenon of activity-dependent strengthening of synaptic transmission, known as long-term potentiation (LTP) is an important mechanism of learning and memory as well as many other forms of experience-dependent plasticity in the mammalian brain (Malenka & Bear, 2004). LTP has been investigated extensively in animal studies but recently the introduction of transcranial magnetic stimulation (TMS) has provided the opportunity to investigate similar mechanisms in the intact human brain with protocols of repetitive TMS (rTMS) that resemble those used in experimental preparations (Cooke & Bliss, 2006). Thus, repetitive TMS of the motor cortex leads to after-effects on the excitability of corticospinal and corticocortical pathways that remain for periods of an hour or so. Pharmacological studies show that at least some of the protocols are influenced by drugs that act at the NMDA receptor, suggesting that the effects may be due to a change in the effectiveness of synaptic connections (Hallett, 2007). The fact that it may be possible to induce LTP/LTD-like changes in the human brain has important implications for therapeutic applications. The hope is that rTMS-induced changes of synaptic connections will promote recovery of function in parts of the brain damaged by an acute or a chronic lesion (Ridding & Rothwell, 2007).
One approach for producing lasting effects in the brain using rTMS, is the recently introduced theta burst stimulation (TBS) protocol. TBS uses bursts of high frequency stimulation (3 pulses at 50 Hz) repeated at intervals of 200 ms (i.e. 5 Hz, the theta rhythm in EEG nomenclature). Interestingly, different patterns of delivery of TBS have opposite effects on synaptic efficiency of the stimulated motor cortex (Huang et al. 2005). The paradigm termed intermittent theta-burst stimulation (iTBS) produces a persisting increase in the amplitude of motor responses evoked by TMS whereas continuous theta-burst stimulation (cTBS) leads to suppression of TMS evoked responses (Huang et al. 2005). It is assumed that these after-effects are due to changes in neural circuits in the cortex involving processes similar to LTP or to long-term depression (LTD) of cortical synapses (Huang et al. 2007). In a recent study we provided direct evidence for the cortical origin of the inhibitory effects of cTBS by recording the corticospinal volleys evoked by single pulse TMS in conscious human subjects who had received an implanted epidural stimulator for the control of pain (Di Lazzaro et al. 2005). We found that cTBS selectively decreased the amplitude of the earliest I-wave. We also tested the effects of iTBS, the facilitatory TBS protocol in a chronic stroke patient who had a dorsal epidural electrode, and we found that iTBS enhanced the corticospinal descending activity evoked by lower limb area stimulation (Di Lazzaro et al. 2006).
The ability to record descending corticospinal activity in conscious humans provides a very useful insight into the after-effects of rTMS since the synchronous neural volleys are a direct measure of the effectiveness of synaptic input to corticospinal neurones evoked by TMS. Effectively, they can provide information about postsynaptic activity that is reasonably comparable to that recorded in experimental studies of LTP and LTD performed in hippocampal slice preparations. In this study we have used this method to examine the action of iTBS over the hand area motor cortex in three conscious subjects with no structural abnormality of the central nervous system who had a cervical epidural electrode implanted chronically for control of pain. We also took the opportunity to compare the variability of the effects with those in a separate large group of control subjects without the implanted electrodes who were given the same form of iTBS.
Methods
Epidural recordings
As described in previous publications (Di Lazzaro et al. 2004), we recorded descending corticospinal activity evoked by TMS of the motor cortex directly from the high cervical epidural space of three conscious subject (aged 49, 72 and 88 years) with no abnormality of central nervous system who had electrodes inserted for control of intractable dorso-lumbar pain. Because pain was resistant to medical therapy, the implantation of epidural electrodes for spinal cord stimulation, a minimally invasive and effective option for treatment of chronic pain (Lanner & Spendel, 2007), was performed in these patients.
The patients gave their written informed consent. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the Catholic University of Rome.
The patients were taking no centrally acting medication at the time of the experiments. This is because the trial screening period of epidural stimulation, before permanent implantation, is arranged to occur after a period of wash out of any drugs used for pain relief as well as any other central nervous system acting drug. This is well tolerated by the patients because epidural stimulation is considered only in patients resistant to medical treatment and the interruption of medical treatment for a few days does not usually produce any discomfort. It is necessary to do this so that the efficacy of the epidural stimulation on the symptoms of pain can be evaluated before permanent implantation.
Magnetic stimulation was performed with a high power Magstim 200 (Magstim Co., Whitland, Dyfed, UK) producing magnetic stimuli with a monophasic waveform. A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor evoked potentials (MEPs) in the contralateral first dorsal interosseous (FDI) muscle. In subjects 1 and 3, we evaluated the remote effect of right motor cortex iTBS by recording also the epidural activity and MEPs evoked by the contralateral, left, motor cortex stimulation. Active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a liminal MEP (about 200 μV in 50% of 10 trials) during isometric contraction of the tested muscle. Resting motor threshold (RMT) was defined according to the recommendations of the IFCN Committee (Rossini et al. 1994) as the minimum stimulus intensity that produced a liminal MEP (> 50 μV in 50% of 10 trials) with the tested muscle at rest.
Two different orientations of the stimulating coil over the motor strip were used, with the induced current flowing either in a latero-medial (LM) or in a posterior–anterior (PA) direction. RMT was determined separately for LM and PA stimulation. LM magnetic stimulation was used to identify the latency of the earliest (D-wave) descending volley (Di Lazzaro et al. 2004).
The responses to 20 stimuli at an intensity of 150% RMT were averaged at rest, both for LM and PA
Epidural recordings were made between the most proximal and distal of the four electrode contacts on the epidural electrode. These had a surface area of 2.54 mm2 and were 30 mm apart. The distal contact was connected to the reference input of the amplifier. MEPs and epidural activity were band-pass filtered (bandwidth 3 Hz to 3 kHz) (D360 amplifiers, Digitimer, Welwyn Garden City, UK) and each single trial was recorded on computer for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) and associated software with a sampling rate of 10 kHz per channel.
Amplitude of the volleys was measured from onset to peak, where onset was defined either as the immediately preceding trough, or as the initial deflection from baseline. To improve the identification and the measurement of the individual volleys we averaged the single trials in blocks of two consecutive trials
TBS was delivered over the right motor cortex ‘hot spot’ for MEPs in the contralateral FDI muscle using a MagPro stimulator (Medtronic A/S, Copenhagen, Denmark) connected to a figure-of-eight coil (MCF B65). The initial direction of the current induced in the brain was anterior to posterior. The magnetic stimulus had a biphasic waveform with a pulse width of about 280 μs and maximum magnetic field strength of 1.5 T. The stimulation intensity was defined in relation to AMT evaluated using the MagPro stimulator. An intensity of 80% AMT was used. We used the iTBS protocol in which 10 bursts of high frequency stimulation (3 pulses at 50 Hz) are applied at 5 Hz every 10 s for a total of 600 pulses (Huang et al. 2005).
We compared the corticospinal volleys, evoked by single pulse TMS immediately before and starting from 6 min after the end of iTBS, because at this interval there is the maximum of facilitation (Huang et al. 2005). In subjects 1 and 3, the right, stimulated, hemisphere was studied first.
Because the mechanism of the I1 wave is different from that of the later I-waves, as suggested by the differential behaviour of the I1 and later I-waves in several TMS protocols and in inhibitory protocols in particular (Di Lazzaro et al. 2004), the effects of iTBS on the amplitude of the I1 and of the later I-waves (the sum of the amplitude of all the individual waves after the I1 wave) were analysed separately.
MEP recordings
Effects of age on iTBS
The three patients were of very different ages. Since several previous reports noted changes in neurophysiological parameters with age (Peinemann et al. 2001; Sawaki et al. 2003; Pitcher et al. 2003; Hortobágyi et al. 2006; Oliviero et al. 2006; Talelli et al. 2008) we tested whether this could also be an important contributor to the individual differences we described by examining the effect of iTBS on MEPs in 18 healthy subjects of varying ages (mean age 51.2 ± 17.9 (s.d.) years; range 25–74 years) who did not have epidural electrodes implanted. Single pulse TMS and iTBS were performed as described above. We evaluated RMT, AMT and MEP amplitude bilaterally before and after TBS. In order to evaluate any age related effect, we divided the subjects into three groups: six subjects between 20 and 40 years (mean age was 27.3 ± 1.7 years), six subjects between 41 and 60 years (mean age was 58.5 ± 1.5 years) and six older subjects who were more than 60 years old (mean age was 67.8 ± 2.7 years). None of the subjects had been treated with neuroactive drugs in the 60 days prior to participating in this electrophysiological study.
Effects of iTBS on transcallosal inhibition
We evaluated the effects of iTBS on transcallosal inhibition in 10 of the subjects (mean age 26.6 ± 4.1 (s.d.) years; range 20–34 years) with no epidural electrode. Single pulse TMS and iTBS were performed as described above. The effect of iTBS on callosal function was evaluated by measuring the ipsilateral silent period (iSP) of the stimulated hemisphere before and after iTBS since this has been proposed as a simple electrophysiological test of callosal function (Meyer et al. 1999; Chen et al. 2008). Ipsilateral SPs were elicited whilst subjects held a tonic voluntary contraction of approximately 50% of MVC of the FDI ipsilateral to the stimulated hemisphere. Five stimuli at 200% AMT were given. The ipsilateral cortical silent period was measured according to the objective graphical method described by Garvey et al. (2001). This method allows an automated and objective estimation of onset and offset points, based on statistical analysis of variation of the baseline EMG activity (Garvey et al. 2001).
EMG signal was sampled at 5 kHz. One hundred millisonds of rectified averaged prestimulus EMG signal (that is 500 data points) was analysed to calculate the mean EMG level and the mean consecutive difference of the data points. Ipsilateral SP onset was the first point to fall below the lower variation limit if 50% or more of the data points in the following 5 ms window were also below the lower variation limit. Ipsilateral SP offset was the first point to fall above the lower variation limit if 50% or more of the data points in the following 5 ms window were also above the lower variation limit. In order to automate the procedure, we used a self-made function for the Matlab software (The MathWorks, Inc., Natick, MA, USA). Ipsilateral SPs were measured before and after iTBS using the same stimulus intensity.
Statistics
Epidural recording
To improve the identification and the measurement of the individual volleys, we averaged the single trials in blocks of two consecutive trials. Because only three subjects were studied, we compared the subaverages (of two individual trials) before and after iTBS separately for each subject. To analyse the effect of iTBS on corticospinal volleys and MEPs, the corticospinal volleys (I1 wave and later I-waves) and MEP amplitudes were entered into three separate (one for each subject) one-way ANOVAs (analyses of variance) with the factor stimulation condition (pre and post) and parameters of I1, later I-waves and MEP amplitudes.
Epidural recordings (contralateral – not stimulated – hemisphere)
To improve the identification and the measurement of the individual volleys we averaged the single trials in blocks of two consecutive trials. Because only two subjects were studied, we compared the subaverages (of two individual trials) before and after iTBS separately for each subject. For the analysis of the effect of iTBS on corticospinal volleys and MEPs, the corticospinal volleys (I1 wave and later I-waves) and MEP amplitudes were entered into two separate (one for each subject) one-way ANOVAs with the factor stimulation condition (pre and post) and parameters of I1, later I-waves and MEP amplitudes.
MEP recordings
Effects of iTBS on RMT, AMT and MEP amplitude. The effect of iTBS on RMT, AMT and MEP amplitudes in both hemispheres was assessed by analyses of variance with the factors stimulation condition (pre and post) and parameters of RMT, AMT and MEP amplitudes. We evaluated stimulated and not stimulated (contralateral) hemispheres separately.
Effects of ageing on iTBS effects
For each subject we calculated the percentage of facilitation (in the stimulated hemisphere) and inhibition (in the contralateral hemisphere). The percentage facilitation (in stimulated hemisphere) and inhibition (in contralateral hemisphere) of the three groups were compared by ANOVA using Bonferroni correction for multiple post hoc comparisons.
Effects of iTBS on transcallosal inhibition
In control subjects, the iSPs evoked before iTBS were compared with the corresponding iSPs evoked after iTBS using Wilcoxon tests.
Results
Epidural recordings
LM magnetic stimulation evoked the earliest negative potential. It had a latency of 3.1 ms in subject 1, 2.6 ms in subject 2 and 2.9 ms in subject 3. The short latency of this wave is consistent with direct activation of corticospinal axons. We have therefore termed this volley D-wave (Di Lazzaro et al. 2004). PA magnetic stimulation evoked three descending waves in all subjects (Figs 1, 2 and 3); the earliest of these waves had a latency which was 1.1–1.4 ms longer than the volley recruited by LM magnetic stimulation. Since the earliest volley elicited by LM magnetic stimulation is probably a D-wave we have termed the later volleys recruited by PA magnetic stimulation I-waves, numbered in order of their appearance (Di Lazzaro et al. 2004).
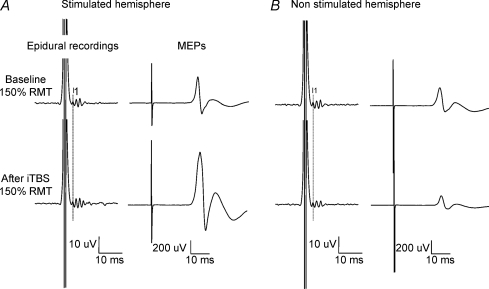
Figure 1. Corticospinal volleys and motor evoked potentials evoked by single pulse magnetic stimulation in baseline conditions and after right motor cortex intermittent theta burst stimulation (iTBS) in subjects 1 after stimulation of the ipsilateral (left panel) and contralateral (right panel) hemisphere.
Each trace is the average of 20 sweeps. A, magnetic stimulation evokes three descending waves. The latency of the earliest (I1) wave is indicated by the vertical line. After iTBS, a further I-wave is recruited (I4), the size of the I2 and I3 waves is increased (F1,18 = 26.53, P < 0.000), and the amplitude of the I1 wave is unchanged (F1,18 = 0.606, P = 0.446). The amplitude of MEP is significantly increased after iTBS (F1,18 = 30.1, P < 0.0001). B, magnetic stimulation evokes three descending waves. After iTBS, the size of the latest (I3) wave is decreased (F1,18 = 7.116, P = 0.016), the amplitude of the I1 wave is unchanged (F1,18 = 4.08, P = 0.058), and the amplitude of MEP is decreased (F1,18 = 14.254, P = 0.001).
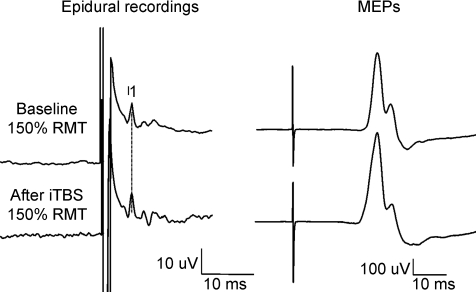
Figure 2. Corticospinal volleys and motor evoked potentials evoked by single pulse magnetic stimulation in baseline conditions and after right motor cortex intermittent theta burst stimulation (iTBS) in subject 2.
Each trace is the average of 20 sweeps. Magnetic stimulation evokes three descending waves. After iTBS, the size of the I2 and I3 waves is increased (F1,18 = 5.9, P = 0.026), and the amplitude of the I1 wave is unchanged (F1,18 = 2.4, P = 0.138). The amplitude of MEP is slightly increased after iTBS, but the change is not significant (F1,18 = 0.57, P = 0.459).
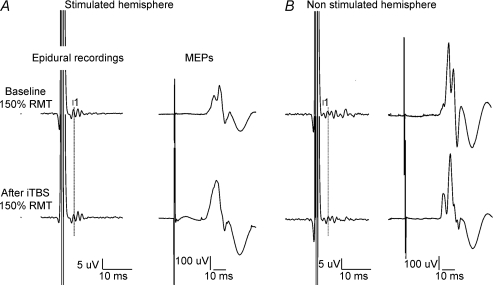
Figure 3. Corticospinal volleys and motor evoked potentials evoked by single pulse magnetic stimulation in baseline conditions and after right motor cortex intermittent theta burst stimulation (iTBS) in subjects 3 after stimulation of the ipsilateral (left panel) and contralateral (right panel) hemisphere.
Each trace is the average of 20 sweeps. A, magnetic stimulation evokes three descending waves. The latency of the earliest (I1) wave is indicated by the vertical line. After iTBS, the size of the I2 and I3 waves is increased (F1,18 = 9.04, P = 0.008), the amplitude of the I1 wave is unchanged (F1,18 = 0.137, P = 0.716). The amplitude of MEP is significantly increased after iTBS (F1,18 = 6.35, P = 0.021). B, magnetic stimulation evokes several descending waves. After iTBS, the size of the later waves is significantly decreased (F1,18 = 10.74, P = 0.004), and the amplitude of the I1 wave is unchanged (F1,18 = 1.3, P = 0.268). The amplitude of MEP is decreased after iTBS, but the change is not significant (F1,18 = 0.59, P = 0.453).
Figure 1 shows the effect of iTBS on the amplitudes of the I-waves and of MEPs in subject 1. The I1 wave was not significantly modified by iTBS (4.5 μV baseline and 5.3 μV after iTBS; F1,18 = 0.606, P = 0.446), but the mean amplitude of later waves increased significantly (F1,18 = 26.53, P < 0.000) by 64% (14.3 μV baseline and 23.5 μV after iTBS). After iTBS a further I-wave (I4) appeared with a mean amplitude of 5.3 μV.
The consequence of these changes can be observed in MEPs we recorded in the FDI muscle. These increased after iTBS by 144% of their pre-rTMS size (1.12 mV baseline and 2.74 mV after iTBS; F1,18 = 30.1, P < 0.0001; Fig. 1).
Figure 2 shows the effect of iTBS on the amplitudes of the I-waves and MEP in subject 2. The I1 wave was not significantly modified (8.9 μV baseline and 10.1 μV after iTBS; F1,18 = 2.4, P = 0.138). The mean amplitude of later waves increased significantly after iTBS (F1,18 = 5.9, P = 0.026) by 45% (7.9 μV baseline and 11.6 μV after iTBS). The consequence of these changes can be observed in MEPs we recorded in the FDI muscle. These increased by 16% of their pre-rTMS size after iTBS (Fig. 2). However, the increase in MEP was not significant (0.59 mV baseline and 0.685 mV after iTBS; F1,18 = 0.57, P = 0.459).
Figure 3 shows the effect of iTBS on the amplitudes of the I-waves and MEP in subject 3. The I1 wave was not significantly modified (6 μV baseline and 6.3 μV after iTBS; F1,18 = 0.137, P = 0.716). The mean amplitude of later waves increased significantly (F1,18 = 9.04, P = 0.008) after iTBS by 22% (11.6 μV baseline and 14.2 μV after iTBS). The consequence of these changes can be observed in MEPs we recorded in the FDI muscle. These increased by 46% of their pre-rTMS size after iTBS (0.39 mV baseline and 0.56 mV after iTBS; F1,18 = 6.35, P = 0.021). The mean results obtained in the three subjects are shown in Fig. 4.
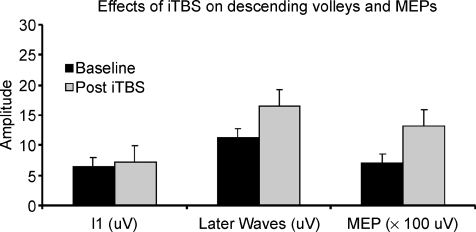
Figure 4. Bar graphs showing grand mean amplitudes of the I1 wave, of the later I-waves (the sum of the amplitudes of waves following I1) and of motor evoked potentials in baseline conditions and after iTBS in the three subjects studied.
The amplitude of later I-waves is increased by about 46% after iTBS, and the amplitude of MEPs is increased by about 90% after iTBS.
In subjects 1 and 3, we evaluated the effects of iTBS on responses evoked by stimulation of the opposite hemisphere (Figs 1 and 3). The increase of corticospinal volleys and MEPs evoked by stimulation of the right hemisphere was associated with a decrease in the amplitude of later volleys and of MEPs recorded after stimulation of the left hemisphere. In subject 1, the I1 wave was not significantly modified by iTBS (F1,18 = 4.08, P = 0.058; mean amplitude of the I1: 6.2 μV baseline and 4.6 μV after iTBS). The mean amplitude of later waves decreased significantly (F1,18 = 7.116, P = 0.016) by 28% (14.7 μV baseline and 10.5 μV after iTBS). The consequence of these changes can be observed in MEPs we recorded in the right FDI muscle. These decreased by 42% of their pre-rTMS size after iTBS (0.82 mV baseline and 0.47 mV after iTBS; F1,18 = 14.254, P = 0.001). Similar findings were obtained in subject 3; the I1 wave was not significantly modified by iTBS (F1,18 = 1.3, P = 0.268; mean amplitude of the I1: 5.9 μV baseline and 5.4 μV after iTBS). The mean amplitude of later waves decreased significantly (F1,18 = 10.74, P = 0.004) by 37% (20.4 μV baseline and 16.6 μV after iTBS). The consequence of these changes can be observed in MEPs we recorded in the right FDI muscle. These decreased by 12% of their pre-rTMS size after iTBS (1 mV baseline and 0.88 mV after iTBS), but this change was not statistically significant (F1,18 = 0.59, P = 0.453).
Effect of age on RMT, AMT and MEP following iTBS in healthy subjects
In the control group of 18 subjects, the mean RMT and AMT of the hemisphere stimulated with iTBS did not change significantly (RMT baseline 51.6 ± 9.7% (s.d.) and after iTBS 50.7 ± 10.36%, F1,34 = 0.062, P = 0.805; AMT baseline 39.5 ± 7.4% (s.d.) and after iTBS 38 ± 7.3%, F1,34 = 0.375, P = 0.545).
Overall, the mean amplitude of MEPs of the hemisphere stimulated with iTBS increased by 70.9 ± 91.2% (s.d.) after iTBS (F1,34 = 7.729, P = 0.009; 0.8 ± 0.3 mV (s.d.) baseline and 1.28 ± 0.65 mV after iTBS). However, the analysis of data showed a high interindividual variability with changes after iTBS ranging from –21% to +302%. Similarly in the hemisphere contralateral to the iTBS, there was no significant change in the mean RMT and AMT (RMT baseline 52.5 ± 10.3% (s.d.) and after iTBS 51.9 ± 10.1%, F1,34 = 0.017, P = 0.896; AMT baseline 40.2 ± 7.3% (s.d.) and after iTBS 40.5 ± 7.3%, F1,34 = 0.019, P = 0.892). However, the mean amplitude of MEPs evoked from the contralateral hemisphere decreased by 19.5 ± 30.6% (s.d.) after iTBS (F1,34 = 4.6, P = 0.039; 0.80 ± 0.35 mV (s.d.) baseline and 0.59 ± 0.26 mV after iTBS). Again, there was a high interindividual variability with changes after iTBS ranging from –85% to +37%.
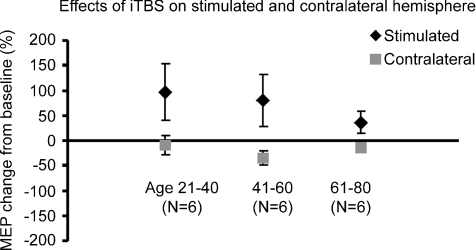
MEP facilitation in the stimulated hemisphere was larger in younger control subjects (group I aged 20–40 and II aged 41–60 years) than in older subjects (group III aged 61–80 years) (facilitation of MEPs: group I 96.4 ± 112% (s.d.), group II 80 ± 105%, and group III 36.3%± 46.9%; Fig. 5), but the difference was not significant (F2,15 = 0.67, P = 0.528). Inhibition of MEPs from the contralateral hemisphere was similar in younger and older subjects (Fig. 5; inhibition of MEPs: group I –9.8 ± 37% (s.d.), group II –35.3 ± 30%, and group III –13.6 ± 21.3%; F2,15 = 1.246, P = 0.316).
Figure 5. Mean increase in MEP amplitude of the stimulated and contralateral hemisphere after iTBS in groups of control subjects of different ages.
The facilitation of MEPs (in stimulated hemisphere) is larger in younger control subjects (groups I aged 20–40 and II aged 41–60 years) than in older subjects (group III aged 61–80 years) (facilitation of MEPs: group I 96.4 ± 112% (s.d.), group II 80 ± 105%, and group III 36.3 ± 46.9%), but the difference is not significant (F2,15 = 0.67, P = 0.528). Inhibition of MEPs (in contralateral hemisphere) is similar in younger and older subjects (F2,15 = 1.246, P = 0.316).
Effects of iTBS on transcallosal inhibition
In the control group of 10 subjects, the mean iSP was not significantly modified by iTBS (37.1 ± 13.6 ms (s.d.) baseline and 30.9 ± 14.9 ms (s.d.) after iTBS; P = 0.83, Wilcoxon test).
Discussion
Recording of corticospinal activity evoked by motor cortex TMS is a valuable method of investigating the after-effects of rTMS protocols on synaptic excitability. This is because the amplitude of the synchronized corticospinal volleys is a direct reflection of the effectiveness of excitatory synaptic input to pyramidal neurones evoked by single pulses of TMS. As such these measures are a good substitute for the population synaptic activity recorded in experimental models of LTP.
The present results demonstrate that rTMS given as iTBS leads to a pronounced increase in the excitability of cortical circuits generating the later I-waves, whilst the earliest I-wave is unaffected. I-waves represent synchronous activity of corticospinal axons originating from trans-synaptic activation of corticospinal cells. Although their origin is still unclear, there is a good deal of evidence to suggest that the early and late I-waves are generated by independent cortical mechanisms (Ziemann & Rothwell, 2000; Di Lazzaro et al. 2004). Thus, our results suggest that iTBS produces its effect by influencing the intrinsic circuitry of the motor cortex that generates later I-waves. The increase in synaptic cortical activity revealed by the increase in corticospinal activity in our patients is consistent with the idea that iTBS may induce LTP-like changes at synaptic connections in motor cortex (Huang et al. 2007).
A careful examination of the recordings of subject 2, shows that the most evident change after iTBS is a more pronounced trough between the I2 and I3 wave. This change might be explained by a more synchronous discharge of the axons of cortical circuits generating the later I-waves. In this subject, the effect of iTBS on MEP amplitude was limited when compared with that observed for epidural activity. One possible explanation is that volleys recorded from the epidural space may be destined for other muscles in addition to FDI so that the increase in epidural volley amplitude may be more pronounced than the effect on the MEP.
The effect of the iTBS on the later I-waves is similar to that observed using other TMS protocols. For example, the suppression seen in short latency intracortical inhibition and the suppression produced via transcallosal inhibition both preferentially affect the later I-waves and leave the I1 wave virtually unchanged (Di Lazzaro et al. 2004). However, the effect of the iTBS contrasts with that of inhibitory cTBS since that protocol preferentially affects the I1 wave and leaves the later I-waves virtually unchanged (Di Lazzaro et al. 2006). This specificity suggests that the two protocols produce their effects by modulating different circuits of the motor cortex.
The effect of iTBS on corticospinal volleys was variable among subjects; it was very pronounced in subject 1 (later waves were increased by about 64% and, after iTBS, a previously absent I4 appeared) while a pronounced smaller effect was observed in subject 3 (later waves were increased by about 20%). Subject 2 showed an intermediate change. Interestingly subject 1 was the youngest and subject 3 the oldest, and it can be hypothesized that there is an age related decline in cortical excitability changes induced by rTMS in agreement with experimental studies that show an age related decline of LTP (Barnes, 1979; Sawaki et al. 2003). Another possibility is that the different degree of facilitation is related to the high interindividual variability of the effects of rTMS (Maeda et al. 2000). In order to investigate the effect of age we measured the effects of iTBS on MEP amplitude in a large population of control subjects. We observed a high variability in the magnitude of iTBS effects. Although there was greater facilitation in younger subjects than in older subjects the difference was not significant. Thus, the data suggest that the different behaviour of corticospinal activity of younger and older patient with the epidural electrode is due mainly to the interindividual variability of the effects of iTBS and only to a minor extent to an age related decline of the response to iTBS.
Interestingly, in subjects 1 and 3 the increase in corticospinal activity and MEPs that was observed after iTBS of the ipsilateral hemisphere was associated with reduced excitability of the contralateral hemisphere. Such interhemispheric effects of standard rTMS protocols have been observed by a number of authors (Gilio et al. 2003; Schambra et al. 2003; Plewnia et al. 2003; Pal et al. 2005; Heide et al. 2006) with variable results. Two more recent studies with cTBS have also reported effects, but again the data are conflicting (Ishikawa et al. 2007; Stefan et al. 2008). Since there have been no published studies of the interhemispheric effects of iTBS we decided to investigate them in a group of 10 healthy subjects. As in the patients, MEPs evoked from the contralateral hemisphere were reduced after iTBS.
The mechanism of this effect is not clear. As noted by others, it could be due to persisting changes in the tonic activity of transcallosal connections from the hemisphere receiving iTBS. Alternatively there could have been a lasting change in the intrinsic excitability of the contralateral hemisphere secondary to changed transcallosal input during the iTBS. We hypothesized that if the former were the case then we might be able to detect it as an increase in the excitability of transcallosal inhibitory connections assessed by means of the iSP. However, there was no change in this following iTBS, so that it seems likely that iTBS caused locally persisting changes in contralateral corticospinal excitability and that these were responsible for the reduction in MEPs.
Finally, it should be noted that the epidural recording was performed in patients with chronic pain. Because noxious stimuli affect motor cortex excitability (Valeriani et al. 1999), we cannot exclude the possibility that the presence of pain influenced the response of the motor cortex to iTBS in our patients. However, the facilitation of MEPs after iTBS was similar to that described in normal subjects by Huang et al. (2005), suggesting that this effect is not sensitive to painful inputs. Indeed the changes observed in MEP amplitude were consistent with those observed in corticospinal activity. We conclude that the epidural recordings obtained in our patients provided valuable information about the origin of the facilitation independently from any possible pain-related change in cortical excitability.
In conclusion, we found that iTBS at an intensity of 80% AMT leads to a rapid increase in the excitability of cortical mechanisms that generate later I-waves in response to single TMS pulses. This differs from the results seen after cTBS, which preferentially affects the amplitude of the I1 wave and not later I-waves. The implication is that populations of excitatory cortical circuits are differentially sensitive to the effects of iTBS and cTBS. This may be of importance when testing the therapeutic potential of these methods in rehabilitation of patients with neurological disorders.
References
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Profice P, Pilato F, Cioni B, Meglio M, Capone F, Tonali PA, Rothwell JC. Direct demonstration that repetitive transcranial magnetic stimulation can enhance corticospinal excitability in stroke. Stroke. 2006;37:2850–2853. doi: 10.1161/01.STR.0000244824.53873.2c. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner H, Rothwell JC. Effects on the right motor hand area excitability produced by low-frequency rTMS over the contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–189. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Heide G, Witte OW, Ziemann U. Physiology of modulation of motor cortex excitability by low-frequency suprathreshold repetitive transcranial magnetic stimulation. Exp Brain Res. 2006;171:26–34. doi: 10.1007/s00221-005-0262-0. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171:322–329. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol. 2007;118:1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lanner G, Spendel MC. Spinal cord stimulation for the treatment of chronic non-malignant pain. Acta Neurochir Suppl. 2007;97:79–84. doi: 10.1007/978-3-211-33079-1_10. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Schmierer K, Irlbacher K, Meierkord H, Niehaus L, Grosse P. First diagnostic applications of transcallosal inhibition in diseases affecting callosal neurones (multiple sclerosis, hydrocephalus, Huntington's disease) Electroencephalogr Clin Neurophysiol Suppl. 1999;51:233–242. [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pal PK, Hanajima R, Gunraj CA, Li JY, Wagle-Shukla A, Morgante F, Chen R. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J Neurophysiol. 2005;94:1668–1675. doi: 10.1152/jn.01306.2004. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol. 2003;546:605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport. 2003;14:609–612. doi: 10.1097/00001756-200303240-00017. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lücking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN Committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53:521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, Classen J. Theta-burst stimulation: Remote physiological and local behavioral after-effects. Neuroimage. 2008;40:265–274. doi: 10.1016/j.neuroimage.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Profice P, Le Pera D, Saturno E, Tonali P. Inhibition of the human primary motor area by painful heat timulation of the skin. Clin Neurophysiol. 1999;110:1475–1480. doi: 10.1016/s1388-2457(99)00075-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]