Abstract
Progenitor cells expressing the proteoglycan NG2 represent approximately 5% of the total cells in the adult brain, and are found both in grey and white matter regions where they give rise to oligodendrocytes. The finding that these cells receive synaptic contacts from excitatory and inhibitory neurons has not only raised major interest in the possible roles of these synapses, but also stimulated further research on the developmental and cellular functions of NG2-expressing (NG2+) progenitors themselves in the context of neural circuit physiology. Here we review recent findings on the functional properties of the synapses on NG2+ cells in grey and white matter regions of the brain. In this review article we make an attempt to integrate current knowledge on the cellular and developmental properties of NG2+ progenitors with the functional attributes of their synapses, in order to understand the physiological relevance of neuron–NG2+ progenitor signal transmission. We propose that, although NG2+ progenitors receive synaptic contact in all brain regions where they are found, their synapses might have different developmental and functional roles, probably reflecting the distinct functions of NG2+ progenitors in the brain.
The adult brain contains a significant reservoir of progenitor cells that can generate both neurons and glia (Emsley et al. 2005; Duan et al. 2008). Since its first identification (Stallcup & Beasley, 1987; Nishiyama et al. 1999), the progenitor population expressing the proteoglycan NG2 (referred here as NG2+ progenitors or NG2+ cells) has been extensively studied based on the following important attributes: (i) NG2+ cells constitute a significant percentage (5%) of the total cells in the mammalian brain (Dawson et al. 2003); (ii) these cells are found as proliferative cells in the developing postnatal and in the adult brain, both in grey and white matter regions (Nishiyama et al. 2002; Dawson et al. 2003; Aguirre et al. 2004a; Ligon et al. 2006); (iii) NG2+ cells generate white and grey matter oligodendrocytes of the brain and are therefore sometimes referred to as OPCs (Levine & Stallcup, 1987; Levine et al. 2001; Polito & Reynolds, 2005; Zhu et al. 2008); (iv) NG2+ cells are often anatomically associated to neurons and receive excitatory and inhibitory synaptic contacts (Bergles et al. 2000; Lin & Bergles, 2004; Dayer et al. 2005; Karadottir et al. 2008; Mangin et al. 2008); and (v) NG2+ cells are able to generate astrocytic and neuronal progenies under normal or pathological conditions (Belachew et al. 2003; Aguirre et al. 2007; Tamura et al. 2007; Zhu et al. 2008). These findings have understandably generated significant interest, particularly for the potential of endogenous pools of NG2+ cells to be targeted in cell regeneration and cell repair therapies.
There is now extensive evidence that most if not all NG2+ progenitors are contacted by functional synapses in the postnatal brain, both in grey and white matter areas. However, the cellular and physiological functions of these synaptic inputs are still unknown. Here, we review recent developments and different hypotheses that have been proposed since the initial discovery of direct synaptic transmission on NG2+ cells by Bergles et al. (2000). Several review articles have been recently published on this topic (Lin & Bergles, 2002, 2004; Paukert & Bergles, 2006), and therefore rather than attempting a comprehensive review of the literature, we will focus the present article on specific questions that are currently pending on the possible function of synaptic transmission on NG2+ progenitors in the brain. The recent work on the physiology of these neuron–NG2+ progenitor synapses demonstrates that the existing dogma of the exclusivity of synapses between neurons needs to be revised, and that synapses between neurons and undifferentiated progenitor cells are likely to mediate a different type of information processing that is distinct from that occurring between neurons. We extensively discuss this notion and speculate on the specific functions that neuron–NG2+ cell synapses might have in distinct brain regions.
Properties of neuron–NG2+ cells synapses
It has been known for some time that various types of glial cells, including oligodendrocyte progenitor cells (OPCs) in white matter, express glutamate binding proteins and generate electrical currents in response to glutamate application, or in response to neuronal activity (e.g. Kriegler & Chiu, 1993; Mennerick & Zorumski, 1994; Bergles & Jahr, 1997). However, a conclusion common to all these studies is that, while glial cells are able to detect transmitter molecules that have accumulated in the extracellular space, quantal synaptic transmission is an exclusive property of neuronal communication. Therefore, the surprising finding that NG2+ cells are able to discern single quanta of neurotransmitter released from individual neurons challenged one of the fundamental dogmas of synaptic transmission in the brain (Bergles et al. 2000; Lin & Bergles, 2004).
Quantal transmission on NG2+ cells
Bergles and colleagues patch-clamped NG2-expressing glial cells in the hippocampal CA1 region, and recorded currents upon stimulation of CA3 pyramidal cells or local interneurons which were kinetically and pharmacologically indistinguishable from EPSCs and IPSCs typically recorded in neighbouring CA1 pyramidal neurons under identical conditions. Figure 1 shows a 3D reconstruction of an NG2-expressing cell from CA1 stratum radiatum. Consistent with the idea that currents on NG2+ cells were due to synaptic events, it was demonstrated that they were due to action potential-mediated and calcium-dependent vesicular release of glutamate or GABA, and were not caused by spill-over from neighbouring synapses (Bergles et al. 2000; Lin & Bergles, 2004).
Figure 1. Three-dimensional reconstruction of a dye filled hippocampal NG2+ cell from CA1 stratum radiatum.
Note the parallel orientation of some processes in the direction of the Schaffer collaterals. The polygonal soma of the cell is hidden in the background. Isosurface rendering from a stack of confocal laser scans. Outer box, 30 × 60 × 80 μm3. Cube, 5 × 5 × 5 μm3.
The mechanism of transmitter release onto NG2+ cells has been recently analysed in more detail and it was shown to consist of dedicated molecular machinery similar to that previously defined for classical synaptic transmitter release from neurons onto other neurons (Kukley et al. 2007), including (i) microdomain calcium signalling, (ii) calcium-dependent, cooperative and highly synchronous fusion events, (iii) release probability comparable to that observed in interneuronal communication, and (iv) a defined pool of readily releasable vesicles and a high rate of vesicle recycling allowing stable signalling even during enduring trains of action potential activity. Based on this mechanistic similarity to neuronal EPSCs and IPSCs, in the present review we will call these currents in NG2+ cells ‘synaptic’; however, this shall not imply a complete equivalence of the underlying ultra-structure or of the physiological role (see below).
Synaptic currents in NG2+ cells have been described by several groups and in different brain regions, including cerebral cortex (Chittajallu et al. 2004; Kukley et al. 2008), cerebellar molecular layer (Lin et al. 2005), hippocampus (Bergles et al. 2000; Lin & Bergles, 2004; Ge et al. 2006; Kukley et al. 2008; Mangin et al. 2008) and corpus callosum (Kukley et al. 2007; Ziskin et al. 2007). What could be the physiological significance of detecting vesicular neurotransmitter release on NG2+ cells, as compared to the activation of membrane receptors by ambient, extracellular neurotransmitter? A first advantage is that quantal transmission enables a given postsynaptic NG2+ cell to determine the origin of this activity with excellent spatial resolution. A second advantage is the high degree of temporal resolution enabled by the high synchronicity of vesicle release with action potentials, and the rapid decay of synaptic currents. This allows NG2+ cells to potentially discriminate frequencies of neuronal activity of up to several hundred hertz. In contrast, an elevation of the ambient transmitter concentration could equally be caused by high frequency firing of a population of remote neurons, or by low frequency firing of a few neighbouring cells.
The existence of quantal synaptic currents in NG2+ progenitors in grey matter regions could be viewed as misguided target selection during synaptogenesis, as the density of synapses in grey matter is generally very high. However, in white matter areas, the formation of specific synapses on NG2+ cells from axon collaterals occurs in the absence of other synaptic targets, i.e. in brain regions devoid of neurons (Ziskin et al. 2007; Kukley et al. 2007; Karadottir et al. 2008). As white matter areas such as the optic nerve only consist of glial cells, axons and blood vessels, these findings raise the possibility that synaptic currents in NG2+ cells represent an integral part of widespread signalling occurring between axons and NG2+ progenitors. This view is also supported by in vitro findings demonstrating that, in coculture of hippocampal neurons with NG2+ cells and oligodendrocytes, growing axons are targeting NG2+ cells, while they avoid mature oligodendrocytes (Yang et al. 2006).
Different neurotransmitters are synaptically released onto NG2+ cells
Experimental evidence accumulated so far demonstrates that the two major neurotransmitters in the brain, glutamate and GABA, mediate synaptic transmission onto NG2+ cells. In some white matter areas, such as optic nerve and corpus callosum – which primarily contain glutamatergic fibres – NG2+ progenitors display only glutamatergic currents (Kukley et al. 2007; Ziskin et al. 2007). Conversely, in cerebellar white matter NG2+ cells display both glutamatergic and GABAergic currents, the latter most likely arising from Purkinje cell axons (Karadottir et al. 2008). Gray matter NG2+ cells display both glutamatergic and GABAergic synaptic currents, consistent with the presence of axons of both excitatory principal neurons and inhibitory interneurons (Lin & Bergles, 2004; Kukley et al. 2008). The finding that two transmitter signalling systems converge onto individual NG2+ progenitors in areas where they are both present (Lin & Bergles, 2004; Karadottir et al. 2008; Kukley et al. 2008) raises the question of how GABAergic and glutamatergic signals are integrated in individual NG2+ progenitors and how these cells can discriminate between them.
Putative mechanisms of signal transduction in NG2+ cells
By using perforated patch recordings on NG2+ cells, Lin & Bergles (2004) showed that these cells do not possess the low intracellular chloride concentration that is typically observed in neurons and that, consistent with this observation, GABAergic currents in NG2+ cells reverse at around –40 mV (Lin & Bergles, 2004). As NG2+ cells display relatively negative resting potentials (approximately –80 mV) both glutamatergic and GABAergic synaptic inputs depolarize their membrane. Notably, NG2+ cells can have a high input resistance in the range of several gigaohms, implying that even small synaptic inputs can cause strong depolarization of their membrane (Lin & Bergles, 2004). The consequences of this depolarization are currently unclear, although it could cause opening of voltage gated calcium channels present in NG2+ cells (Berger et al. 1992), or changes in DNA synthesis, as reported for neuronal progenitors (LoTurco et al. 1995).
It has been recently reported that a subpopulation of NG2+ progenitors in the rat white matter expresses a higher density of voltage-dependent Na+ channels and can generate mature and fully developed action potentials (Karadottir et al. 2008). Immature action potentials were also observed in a subpopulation of cortical NG2+ cells (Chittajallu et al. 2004). Therefore, it seems conceivable that membrane depolarization could activate Na+ channels to generate action potentials in these NG2+ cells. However, independent studies in mouse white and grey matter regions showed that NG2+ progenitors do not spike, and express sodium currents which are consistently smaller than potassium currents (Berger et al. 1992; Bergles et al. 2000; Chittajallu et al. 2004; Ge et al. 2006; Ziskin et al. 2007; Kukley et al. 2007, 2008). Future comparative studies performed under the same recording conditions will further define whether subpopulations of NG2+ progenitors can be identified based on these functional properties and will address this discrepancy.
As an alternative mechanism, the synaptic input may also induce a chemical rather than a solely electrical signal. The charge transferred by a glutamatergic or GABAergic quantal current is expected to produce tens of millimolar ion concentration changes in short segments of the thin (∼200 nm) processes of NG2+ cells. Such ion concentration changes may modulate sodium- or chloride-dependent processes, e.g. lead to intracellular Ca2+ accumulation via the Na+/Ca2+ exchanger (Blaustein & Lederer, 1999), and would allow the cell to discriminate the type of transmitter released.
Finally, it has been suggested that synaptic release of glutamate might induce Ca2+ elevation in NG2+ cells via Ca2+ permeable AMPARs (Bergles et al. 2000; Lin et al. 2005; Ge et al. 2006; Mangin et al. 2008). Therefore, glutamatergic synapses could influence NG2+ cell physiology via Ca2+-dependent intracellular signalling pathways. Additionally, synaptic release of transmitter could be transduced by metabotropic receptors on NG2+ progenitors, as suggested by evidence that NG2+ cells express mGluR3 and mGluR5a metabotropic glutamate receptors (Luyt et al. 2003). Whether these receptors are activated by synaptically released glutamate still remains to be established.
How widespread are neuron–NG2+ cell synapses?
A crucial question is whether synaptic currents have been detected only in some NG2+ progenitors owing to the use of electrophysiological methods that allow the measurement of very small currents, or whether synaptic inputs are a common feature of the entire NG2+ cell population. This is an important question, as not all NG2+ cells differentiate into oligodendrocytes; many persist in an undifferentiated state throughout life (Dawson et al. 2003; Reynolds & Hardy, 1997) and some differentiate into astrocytes or neurons (Zhu et al. 2008; Aguirre & Gallo, 2004; Belachew et al. 2003; Aguirre et al. 2004a). Therefore, it is important to define whether synaptic innervations may be restricted to a subpopulation of NG2+ progenitors.
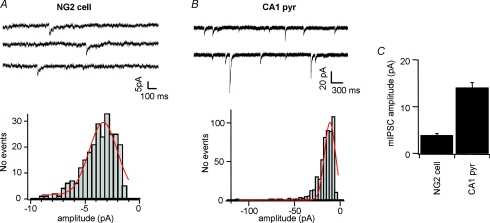
Directly addressing this question has been challenging, since synaptic currents in NG2+ progenitors are generally small, due to tiny quantal amplitudes (Fig. 2) and a smaller number of input release sites per cell (although the surface density may be similar to that found in neurons). Therefore, spontaneously occurring synaptic currents are usually only detectable in a fraction of NG2+ cells in which they appear at high frequency and display large amplitudes. However, where the frequency of spontaneous vesicles fusion is increased by a secretagogue (e.g. ruthenium red), and where ion concentrations and recording conditions are optimized, quantal glutamatergic and GABAergic currents have been demonstrated in practically all NG2+ cells tested in grey and white matter regions (Lin et al. 2005; Kukley et al. 2007, 2008; Ziskin et al. 2007). Bearing in mind that the vast majority of oligodendrocytes are derived from NG2+ cells (Zhu et al. 2008), this implies not only that synaptic currents are a consistent feature of NG2+ cells, but also that most myelinating oligodendrocytes go through a stage of synaptic innervation. Even though most NG2+ cells appear to receive at least one functional synaptic input, it is clear that synaptic density and efficacy vary between different developmental stages of NG2+ cells (Ziskin et al. 2007; Kukley et al. 2008). It is at present unknown whether mature oligodendrocytes retain functional synapses, although it has been demonstrated that functional NMDA receptor channels are expressed in these cells (Karadottir et al. 2005; Salter & Fern, 2005; Micu et al. 2006). Therefore, it will be important to determine under which conditions glutamate activates NMDA receptors in mature oligodendrocytes.
Figure 2. Quantal amplitude of GABAergic synaptic currents in NG2+ cells is small when compared to neurons.
A, top panel, whole-cell recording of an NG2+ cell in the CA1 region of a murine hippocampal brain slice at postnatal day 10. TTX and glutamate receptor antagonists were present. Chloride reversal potential is –35 mV, and the cell was voltage clamped at –80 mV. Note the small amplitude and low frequency of unprovoked spontaneous synaptic currents. Bottom panel. Pooled histograms (from 4 NG2+ cells) of peak current amplitudes. Most currents are smaller than 5 pA and it is likely that the smallest currents were lost in the recording noise. B, identical conditions as in A, but a whole-cell recording of a CA1 pyramidal cell is shown. Notice that the current scaling is different and that miniature synaptic currents are much more frequent and larger in amplitude than in NG2+ cells. Histogram represents an average from 4 cells. C, summary bar graph of the mean miniature synaptic current amplitude in NG2+ and in CA1 pyramidal cells under identical conditions, demonstrating that the quantal amplitude in NG2+ cells is 3- to 4-fold smaller than in neurons.
Morphology of neuron–NG2+ cell synapses
From the functional point of view, synaptic currents in NG2+ cells do not differ from synaptic currents in neurons. However, it is less clear whether the underlying cellular structures that allow synaptic transmission between neurons and NG2+ progenitors are also similar to those found in neuronal synapses. Electron micrographs of the ultrastructural features of synaptic contacts between neurons and NG2+ cells in grey matter demonstrate a degree of specialization similar to that found in neuron–neuron synapses (Bergles et al. 2000; Lin & Bergles, 2004; Kukley et al. 2008). The presynaptic compartment appears as a bouton and contains a large number of vesicles. The pre- and postsynaptic membranes are well aligned, and submembraneous electron dense material is found pre- and postsynaptically at the putative sites of vesicle release. Notably, the same micrographs also indicate that NG2+ cells frequently share presynaptic boutons, but not the release sites with neighbouring neuronal postsynaptic spines and dendrites.
The ultrastructural features of neuron–NG2+ cell synapses appear to be different in distinct brain regions. In particular, in white matter regions the degree of synaptic specialization appears to be lower (Kukley et al. 2007). The alignment of the two membranes and the electron dense material is less prominent, the number of vesicles appears to be much smaller, and vesicles are contained either in axonal shafts or in inconspicuous varicosities. This may indicate that a sole axon–NG2+ cell contact, i.e. when given NG2+ cell does not share a presynaptic bouton with a neuron, is not sufficient to generate formation of a complete presynaptic specialization. It has been proposed that axons may release transmitters at discrete but arbitrary sites, which may be contacted by a process of an NG2+ cell in a subsequent step. This speculative idea was generated by the finding that, in young optic nerve axons, small clusters of vesicles and fusion proteins are frequently found not to be associated with NG2+ cells (Kukley et al. 2007). Similar findings of functional clusters of synaptic vesicles in the absence of an axodendritic contact were also reported in mature cultured neurons and were termed ‘orphan release sites’ (Krueger et al. 2003). Hence, it would be interesting to test whether glutamate, released either from axons or from existing neuronal synapses, can act as a chemoattractant for NG2+ cell processes to initiate the formation of neuron–NG2+ cell synapses.
Do cellular and developmental properties of NG2+ cells suggest possible roles for their synapses?
It is now well established that glial cells are direct participants in synaptic transmission (Haydon & Carmignoto, 2006; Ni et al. 2007; Perea & Araque, 2007), and glial morphology appears to have evolved to fulfil this function. Astrocytes are morphologically specialized to modulate synaptic communication between neurons, as they extend their cellular processes into the synaptic cleft and participate in the control of the cellular microenvironment of synapses (Haydon, 2001; Haydon & Carmignoto, 2006). The majority of NG2+ progenitors exhibit a stellate morphology with small cell body and many radial processes (Fig. 1) (Chittajallu et al. 2004, 2005; Polito & Reynolds, 2005; Kukley et al. 2007; Ziskin et al. 2007). These morphological features were originally determined based on immunostaining with anti-NG2 antibodies, and have been confirmed more recently after biocytin filling of electrophysiologically and immunocytochemically identified NG2+ cells (Chittajallu et al. 2004, 2005; Kukley et al. 2007; Ziskin et al. 2007).
The presence of many cellular processes allows NG2+ cells to receive multiple synaptic contacts and to rapidly detect neurotransmitter signals released by neurons through ligand-gated ionic channels (Belachew & Gallo, 2004). Furthermore, as NG2+ progenitors also express a wide array of voltage-gated ionic channels (Lin & Bergles, 2002), neurotransmitter signalling most likely modulates the activity of other membrane channels in these cells. It is important to note that, although NG2+ cells are equipped to receive a variety of signals and even display action potentials (Chittajallu et al. 2004; Karadottir et al. 2008), they possess no specialized functional outputs comparable to axons. Therefore, the cellular and physiological changes triggered by synaptic inputs on NG2+ cells are not integrated to generate new signals that are forwarded to other cells through axon terminals.
Are neuron–NG2+ cell synapses stable?
In vivo imaging of OPCs in the zebrafish demonstrate that the extension and retraction of their cellular processes is very dynamic and happens on the scale of several minutes (Kirby et al. 2006). This raises the question of whether synapses on NG2+ cells are stable or undergo continuous dynamic changes. In vivo imaging analysis in rodents similar to that performed in zebrafish is not currently available; nevertheless there is conspicuous ultrastructural specialization frequently found at more differentiated neuron–NG2+ cell synapses, which would unlikely fully develop during short-lived intercellular contacts. These structures have been called glial invaginations and consist of 50–100 nm wide, drop-like protrusions of axonal cytoplasm into the NG2+ cell (Kukley et al. 2007). They are found in both grey and white matter regions, and can be identified in many electron micrographs (e.g. see Bergles et al. 2000– Fig. 5a; Lin et al. 2005– Fig. 7b). While the function of these structures is at present unknown, their existence suggests that the neuron–NG2+ cell contacts last longer than a few minutes.
Further evidence in support of the idea of stable neuron–NG2+ cell synapses stems from the observation that synaptic currents appear to mature during the first few postnatal weeks (Mangin et al. 2008). The changes in the kinetics and amplitudes of quantal synaptic events suggest that the association of the pre- and postsynaptic membranes becomes tighter over that time frame. Based on the current ultrastructural evidence, it is too early to define the typical morphological substrate of neuron–NG2+ cell synapses and the developmental sequence of events that culminates in their formation. Future studies employing a complete 3D reconstruction of these structures from serial sections are needed to address these issues. Analysis of synapse formation and structure in vitro could also provide interesting information concerning the clustering mechanism of glutamate and GABA receptors on NG2+ cell postsynaptic membrane, and the dependence of this phenomenon on the presynaptic neurotransmitter input itself.
Synapses and NG2+ cell proliferation
As NG2+ progenitors represent the most proliferative cell population in the postnatal and adult bran, it is plausible that the synaptic contacts that they receive might regulate their proliferation potential in vivo. In the developing postnatal brain, the NG2+ progenitor cell population is continuously expanding, as approximately 50% of the cells are actively dividing every 3 days (Kukley et al. 2008). Considering that newborn neurons require more than 7 days to acquire a synaptic input (Carleton et al. 2003; Ge et al. 2005), it may appear puzzling how all these immature newborn NG2+ cells can get rapidly connected to functional release sites. The key difference may be that NG2+ cells are actually born with synapses, i.e. that newly born NG2+ cells are already ‘wired’ by new synaptic contacts while they divide and relocate (Kukley et al. 2008). This notion would imply that, when entering mitosis, the parent NG2+ cell is able to keep its processes linked to the presynaptic release sites and to transfer these to the daughter cells upon cytokinesis. This mechanism would ascertain that synaptic contacts are established in all NG2+ cells independently of their maturational stage, and would enable these cells to expand their pool and migrate in the brain while still monitoring synaptic activity arising in local networks.
Previous work performed in cultured cells and in organotypic slice cultures showed that glutamate inhibits NG2+ cell proliferation by depolarizing the cell membrane and by blocking outward K+ currents (Gallo et al. 1996), which are characteristically expressed only in dividing NG2+ cells and not in differentiated oligodendrocytes (Gallo et al. 1996; Knutson et al. 1997). Endogenous sources of glutamate are themselves sufficient to inhibit the proliferation and lineage progression of NG2+ cells via AMPAR activation in cerebellar slice cultures (Yuan et al. 1998). While this study did not define the exact mode of activation of their AMPAR, cerebellar NG2+ are known to exhibit AMPAR-mediated synaptic currents at this stage (Lin et al. 2005; Karadottir et al. 2008). In order for glutamatergic inputs to regulate NG2+ progenitor proliferation in vivo, EPSCs arising in different cellular compartments (e.g. cell body versus distant cellular processes) must be integrated in the cell soma, where patterns of activity are likely to depend not only on the number of synapses being activated, but also on their anatomical position in the NG2+ cell. Moreover, the influence of glutamatergic inputs needs also to be integrated in concert with other signals that either promote or inhibit cell division, to modify NG2+ cell proliferation rate. Previous findings that glutamate receptor activation in NG2+ cells results in specific changes in the expression levels of cell cycle regulatory proteins, in particular p27 and p21 (Ghiani et al. 1999a,b), are consistent with the hypothesis that graded synaptic activation can be integrated with other cellular signals in NG2+ progenitors to alter expression of regulators of cell cycle progression.
In conclusion, although the specific mechanism of EPSC integration in NG2+ cells is still undefined, this is likely to depend on spatio-temporal summation, similar to the generation of action potentials in neurons. In other words, the influence of EPSCs on cell proliferation, as well as on other NG2+ progenitor functions, would depend on their degree of synchronization, i.e. a higher degree of synchronization leading to a stronger summation. Thus, it remains to be determined whether synchronized activity actually inhibits or promotes NG2+ cell proliferation, since it is debatable whether continuous application of agonists, as used in previous studies (Gallo et al. 1996; Yuan et al. 1998), is more likely to simulate synchronized over asynchronized stimulation.
Synapses and NG2+ cell migration
NG2+ progenitors are still migrating in the developing postnatal brain (Levison & Goldman, 1993; Zhu et al. 2008) while they exhibit synaptic activity (Kukley et al. 2008). Thus, NG2+ progenitors are likely to migrate while they receive functional synaptic contacts, similar to interneurons in the molecular layer of the dentate gyrus (Morozov et al. 2006; Chittajallu et al. 2007). Synaptic release of neurotransmitter may act as a positional clue regulating the migration process, and glutamate has been shown to promote OPC migration via an αv integrin–myelin proteolipid protein complex (Gudz et al. 2006). It has been recently shown in the mouse hilus that synaptic activity in NG2+ cells is partially synchronized with local neurons (Mangin et al. 2008). Since NG2+ cells are likely to integrate the activity arising in several neighbouring neurons, they could assess the degree of synchrony arising in any given set of contacted neurons while migrating. Since such synchrony is more likely to occur in neurons receiving inputs from the same areas and belonging to the same structure, it can be speculated that NG2+ cells would be able to recognize functionally homogeneous group of neurons while migrating and that the neurotransmitter released from these neurons acts as a positional clue.
Synapses and NG2+ cell differentiation
Functional synapses are maintained on NG2+ progenitors also at non-migratory developmental stages, i.e. when they undergo cell cycle exit and initiation of cell differentiation (Kukley et al. 2008; J.-M. Mangin and V. Gallo, unpublished observations). Moreover, glutamatergic synapses on NG2+ progenitors promote Ca2+ influx through the cell membrane, as a significant percentage of the AMPA channels on NG2+ cells conduct Ca2+ ions (Bergles et al. 2000; Lin et al. 2005; Ge et al. 2006; Mangin et al. 2008). Permeability of AMPA channels to this cation appears to change with age (Itoh et al. 2002; Ge et al. 2006; Ziskin et al. 2007). Altogether, these observations suggest that synaptic activity might also play a role in coordinating cell cycle withdrawal and cell differentiation through Ca2+-dependent events. Several transcription factors that play important roles in NG2+ cell maturation along the oligodendrocyte lineage have been identified, including Yin Yang1 (YY1; He et al. 2007), Sox17 (Sohn et al. 2006), and Sox9 and 10 (Stolt et al. 2003, 2004; Wegner, 2008). In future studies, it will be important to determine whether different patterns of synaptic activity could regulate the signalling pathways that control expression of these transcription factors, and/or transcription of oligodendrocyte genes controlled by these proteins.
Putative roles of synapses on NG2+ cells in grey matter
A difference in cell morphology is probably the more direct and less controversial distinction between grey and white matter NG2+ cells (Chittajallu et al. 2004; Butt et al. 2005; Dayer et al. 2005). While white matter NG2+ cells usually exhibit a fusiform soma and polarized processes aligned with axons bundles, grey matter NG2+ cells will display a non-polarized, radial arborization (Butt et al. 2005). The main reason explaining these morphological differences may be the particular affinity that grey matter NG2+ cells display for neurons present in their vicinity. Indeed, their soma and processes are frequently found associated to neuronal soma and neurites in cortex, hippocampus and cerebellum (Butt et al. 2005; Dayer et al. 2005), and some of the grey matter NG2+ cells are so closely apposed to neurons that they could be considered as satellite cells (Dayer et al. 2005) (Fig. 3A and B). By analysing the spatial distribution of NG2+ cells and neurons in the mouse hilus, it was recently demonstrated that NG2+ cell somata are indeed statistically closer to neuronal somata than would be expected by a random distribution of both cell types (Mangin et al. 2008).
Figure 3.
Anatomically associated pairs of hilar NG2+ cell and interneurons exhibit synchronized synaptic activity A, single-plane merged confocal image showing the dentate gyrus of a P15 CNP-EGFP (green) transgenic mouse immunostained for the proteoglycan NG2 (blue) and the neuronal marker NeuN (red). The dotted box indicates an NG2+EGFP+ cell associated to hilar neurons. Right panels show the individual staining for the area delimited by the dotted box. B, confocal images showing a hilar interneuron (red) and its sNG2+ cell (green) filled with biocytin (red in B and grey in right lower panel) and tetramethylrhodamine dextran (green in B and grey in right upper panel), respectively. C, example of synchronized bursts observed in a hilar interneuron (Vh=–60 mV, upper trace) and its sNG2+ cell (Vh=–80 mV, lower trace) recorded in a whole hippocampal slice preparation in the presence of 100 μm picrotoxin. The grey boxes indicate the interval of the trace in A magnified in the right panels. In the right panels, dotted lines indicate recurrent EPSCs, which are usually observed after the initial discharge of EPSCs indicated by a continuous line. D, example of spontaneous activity recorded in a hilar interneuron (Vh=–60 mV, upper trace) and its sNG2+ cell (Vh=–80 mV, lower trace) by using a specific caesium methanesulphonate internal solution to isolate EPSCs from GABAergic activity (ECl=–80 mV and Ecation= 0 mV). The application of 20 μm carbachol was used to increase the spontaneous EPSC activity in both cells. Asterisks indicate synaptic current that are synchronized between both cells.
Since most NG2+ cells exhibit glutamatergic and/or GABAergic synaptic currents in grey matter areas, one could expect that spatial proximity would facilitate synaptic connectivity between NG2+ cell and their associated neurons. However, pair recordings of ‘satellite’ NG2+ cells (sNG2+ cells) associated to GABAergic interneurons in the mouse hilus revealed no functional connectivity between closely associated cells (Mangin et al. 2008). While this absence of connectivity may be specific to hilar interneurons, it clearly shows that anatomical proximity between NG2+ cells and interneurons is serving other purposes than facilitating connectivity between cells.
In the same study, it was also shown that associated NG2+ cell–interneuron pairs are more likely to receive glutamatergic inputs from the same presynaptic neuron (Fig. 3C and D), as compared to pairs separated by more than 200 μm (Mangin et al. 2008). However, the frequency of such shared inputs in a given NG2+ cell–interneuron pair remains modest. On average, around 15% of all EPSCs observed in an NG2+ cell are synchronized with an EPSC in a given associated interneuron. In a similar fashion, it has been previously shown in cerebellum that a small fraction (3/77) of NG2+ cell–Purkinje cell pairs located in the same folia are connected by the same climbing fibres (Lin et al. 2005). Interestingly, while each Purkinje cell is contacted by only one climbing fibre, NG2+ cells can receive inputs from several climbing fibres, providing them with the opportunity to monitor and integrate the activity arising in several neighbouring Purkinje cells. Similarly, NG2+ cells in grey matter areas display a dense arborization contacting multiple neighbouring neurons (Butt et al. 2005), and are thus likely to integrate synaptic activity arising in neighbouring groups of neurons.
Do synapses inhibit the differentiation of grey matter NG2+ cells into oligodendrocytes?
It has been reported that glutamatergic synapses on white matter NG2+ cells are made by constitutively unmyelinated fibres in adult mice (Ziskin et al. 2007). Similarly, most grey matter NG2+ cells are synaptically connected, but do not differentiate into oligodendrocyte, myelination being minimal in grey matter areas. Moreover, since glutamate inhibits NG2+ cell differentiation into oligodendrocyte (Gallo et al. 1996; Yuan et al. 1998), it is tempting to suggest that glutamate released from synapses acts as an antimyelination signal. One could even generalize that, since most GABAergic synapses are made by non-myelinated local interneurons, the synaptically released neurotransmitter acts as a myelination inhibitor. However, this hypothesis cannot explain why NG2+ cells are found in poorly myelinated grey matter areas in the first place, if their lineage potential is limited to the generation of oligodendrocytes. Moreover, synaptic quantal transmission appears designed to carry information with a high degree of spatio-temporal resolution; it is thus hard to reconcile synaptic transmission on NG2+ cells with such a broad function. Therefore, even if future studies demonstrate that neurotransmitters released onto NG2+ cells regulate myelination in vivo, synaptic integration of NG2+ cells in grey matter areas is likely to have additional roles than merely inhibiting myelination.
Are synapses predisposing NG2+ cells to a neuronal fate?
NG2+ progenitors are able to give rise to neurons in vitro (Kondo & Raff, 2000; Belachew et al. 2003) and in vivo after transplantation (Shihabuddin et al. 2000; Aguirre et al. 2004). There is also evidence that endogenous NG2+ cells may give rise to interneurons in the hippocampus (Belachew et al. 2003; Aguirre et al. 2004), olfactory bulb (Aguirre & Gallo, 2004) and cortex (Dayer et al. 2005). It has also been shown that a subpopulation of NG2+ cells express a density of Na+ channels sufficient to generate immature single action potentials or, more rarely, bursts of spikes in the mouse cortex (Chittajallu et al. 2004). These findings support the hypothesis that oligodendrocyte and interneuron lineages are more tightly connected than one would initially expect. Such observations are themselves derived from studies showing that interneurons and oligodendrocytes share a common origin in the embryonic mammal telencephalon (He et al. 2001; Marshall & Goldman, 2002) as well as in the adult SVZ (Aguirre et al. 2004).
A recent study suggests that new interneurons could be generated from ‘satellite’ NG2+ cells in the cortex (Dayer et al. 2005). In this context, it is particularly significant that satellite NG2+ cells associated with interneurons in the mouse hilus (Mangin et al. 2008) are found to share common presynaptic inputs with these neurons, a property which would facilitate further differentiation steps towards an interneuron fate. However, a recent study using an NG2-Cre transgenic mouse model suggests that the ability of endogenous NG2+ cells to generate interneurons during normal development in vivo is at best a marginal phenomenon (Zhu et al. 2008). In spite of these findings, the opportunity of manipulating endogenous NG2+ cells to replace interneurons in order to strengthen inhibitory pathways damaged under pathological conditions may be less unrealistic than previously thought.
Does synaptic integration allow NG2+ cells to modulate neuronal activity?
Because many NG2+ cells persist in the adult brain in an apparently ‘quiescent state’, it has been proposed that these cells could actually correspond to a new class of mature glial cells whose exact physiological function(s) remain to be understood (Butt et al. 2005). NG2+ cells are known to produce several extracellular matrix (ECM) molecules, such as hyaluronan, versican, phosphacan, neurocan and tenascins, which function to stabilize synapses by interacting with spectrin and ankyrin, two neuronal cytoskeleton components known to anchor ion channels and neurotransmitter receptors at synapses (Butt et al. 2005). Indeed, NG2+ cell processes are known to interdigitate between glutamatergic pre- and postsynaptic elements (Ong & Levine, 1999; Bergles et al. 2000). Moreover, electron microscopy observations made in the CA1 stratum radiatum suggest that presynaptic boutons that make contacts with NG2+ cells are frequently shared with a neighbouring neuron (Bergles et al. 2000). Indeed, it has been recently confirmed that anatomically associated pairs of NG2+ cell–neuron tend to receive functional glutamatergic inputs from the same presynaptic neurons (Mangin et al. 2008). Taken together, these data suggest that NG2+ cells can ‘eavesdrop’ on synapses made on neighbouring neurons via a dedicated presynaptic structure in which the presynaptic release site has been duplicated. Such a configuration would allow NG2+ cells to monitor and regulate neuron–neuron synapses, either individually or by comparing their activity with other synapses contacted by NG2+ cells.
Our understanding of neuron–NG2+ cell synapses could also benefit from a parallel with cerebellar Bergmann glia. Similar to NG2+ cells, Bergmann glia cell bodies are found tightly apposed to Purkinje cell somata and contact their dendrites via multiple processes. Bergmann glial cells receive multiple inputs from the same climbing fibres that innervate Purkinje cells, but do not share identical presynaptic release sites (Matsui & Jahr, 2003). Finally, in both Bergmann glia and NG2+ cells, synaptic release of glutamate activates Ca2+-permeable AMPA-Rs (Bergles et al. 2000; Matsui et al. 2007). The conversion of these receptors into Ca2+-impermeable receptors by a viral transfection of GluR2 subunit (Iino et al. 2001) induces retraction of Bergmann glia processes, allowing climbing fibres to form aberrant multiple contacts onto Purkinje cells. Therefore, AMPAR-mediated Ca2+ entry appears to influence the morphology of glial cells, and by this means can regulate the connectivity of associated neurons. In the context of duplicated and shared presynaptic terminals mentioned above, it is possible that small changes in the morphology of NG2+ cell postsynaptic processes may allow these cells to finely regulate the function of a neighbouring neuron–neuron contact. Indeed, it has been shown that the acquisition of faster kinetics that occurs in glutamatergic synapses during postnatal development is probably driven by modifications of the synaptic structure itself (Cathala et al. 2005). It can be also speculated that NG2+ cells may influence synaptic activity by releasing neuromodulators in response to synaptic activation, as has been shown in astrocytes (Perea & Araque, 2007). Because NG2+ cells are present in grey matter areas and receive synaptic contact as early as 4–5 postnatal day in mice (Bergles et al. 2000; Lin & Bergles, 2004; Mangin et al. 2008; Kukley et al. 2008), their ability to monitor individual neuron–neuron synapses would allow them to modulate the formation and the refinement of synaptic networks occurring during the first postnatal weeks. Moreover, by modulating the formation and stability of synapses received by neighbouring neurons during development, NG2+ cells may regulate the emergence of synchronized and functionally equivalent groups of neurons.
Do synapses allow NG2+ cells to detect ‘pathological’ patterns of neuronal activity?
The long-term consequences of epilepsy on NG2+ cells and the physiological role of NG2+ cells themselves in epilepsy are still largely unexplored. We have recently shown that hilar NG2+ cells exhibit a distinct pattern of activity when presynaptic CA3 pyramidal neurons are pharmacologically synchronized by carbachol and by GABAergic antagonists (Mangin et al. 2008), two drugs which, respectively, induce gamma oscillations and epileptic-like ictal activity. Gamma oscillations are known to occur in vivo under physiological conditions (Fischer, 2004). In hilar NG2+ cells, gamma oscillations are associated with a uniform fourfold increase in sEPSC frequency (0.15–0.65 Hz), without significant EPSC summation, as compared to basal conditions. By contrast, during epileptic-like ictal events, NG2+ cells exhibit transient bursts of EPSCs at high frequency (40–100 Hz; Fig. 3B), which display significant summation and are separated by 20–60 s periods of low frequency activity (0.1–0.2 Hz) (see Fig. 3B). Such short bursts of high frequency activity might induce larger and transient increases of intracellular Ca2+, which may be more likely to influence overall NG2+ cell function (e.g. proliferation and differentiation) than the milder and uniform increase in EPSC frequency observed during gamma oscillations. Consistent with this hypothesis, it has been shown that hippocampal NG2+ cells actively proliferate after electroconvulsive seizures (Wennström et al. 2003), suggesting that synaptic integration may allow NG2+ cells to detect abnormal pattern of neuronal activity that will induce a functional response.
Putative roles of synapses on NG2+ cells in white matter
Why have neurons developed dedicated release machinery at specific sites along their axons in the white matter, as these specialized structures are not used for synaptic communication with other neurons?
Oligodendrocytes are responsible for enwrapping axons in myelin sheaths to permit rapid action potential propagation by saltatory conduction. Since NG2+ progenitors residing in white matter regions have been shown to generate only oligodendrocytes and other NG2+ cells under normal physiological conditions (Zhu et al. 2008), we mainly consider in this section their function as OPCs. As highlighted in recent reviews, the process of myelination during development of the brain does not follow a rigid stereotypical programme, but is strongly modulated by neuronal activity (Fields, 2005; Fields, 2008). For example, raising animals in enriched environments has a supportive influence on the number of oligodendroglial cells and on the speed of myelination (e.g. Szeligo & Leblond, 1977; Sirevaag & Greenough, 1987; Juraska & Kopcik, 1988). At the cellular level (for review see Zalc & Fields, 2000), it has been reported that axonal activity is an important modulator not only of the generation of oligodendroglial cells, but also of the onset and speed of myelination (e.g. Barres & Raff, 1993; Demerens et al. 1996).
These results generate the question of how NG2+ cells in white matter regions can detect that specific, electrically active neurons located at a considerable distance require myelination. Thus, there is a clear need for a local signal with a high spatial and temporal resolution, which enables NG2+ cells to recognize different firing frequencies of individual neighbouring axons. As reasoned above, vesicular glutamate release from axons would be ideally suited to fulfilling this task and guiding NG2+ cells to axons requiring myelination. In fact, the findings in developing white matter that NG2+ cells detect glutamate concentrations at lower levels than postsynaptic neurons and that there are putative ‘orphan’ release sites in unmyelinated axons (Kukley et al. 2007) suggest the hypothesis that NG2+ cells might be in the process of approaching these axons along a concentration gradient of glutamate. Although a chemoattractant effect of axonally released glutamate on NG2+ cells is as yet pure speculation, there is substantial evidence from culture systems that pharmacological activation of ionotropic glutamate receptors (AMPA/kainate type) influences their proliferation, migration and differentiation (e.g. Yuan et al. 1998; reviewed by Gallo & Ghiani, 2000).
Finally, the question of the final fate of the axon–NG2+ cell junctions when NG2+ progenitors differentiate is still pending. Are these structures lost, transformed into nodes of Ranvier, or used to initiate enwrapping of axons? Progressive loss of synapses on NG2+ progenitors during cell differentiation would strongly suggest specific functions that relate to early developmental events, i.e. cell proliferation, migration and lineage progression to a committed oligodendrocyte stage.
Importantly, myelination is not only an early developmental phenomenon, but it continues until adulthood, when it may underlie certain forms of learning and memory (Fields, 2008; Fields, 2005). For example, concert pianists show an increased white matter signal in specific fibre tracts proportional to the amount of time spent practicing their instrument (Bengtsson et al. 2005). It is noteworthy that synaptic signals in white matter NG2+ cells are present even in adult human brain (Fig. 4), suggesting that electrical activity in specific fibre tracts might be one of the mechanisms that regulate myelination during postnatal development and in adulthood also in humans.
Figure 4. NG2+ cells in adult human white matter display synaptic currents.
Recording was performed in the fimbria of a human hippocampal brain slice prepared from a neurosurgical specimen. The hippocampus was removed for the treatment of medically resistant temporal lobe epilepsy (35 years of age). A, whole cell current pattern in response to depolarizing voltage steps (ΔV = 10 mV) from a holding potential of –80 mV. The current pattern is very similar to rodent NG2+ cells. B, spontaneous glutamatergic synaptic currents in the presence of Ruthenium Red (100 μm). Note the fast kinetics of currents, which are typical for glutamatergic synaptic currents in rodent NG2+ cells.
The demonstration of axonal release of glutamate onto NG2+ progenitors in white matter is likely to have important implications for our understanding of a variety of neurological and developmental disorders. Widespread glutamate release from axonal shafts is likely to be harmful and to cause significant cellular damage in a variety of pathological conditions, including ischaemia, vascular dementia, cerebral palsy and traumatic brain injury (Matute et al. 2001; Deng et al. 2003; Dewar et al. 2003; Follett et al. 2004; Stys, 2004). Interestingly, during ischaemia glutamate damages oligodendoglial cells not only by acting on AMPA receptors (McDonald et al. 1998; Tekkok & Goldberg, 2001), but also by activating NMDA receptors situated in myelin sheaths (Karadottir et al. 2005; Salter & Fern, 2005; Micu et al. 2006; Bakiri et al. 2008). The source of glutamate released in white matter under pathological conditions has been intensely debated, but it seems likely that vesicular release from axons plays a major role in contributing to the excessive extracellular levels of glutamate that cause damage in oligodendroglial cells.
Conclusion and future directions
As typically occurs after fundamental observations are made, the original finding that NG2+ progenitors of the postnatal brain receive functional synapses similar to those existing between neurons (Bergles et al. 2000) has generated enormous interest and initiated a new field of research. Furthermore, the recent studies demonstrating that NG2+ cells also respond to glutamate synaptically released from axons in white matter (Kukley et al. 2007; Ziskin et al. 2007) have not only further challenged existing dogmas on synaptic communication in the brain, but also opened up new perspectives on cellular interactions in white matter during normal development and in pathological conditions. In this review, we have summarized our view and speculated on future research directions that will likely move this exciting field forward. These directions are based on fundamental questions that relate to the function of neuron–NG2+ cell synapses in the context of synaptic communication within specific neural networks, and in the developmental context of lineage fate decision of this progenitor cell population. Other important issues to be addressed in future studies might be more specific for NG2+ cells found in white matter regions and relate to the interaction between NG2+ cells and axons. What is the fate of synaptic inputs to NG2+ cells when they differentiate to myelinating oligodendrocytes? What is the fate of axonal release sites/synapses contacting NG2+ cells as axons become myelinated? Do unmyelinated axons that release glutamate on NG2+ cells ever become myelinated? It will be interesting to find out whether a better understanding of the role of synapses in NG2+ cells will result in elucidating the function of non-myelinating NG2+ cells in the postnatal and adult brain.
Acknowledgments
The authors of this review were supported by National Institutes of Health grants R01NS045702 and R01NS1056427 (V.G.), NIH R21NS050463 (V.G.), C.U.R.E. (Citizens United for Research on Epilepsy; V.G.), by grant RG4019 (V.G.) from the National Multiple Sclerosis Society, by DFG (SFB TR3, DI853–2; D.D.) and by NIH IDDRC P30HD40677. V.G. was also partially supported by the European Leukodystrophy Association. We are grateful to J. Schramm for kindly providing neurosurgical specimen. We thank Dr Tarik Haydar for assistance and training in the use of the Zeiss LSM-510 confocal microscope.
References
- Aguirre A, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Hamilton NB, Karadottir R, Attwell D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56:233–240. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Gallo V. Synaptic and extrasynaptic neurotransmitter receptors in glial precursors' quest for identity. Glia. 2004;48:185–196. doi: 10.1002/glia.20077. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Orkand PM, Kettenmann H. Sodium and calcium currents in glial cells of the mouse corpus callosum slice. Eur J Neurosci. 1992;4:1271–1284. doi: 10.1111/j.1460-9568.1992.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cathala L, Holderith NB, Nusser Z, DiGregorio DA, Cull-Candy SG. Changes in synaptic structure underlie the developmental speeding of AMPA receptor-mediated EPSCs. Nat Neurosci. 2005;8:1310–1318. doi: 10.1038/nn1534. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. Downregulation of platelet-derived growth factor-α receptor mediated tyrosine kinase activity as a cellular mechanism for K+ channel regulation during oligodendrocyte lineage progression in situ. J Neurosci. 2005;25:8601–8610. doi: 10.1523/JNEUROSCI.2122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Kunze A, Mangin JM, Gallo V. Differential synaptic integration of interneurons in the outer and inner molecular layers of the developing dentate gyrus. J Neurosci. 2007;27:8219–8225. doi: 10.1523/JNEUROSCI.2476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity. Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Y. The hippocampal intrinsic network oscillator. J Physiol. 2004;554:156–174. doi: 10.1113/jphysiol.2003.055558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2005;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Eisen AM, Yuan X, DePinho RA, McBain CJ, Gallo V. Neurotransmitter receptor activation triggers p27Kip1 and p21CIP1 accumulation in oligodendrocyte progenitors and cell cycle arrest in G1. Development. 1999b;126:1077–1090. doi: 10.1242/dev.126.5.1077. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Yuan X, Eisen A, Knutson P, DePinho R, McBain CJ, Gallo V. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27Kip1 and p21CIP1 in glial progenitor cells. J Neurosci. 1999a;19:5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an αv integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casacci-Bonnedil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia–synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Knutson P, Ghiani C, Zhou JM, Gallo V, McBain C. K+ channel expression and proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger SR, Kolar A, Fitzsimonds RM. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–957. doi: 10.1016/s0896-6273(03)00729-3. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2008 doi: 10.1096/fj.07-090985. in press. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of the rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luyt K, Varadi A, Molnar E. Functional metabotropic glutamate receptors are expressed in oligodendrocyte progenitor cells. J Neurochem. 2003;84:1452–1464. doi: 10.1046/j.1471-4159.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2+ progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.1355-08.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Ayoub AE, Rakic P. Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci. 2006;26:5017–5027. doi: 10.1523/JNEUROSCI.0272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watyanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Sohn J, Natale J, Chew LJ, Belachew S, Cheng Y, Aguirre A, Lytle J, Nait-Oumesmar B, Kerninon C, Kanai-Azuma M, Kanai Y, Gallo V. Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J Neurosci. 2006;26:9722–9735. doi: 10.1523/JNEUROSCI.1716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factor Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Muli-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci. 2008;35:3–12. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- Wennström M, Hellsten J, Ekdahl CT, Tingström A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat hippocampus. Biol Psychiatry. 2003;54:1015–1024. doi: 10.1016/s0006-3223(03)00693-0. [DOI] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zalc B, Fields RD. Do action potentials regulate myelination. Neuroscientist. 2000;6:5–13. doi: 10.1177/107385840000600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]