Abstract
Chemokines have been implicated in the promotion of leucocyte trafficking to diseased muscle. The purpose of this study was to determine whether a subset of inflammatory chemokines are able to directly drive myoblast proliferation, an essential early component of muscle regeneration, in a manner which is entirely independent of leucocytes. Cultured myoblasts (C2C12) were exposed to monocyte chemoattractant protein-1 (MCP-1; CCL2), macrophage inflammatory protein-1α(MIP-1α; CCL3) or MIP-1β (CCL4). All chemokines induced phosphorylation of extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) and greatly increased myoblast proliferative responses. Chemokine-induced myoblast proliferation was abolished by pertussis toxin and the MEK1/2 inhibitor U0126, implicating both Gαi-coupled receptors and ERK1/2-dependent signalling. Myoblasts expressed receptors for all of the chemokines tested, and mitogenic responses were specifically inhibited by antibodies directed against CC family chemokine receptors 2 and 5 (CCR2 and CCR5). Within an in vitro myogenic wound healing assay devoid of leucocytes, all chemokines significantly accelerated the time course of myoblast wound closure after mechanical injury. Injections of MCP-1 into cardiotoxin-injured skeletal muscles in vivo also suppressed expression of the differentiation marker myogenin, consistent with a mitogenic effect. Taken together, our results indicate that CC chemokines have potent and direct effects on myoblast behaviour, thus indicating a novel role in muscle repair beyond leucocyte chemoattraction. Therefore, interventions aimed at modulating the balance between myoblast and leucocyte effects of CC chemokines in injured muscle could represent a novel strategy for the treatment of destructive muscle pathologies.
Skeletal muscle regeneration is an essential compensatory response to both genetic and acquired forms of muscle fibre damage and loss. Damaged muscle regenerates itself by activating a population of undifferentiated muscle precursor cells, commonly referred to as satellite cells (Hawke & Garry, 2001; Charge & Rudnicki, 2004), which contribute to the formation of new healthy fibres. The satellite cells normally reside between the plasma membrane and basal lamina in a relatively quiescent, non-proliferative state. However, once the satellite cells are activated by muscle injury, they undergo intense proliferation as well as migration to sites of muscle fibre damage (Hawke & Garry, 2001). After several rounds of cellular division, the majority of these cells (now considered adult myoblasts) will exit the cell cycle and differentiate into post-mitotic myotubes, which then evolve into mature adult fibres. In experimental models of skeletal muscle injury, major leucocyte accumulation also occurs at sites of muscle regeneration, consisting initially of neutrophils and then primarily of macrophages (Tidball, 2005). It has been shown that interference with macrophage influx will delay subsequent muscle repair (Lescaudron et al. 1999; Summan et al. 2006; Tidball & Wehling-Henricks, 2007). Moreover, a number of cytokines and growth factors with the capacity to induce satellite cell proliferation or migration can be produced by macrophages (Hawke & Garry, 2001; Tidball, 2005), and macrophage-derived soluble factors have been shown to favour the muscle regeneration process (Robertson et al. 1993; Merly et al. 1999; Wehling et al. 2001; Cantini et al. 2002).
Recently, it has been suggested that chemokines are important actors in skeletal muscle regeneration (Warren et al. 2004, 2005; Contreras-Shannon et al. 2006). The chemokines are subdivided into four main families (CXC, CC, C and CX3C), based upon the number and arrangement of their amino-terminal cysteine residues (Zlotnik & Yoshie, 2000). Increased expression levels of several chemokine ligands and their cognate receptors have been found in muscle biopsies obtained from animal models and human patients suffering from muscular dystrophy or inflammatory myopathies. In particular, a predominant up-regulation of the CC chemokines, including MCP-1 (CCL2), MIP-1α (CCL3) and MIP-1β (CCL4), has been reported (Confalonieri et al. 2000; Reyes-Reyna et al. 2002; Porter et al. 2003; Demoule et al. 2005). In addition, skeletal muscle cells constitutively express MCP-1 and MIP-1α (Nagaraju et al. 2000; De Rossi et al. 2000; Reyes-Reyna & Krolick, 2000), and CC chemokines are greatly up-regulated following experimental muscle injury (Hirata et al. 2003; Warren et al. 2004; Contreras-Shannon et al. 2006) or exposure to pro-inflammatory cytokines such as TNF-α (De Rossi et al. 2000; Demoule et al. 2005). Interestingly, the up-regulation of CC chemokines and their receptors observed following muscle damage (Hirata et al. 2003) appears to correspond temporally with the period of satellite cell proliferation found in experimental models of muscle injury (Grounds & McGeachie, 1992). More direct evidence for the importance of CC chemokines in muscle regeneration has been provided by the demonstration that recovery of normal muscle structure and force production after acute injury in vivo is significantly impaired in mice receiving antibodies against MCP-1 or lacking its primary receptor, CCR2 (Warren et al. 2004, 2005; Contreras-Shannon et al. 2006).
As the chemokines are classically associated with the induction of leucocyte trafficking to sites of tissue inflammation, one interpretation of these findings is that the purpose of CC chemokine up-regulation within damaged muscle is to direct leucocytes to sites of muscle injury. This could favour muscle repair in an indirect fashion, such as through the release of macrophage-derived mediators which favour muscle regeneration, as mentioned earlier. However, another possibility is that the CC chemokines expressed within injured skeletal muscle are capable of acting directly on the muscle cells per se, by regulating myoblast behaviour during one or more steps associated with the formation of new muscle fibres. Therefore, the purpose of the present study was to determine whether CC chemokines are capable of playing a role in the muscle regeneration process through mechanisms that are entirely independent of their effects on leucocyte trafficking.
Methods
Reagents
Murine MCP-1, MIP-1α and MIP-1β were purchased from R&D (La Jolla, CA, USA). Functional blocking antibodies directed against CCR2 and CCR5 were generated by M. Mack (University of Munich, Germany), as previously described (Mack et al. 2001). Polyclonal antibodies recognizing the total and phosphorylated forms of ERK1/2 MAPK were purchased from Cell Signalling Technology (Beverly, MA, USA), and the corresponding secondary anti-rabbit secondary antibody coupled to horseradish peroxidase as well as enhanced chemiluminescence kit, were purchased from Amersham Biosciences (Piscataway, NJ, USA). Monoclonal antibody directed against myogenin was purchased from Santa Cruz (Santa Cruz, CA, USA). Pertussis toxin (PTX) and the MEK1/2 inhibitor U0126 were purchased from EMD Biosciences Inc. (San Diego, CA, USA). For cell proliferation analyses, a 5-bromo-2′-deoxyuridine (BrdU) incorporation kit was purchased from BD Pharmingen (Mississauga, ON, Canada). Mouse C2C12 myoblasts were obtained from the American Type Culture Collection (Manassas, VA, USA) and use was limited to less than 10 passages. Tissue culture media components, Trizol reagent, random hexamers, M-MLV reverse transcriptase and Taq DNA polymerase, were all obtained from Invitrogen (Rockville, MD, USA).
Chemokine receptor expression
Total RNA was extracted from skeletal muscle cells using Trizol reagent according to the manufacturer's protocol. For reverse transcription, 2 μg of total RNA and random hexamers (1 μg μl−1) were employed. PCR amplification was performed for 40 cycles. The following primer sets (5′ to 3′) were employed: CCR1 forward cagaagcctaccccacaactac, reverse aatcagaagccagcagagag; CCR2 forward gtatccaagagcttgatgaaggg, reverse gtgtaatggtgatcatcttgtttgga; CCR3 forward gcaccaccctg-tgaaaaagt, reverse cgaggactgcaggaaaactc; CCR4 forward gcaccaaggaaggtatcaag, reverse gttctagcaaagtacctaca; CCR5 forward gattttcaagggtcagttcc, reverse ccagtagaaacttcatgttc. In all cases, negative controls lacking reverse transcriptase were included in order to exclude genomic DNA contamination.
Cell proliferation assays
Myoblasts were expanded in growth medium (GM) composed of low glucose Dulbecco's modified Eagle's medium (DMEM) and 10% (v/v) fetal bovine serum (FBS). DNA synthesis in C2C12 cells was evaluated by measuring BrdU incorporation with the use of flow cytometry. Briefly, myoblasts were plated at 5 × 105 cells per 60 mm well and cultured overnight in GM. Cells were subsequently switched to stimulation medium (SM), consisting of DMEM supplemented with 5% FBS (in preliminary studies, we found that higher serum concentrations caused unacceptably high background levels of myoblast proliferation, whereas lower serum concentrations impaired chemokine-induced proliferative effects). This is consistent with other studies showing that 5% FBS maintains myoblasts in a state of low-level proliferation without inducing differentiation (Spangenburg & Booth, 2002; Machida et al. 2003). Myoblasts were maintained in SM for 24 h, and then stimulated for the next 24 h with murine MIP-1α, MIP-1β or MCP-1 (100 ng ml−1 in SM). The cells were labelled with BrdU during the final 16 h of stimulation. The myoblasts were then permeabilized, fixed and stained with an anti-BrdU antibody coupled to FITC according to the manufacturer's protocol. Flow cytometry data were collected using a logarithmic scale (FACSCalibur, BD Biosciences, San Diego, CA, USA), and the percentage of BrdU-positive cells was determined.
Cell counting was performed daily in cultures of C2C12 myoblasts exposed to the different CC chemokines for a period up to 4 days. Subconfluent myoblasts were plated at 5 × 103 cells per well in 24-well plates, and maintained in SM for 24 h prior to being stimulated. The individual chemokines were then added to the medium at 100 ng ml−1, and freshly prepared media containing the chemokines were used daily. At each time point analysed, the myoblasts were trypsinized and resuspended in medium to attain a total volume of 500 μl. Cell counting was performed in triplicate on separate 10 μl aliquots using a haemacytometer.
Western blot analysis
The protein levels contained in myoblast lysates were assessed by the Bradford method, and 20–50 μg of total protein per lane was loaded on a 10% polyacrylamide gel under reducing conditions (Mini Protean System, Bio-Rad, Hercules, USA). After protein transfer to nitrocellulose by electroblotting, the membranes were incubated with primary antibodies against pERK1/2, total ERK1/2 or myogenin. This was followed by incubation with a peroxidase-conjugated secondary antibody, and visualization with enhanced chemiluminescence according to manufacturer's instructions. Equal loading across lanes was verified by staining of the membranes with Ponceau red. Band intensities were determined using an image analysis system (FluorChem 8000, Alpha Innotech Corp., San Leandro, CA, USA).
Myogenic wound healing assay
In vitro muscle wounding was performed by mechanical disruption of confluent C2C12 myoblasts as previously described (Yeow et al. 2002; Harfouche & Hussain, 2006), with minor modifications. The myoblasts were plated in 6-well plates and placed in GM until reaching confluence. The confluent cell layer was wounded using a Pasteur pipette to generate a continuous and well-delineated area of cellular disruption along the surface of each well. After wounding, the cells were rinsed twice with DMEM to remove cellular debris, and then switched to SM containing either vehicle or individual chemokines (100 ng ml−1), each in triplicate. Using an image analysis software program (ImagePro Plus, Media Cybernetics, Silver Springs, MD, USA), the size of the wound area (i.e. the empty space between confluent cells) at the identical region of the tissue culture plate was quantified at different time points. Care was taken to ensure that the initial wound area at time zero was comparable (< 10% variation) among the different experimental groups. For each well analysed, a 100-point grid was superimposed over three non-overlapping microscopic images (4× objective) captured to computer, and the area of the wound space devoid of cells was quantified using a standard point-counting technique (Harfouche & Hussain, 2006). For each time point examined, the data were expressed as a percentage of the mean wound space area values obtained at time zero (defined as 100%).
Cardiotoxin-induced skeletal muscle injury
Adult C57/BL6 mice received injections of cardiotoxin (10 μm in 50 μl PBS) into both tibialis anterior (TA) muscles, in order to induce skeletal muscle injury in vivo as previously described (Hirata et al. 2003). The mice were anaesthetized using ketamine–xylazine–acepromazine (50–5–1 mg kg−1) by intraperitoneal injection prior to the induction of skeletal muscle injury. Following cardiotoxin-induced injury, one TA muscle was injected with recombinant MCP-1 murine (0.5 μg in 50 μl PBS) at 12 h intervals, while the contralateral TA muscle received injections of PBS vehicle at the same time points in each animal. Mice were killed by anaesthetic overdose at 24 h (n = 6), 48 h (n = 6) or 72 h (n = 5) after cardiotoxin-induced injury, and the two TA muscles were removed from each animal for protein extraction. The experiments were approved by the McGill University animal ethics committee, in accordance with standards established by the Canadian Council on Animal Care.
Statistical analysis
Data are presented as group mean values ±s.e.m. All data from in vitro studies represent the average values obtained from at least three independent experiments performed on separate occasions. Statistical comparisons were performed using Student's t test for independent samples or ANOVA in the case of multiple comparisons. A value of P < 0.05 was considered significant.
Results
Myoblasts express CCRs and proliferate in response to chemokine ligands
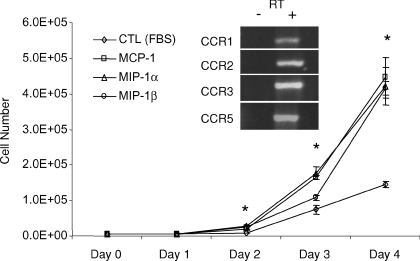
MIP-1α is a ligand for CCR1 and CCR5, MIP-β signalling occurs via CCR5, and MCP-1 is the ligand for CCR2. To determine the pattern of CC chemokine receptor expression in myoblasts, RT-PCR analysis was performed. As shown in Fig. 1, basal expression of CCR1, CCR2, CCR3 and CCR5 could be readily observed, whereas CCR4 expression was not detected (data not shown). To evaluate the effects of CC chemokine stimulation with MIP-1α, MIP-1β or MCP-1 (each at 100 ng ml−1) on myoblast proliferative behaviour, total cell number was determined every 24 h for up to 4 days. There were significant and comparable increases in myoblast cell number as compared to basal control conditions (5% FBS) observed after stimulation with MIP-1α, MIP-1β and MCP-1. As demonstrated in Fig. 1, the increases in myoblast cell number induced by chemokine exposure became statistically significant after 48 h of chemokine stimulation.
Figure 1. Effects of CC chemokines on myoblast cell number.
Time-dependent increases in myoblasts in the presence of control vehicle or stimulation by the individual CC chemokines (each at 100 ng ml−1) are depicted. Note that by 48 h, all chemokines were associated with significantly increased numbers of myoblasts in comparison to the control conditions. In keeping with the above, expression of CCR1, CCR2, CCR3 and CCR5 was demonstrated by RT-PCR on RNA isolated from C2C12 myoblasts. In all cases, negative controls lacking reverse transcriptase were included in order to exclude genomic DNA contamination (RT–).
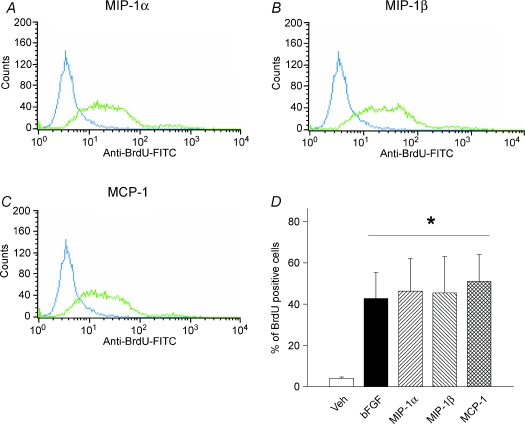
To further confirm that the above CC chemokines are capable of inducing mitogenic responses in myoblasts, we next monitored BrdU incorporation in C2C12 myoblasts incubated with MIP-1α, MIP-1β or MCP-1 for 24 h. The myoblast population undergoing active DNA synthesis was quantified by flow cytometry, as shown in Fig. 2. Under basal control conditions, only 4 ± 0.6% of myoblasts were BrdU-positive, while basic fibroblast growth factor (bFGF; also known as FGF2; at 10 ng ml−1), a classical mitogen for skeletal muscle cells, greatly increased the population of cells incorporating BrdU to 43 ± 13%. In a similar manner, myoblasts exposed to MIP-1α, MIP-1β or MCP-1 also exhibited strong mitogenic responses, with the average percentage of BrdU-positive cells amounting to 46 ± 16%, 45 ± 18% and 51 ± 13%, respectively.
Figure 2. MIP-1α, MIP-1β and MCP-1 stimulate DNA synthesis by cultured myoblasts.
BrdU incorporation was evaluated by flow cytometry after stimulating the cells with control vehicle (blue line) or each of the individual chemokines (green line) at 100 ng ml−1 for 24 h. Representative data are shown for: A, MIP-1α; B, MIP-1β; and C, MCP-1. Group mean values from 3 independent experiments are shown in D. *P < 0.05 for values obtained with chemokine stimulation as compared to its control vehicle.
Roles of CCR2 and CCR5 in chemokine-induced myoblast proliferation
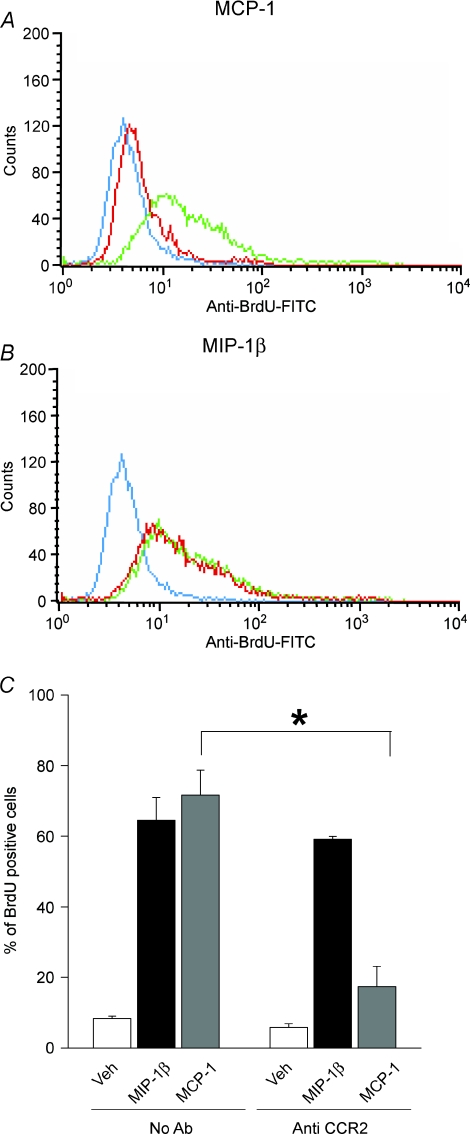
Studies were next performed to confirm that the mitogenic responses of myoblasts to chemokine ligands were in fact mediated by their cognate receptors. The myoblasts were incubated with antibodies directed against CCR2 or CCR5 prior to being stimulated with chemokines and analysed for BrdU incorporation as described earlier. When myoblasts were treated with anti-CCR2 antibody and subsequently exposed to MCP-1, the level of BrdU incorporation induced by MCP-1 was greatly reduced, as indicated in Fig. 3A and C. In contrast, mitogenic responses to ligands which do not bind to CCR2, such as MIP-1β (Fig. 3B and C) and bFGF (data not shown), were not affected by the anti-CCR2 antibody treatment.
Figure 3. Blockade of CCR2 inhibits DNA synthesis triggered by MCP-1 but not MIP-1β.
BrdU incorporation was measured in myoblasts preincubated for 90 min with antibody directed against CCR2 (MC-21, 5 μg ml−1) and subsequently stimulated with MCP-1 or MIP-1β (each at 100 ng ml−1) for 24 h. A and B, representative flow cytometry data (blue line, control; green line, chemokine stimulation; red line, chemokine stimulation + anti-CCR2 antibody treatment). C, corresponding mean values from 3 independent experiments. *P < 0.005 for comparison of values obtained with or without anti-CCR2 antibody.
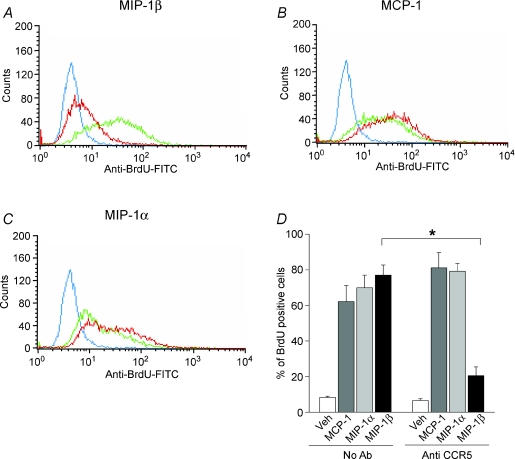
The effects of treating myoblasts with anti-CCR5 antibody prior to addition of the CCR5 ligand, MIP-1β, were also examined. As demonstrated in Fig. 4A and D, the level of BrdU incorporation induced by MIP-1β was reduced severalfold by anti-CCR5 treatment. In addition, the mitogenic responses to ligands which do not signal through CCR5, i.e. MCP-1 (see Fig. 4B and D) and bFGF (not shown), were not affected by the anti-CCR5 antibody treatment. Interestingly, the mitogenic response to MIP-1α, which does employ CCR5 as a receptor, was also unaffected by anti-CCR5 antibody (see Fig. 4C and D). This last finding indicates that MIP-1α is capable of inducing myoblast proliferative responses through an alternative receptor, and is consistent with the RT-PCR data demonstrating that another MIP-1α receptor (CCR1) is also expressed by myoblasts. Therefore, when taken together, our data suggest that myoblast proliferation can be mediated through interactions involving MCP-1–CCR2, MIP-1β–CCR5, and possibly MIP-1α–CCR1.
Figure 4. Blockade of CCR5 inhibits DNA synthesis triggered by MIP-1β but not MCP-1 or MIP-1α.
BrdU incorporation was measured in myoblasts preincubated for 90 min with antibody directed against CCR5 (MC-67, 10 μg ml−1) and subsequently stimulated with each of the above chemokines (all at 100 ng ml−1) for 24 h. A to C, representative flow cytometry data (blue line, control; green line, chemokine stimulation; red line, chemokine stimulation + anti-CCR5 antibody treatment). D, corresponding mean values from 3 independent experiments. *P < 0.005 for comparison of values obtained with or without anti-CCR5 antibody.
Chemokine-induced myoblast proliferation is dependent upon ERK1/2 MAPK and Gαi
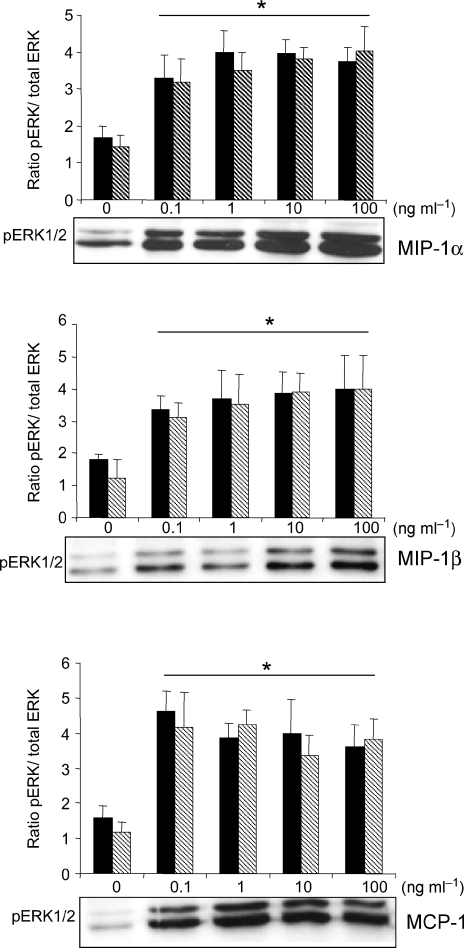
As growth factor-dependent myoblast proliferation has been associated with activation of the extracellular signal-regulated kinases (ERK1/2) of the MAPK family (Milasincic et al. 1996; Wu et al. 2000; Tortorella et al. 2001), experiments were performed to determine whether the mitogenic effects of MIP-1α, MIP-1β and MCP-1 are mediated through this pathway. Figure 5 shows that stimulation of myoblasts with MIP-1α, MIP-1β or MCP-1 induced strong activation of the ERK1/2 MAPK pathway, as indicated by increased levels of phosphorylated ERK1/2 (pERK) relative to total ERK at 10 min post-stimulation. Interestingly, maximal phosphorylation of ERK1/2 was already apparent at 0.1 ng ml−1 for each of the above chemokines.
Figure 5. MIP-1α, MIP-1β and MCP-1 stimulate ERK1/2 phosphorylation in myoblasts.
Cells were maintained in stimulation medium for 24 h and then treated with MIP-1α, MIP-1β or MCP-1 (0.1–100 ng ml−1) for 10 min. Western blot analyses of cellular lysates (20 μg total protein per lane) were carried out using specific antibodies against pERK1/2 and total ERK1/2. Representative immunoblots are shown, along with corresponding group mean data from 3 independent experiments (the left- and right-hand bars represent ERK1 and ERK2, respectively). *P < 0.05 for values obtained with chemokine stimulation as compared to its control vehicle.
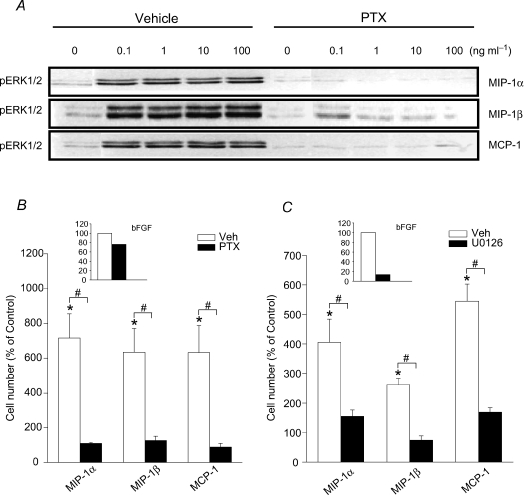
The G protein-coupled chemokine receptors targeted by MIP-1α, MIP-1β and MCP-1 are dependent upon activation of the Gαi subunit. Therefore, the latter was inhibited by pertussis toxin (PTX) prior to chemokine stimulation, in order to determine the relationship between Gαi activation and ERK1/2 MAPK phosphorylation. As shown in Fig. 6A, ERK1/2 phosphorylation by the above chemokines in myoblasts is Gαi subunit-dependent, since PTX was able to abrogate ERK phosphorylation induced by MIP-1α, MIP-1β and MCP-1. At the highest chemokine dosage employed (100 ng ml−1), the changes in pERK/total ERK induced by PTX in chemokine-stimulated myoblasts (expressed as a percentage of non-stimulated control values) amounted to the following: for MIP-1α the pERK/total ERK ratio was reduced from 306% to 158% control; for MIP-1β reduced from 275% to 105% control; and for MCP-1 reduced from 286% to 95% control. Importantly, the increases in myoblast cell number induced by chemokine stimulation were also greatly inhibited by PTX, as shown in Fig. 6B.
Figure 6. Gαi and ERK1/2 MAPK signalling are involved in myoblast proliferation induced by CC chemokines.
A, representative immunoblots demonstrating the ability of PTX (200 ng ml−1 applied overnight) to block chemokine-induced ERK1/2 phosphorylation in C2C12 myoblasts. Total myoblast cell numbers at day 4 were decreased severalfold in chemokine-stimulated cultures treated with either PTX (B) or U0126 (C) (smaller inset graphs within B and C show the percentage inhibition of myoblast cell numbers by PTX or U0126 after stimulation with bFGF under the same conditions). Data are group mean values from 3 independent experiments, expressed as a percentage of the values obtained without chemokine stimulation. *P < 0.05 for comparison of values obtained with or without chemokine stimulation; #P < 0.05 for comparison of values obtained with chemokine stimulation during incubation with PTX and U0126 or their respective vehicles (0.1% BSA for PTX; 0.1% DMSO for U0126).
To more directly establish the requirement for activation of ERK1/2 MAPK in chemokine-induced myoblast proliferation, we also employed the MEK inhibitor U0126 as a method of interfering with the activation of this pathway. As expected, exposure of the cells to U0126 (10 μm) for 30 min prior to chemokine stimulation (100 ng ml−1) greatly reduced the level of ERK1/2 phosphorylation induced by CC chemokines. The magnitude of the reductions in pERK/total ERK induced by U0126 pre-treatment of chemokine-stimulated myoblasts (once again expressed as a percentage of non-stimulated control values) were as follows: from 491% to 102% for MIP-1α, 210% to 73% for MIP-1β and 591% to 70% for MCP-1. As expected, inhibition of ERK1/2 activation by U0126 also interfered with the abilities of MIP-1α, MIP-1β and MCP-1 to induce myoblast proliferation (see Fig. 6C). In bFGF-stimulated cultures, only U0126 had major effects on myoblast cell numbers as shown within the inset graphs in Fig. 6B and C, and neither PTX nor U0126 induced an increase in cell death (determined by trypan blue exclusion). Therefore, our findings collectively indicate that both PTX-sensitive (Gαi) and ERK1/2 MAPK-dependent signalling pathways regulate the myoblast proliferative responses induced by MIP-1α, MIP-1β and MCP-1.
Chemokines accelerate myoblast wound closure in vitro and delay post-mitotic differentiation of myoblasts in vivo
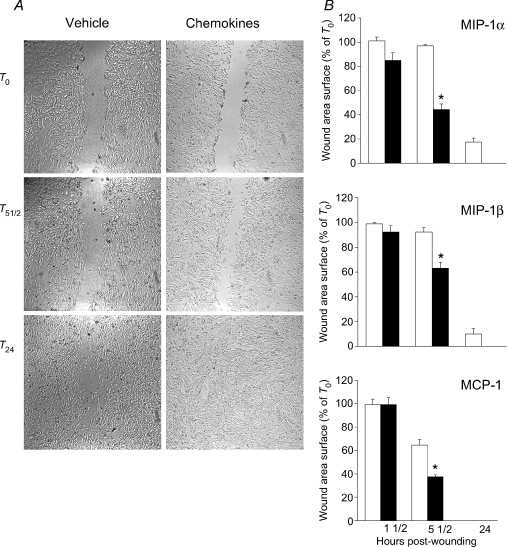
We next evaluated the abilities of MIP-1α, MIP-1β and MCP-1 to impact upon recovery from myogenic wound healing in vitro, using a model of mechanical disruption in a confluent monolayer of cultured myoblasts. Figure 7A shows that mechanical disruption of the myoblasts resulted in a well-delineated wound bed devoid of cells, and the size of the initial wound was not significantly different among groups. At the earliest time point (1½ h) post-wounding, there were no significant differences in the degree of wound space closure among groups. However, by 5½ h post-wounding, there were clear differences between myoblast cultures treated with MIP-1α, MIP-1β or MCP-1, and their corresponding controls. Hence a significantly smaller wound space area was found in the chemokine-treated groups, as shown in Fig. 7B. In addition, while no longer statistically significant at 24 h post-wounding, it is interesting to note that complete closure of the wound space had occurred in all three chemokine-treated groups, whereas closure remained incomplete in 2 of the 3 corresponding control groups at 24 h.
Figure 7. MIP-1α, MIP-1β and MCP-1 promote an acceleration of myogenic wound healing in vitro.
Each of the above chemokines (100 ng ml−1) or its vehicle were added to myoblast cultures immediately after cellular wounding (T0), and microscopic images were then obtained at the identical region of the plate at serial time points post-wounding (51/2 and 24 h; T51/2 and T24, respectively). A, representative images showing the time course of wound space closure obtained with and without the addition of MIP-1β immediately after cellular wounding. B, group mean values from 3 independent experiments, showing the effects of the individual CC chemokines on wound space area closure at different time points. *P < 0.05 for values obtained with chemokine stimulation (filled bars) as compared to its control vehicle (open bars) at each time point.
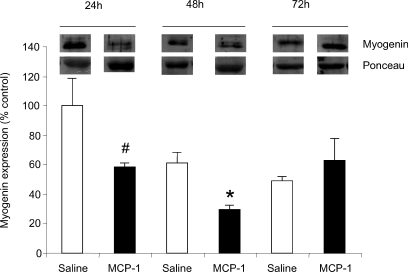
Finally, using a classical cardiotoxin injury-regeneration model, we sought to determine whether a CC chemokine with proliferative effects on myoblasts in vitro is also capable of modifying the muscle regeneration process in vivo. We selected MCP-1 for this experiment because among the chemokines examined in this investigation, it is the most studied in the skeletal muscle injury/regeneration context. Increased myoblast proliferation will necessarily be reflected by a delay in post-mitotic differentiation, and the latter can be readily assessed using specific markers of myoblast differentiation. Therefore, we evaluated the ability of MCP-1 treatment of the injured muscles to modify the expression of myogenin, a muscle-specific differentiation factor that is down-regulated when myoblasts are in the early proliferative stages of the regeneration process (Yablonka-Reuveni & Rivera, 1994; Yablonka-Reuveni & Rivera, 1997). Cardiotoxin injection itself caused a significant decline in myogenin protein expression, which is in keeping with the high prevalence of proliferating myoblasts induced by the injury. However, the superimposition of MCP-1 injections into cardiotoxin-injured skeletal muscles further decreased expression of myogenin at 24 h (42% reduction compared to saline; P = 0.08) and 48 h (51% reduction compared to saline; P < 0.05) post-injury (see Fig. 8), a finding consistent with a mitogenic effect on myoblasts which transiently delays the differentiation gene program under these conditions.
Figure 8. MCP-1 treatment of injured muscle transiently down-regulates the myogenic differentiation marker myogenin in vivo.
Western blot analyses of total muscle homogenates were carried out (50 μg total protein per lane), and the values are expressed as a percentage of the mean 24 h value in saline-treated mice. Ponceau red was used to assure equal protein loading. Although myogenin protein expression was transiently decreased in both saline (n = 3 mice per time point) and MCP-1 (n = 3 for 24 and 48 h; n = 2 for 72 h) groups after cardiotoxin-induced injury, reductions at early time points were greater in MCP-1-treated muscles. #P < 0.1 and *P < 0.05 for values obtained with MCP-1 injection (filled bars) as compared to saline vehicle (open bars) at each time point.
Discussion
Skeletal muscle is able to undergo extensive regeneration and repair after injury. Once activated by injury or other stimuli, satellite cells enter the cell cycle and begin to proliferate, at which point they are considered adult myoblasts. Muscle damage also produces factors that trigger the migration of satellite cells to sites of injury (Schultz et al. 1985; Watt et al. 1987). In this study, we employed C2C12 myoblasts as an in vitro model system, since these cells have been shown to proliferate and migrate to form muscle fibres when implanted into mouse muscles in vivo (Watt et al. 1994), and are frequently used to investigate mechanisms of myogenesis (Milasincic et al. 1996; Wu et al. 2000; Tortorella et al. 2001). Here we provide evidence for a chemokine-mediated signal transduction pathway within myoblasts that can be directly linked to their regenerative function in skeletal muscle, starting from the initial interactions with their relevant chemokine receptors and proceeding downstream to signalling events associated with the mitogenic responses subsequently generated.
We focused our investigation upon several CC chemokines (MCP-1, MIP-1α and MIP-1β) that are highly expressed by skeletal muscle in pathological situations (Zlotnik & Yoshie, 2000). Our results show that myoblasts express receptors for these chemokines, and that they trigger potent mitogenic effects on myoblasts which are critically dependent upon the presence of intact signalling through both G proteins (Gαi) and the extracellular signal-regulated kinases (ERK1/2) of the mitogen-activated protein kinase (MAPK) pathway. In addition, using both in vitro and in vivo models, we demonstrate that CC chemokines are able to significantly modify the muscle repair process after the induction of skeletal muscle injury. Taken together, our findings point to a previously unrecognized role for the CC chemokine system in the direct regulation of myoblast behaviour, which could have significant implications for therapeutic strategies aimed at enhancing the muscle regeneration process in muscular dystrophies and other forms of destructive muscle pathology.
The chemokines exert their biological effects by activating seven transmembrane G protein-coupled receptors (Zlotnik & Yoshie, 2000). It is increasingly evident that chemokines can exert multiple functions that extend well beyond their more established effects on leucocyte activation and trafficking. For example, chemokines have been shown to have important effects on non-myeloid cell types as diverse as endothelial cells (Salcedo et al. 2000), synoviocytes (Garcia-Vicuna et al. 2004), neural cells (Klein et al. 1999), and smooth muscle cells (Chandrasekar et al. 2004; Schecter et al. 2004). More recently, interference with SDF-1 (CXCL12) signalling through its cognate receptor, CXCR4, was found to be associated with impaired migration and increased apoptosis of skeletal muscle progenitor cells during embryogenesis (Vasyutina et al. 2005).
Within a given chemokine family (CXC, CC, C or CX3C), several different chemokine ligands are frequently capable of activating the same receptor, and it is not unusual for a single chemokine ligand to target more than one receptor member within the same family (Zlotnik & Yoshie, 2000; Locati et al. 2005). For example, it is well established that MIP-1α signals through both CCR1 and CCR5, whereas MIP-β signalling occurs via CCR5 (Locati et al. 2005). In addition, while it is generally considered that MCP-1 binds more or less exclusively to CCR2, evidence has been presented for an alternative but as yet unidentified receptor (Schecter et al. 2004). In our study, at least one receptor for each of the CC chemokines tested was found to be expressed in myoblasts, and we found that MCP-1, MIP-1α and MIP-β were all able to stimulate myoblasts to enter the S phase of the cell cycle to an equivalent degree. Moreover, the specificity of mitogenic responses to individual chemokines was confirmed by the ability of neutralizing antibodies directed against their known receptors to block these effects, whereas isotypic antibodies against non-cognate receptors had no significant impact.
In other cell types studied to date, the majority of chemokine-induced responses are inhibited by PTX, which is consistent with Gαi protein family members being the primary transduction partners of the chemokine receptors. In keeping with the above, we found that chemokine-induced myoblast proliferative responses were also inhibited by PTX. Gαi proteins are enriched within the t-tubule invaginations of the muscle fibre membrane (Doucet & Tuana, 1991), and several PTX-sensitive mechanisms in skeletal muscle have been described (Vandenburgh et al. 1995; Fedorov et al. 1998; Gosmanov et al. 2002). Furthermore, our results demonstrate that the effects of MCP-1 and MIP-1β on myoblast proliferation are for the most part mediated through CCR2 and CCR5, respectively. On the other hand, the mitogenic effects of MIP-1α were completely unaffected by inhibition of either CCR2 or CCR5, despite the latter being a major receptor for this ligand. This finding suggests (but does not prove) that under these conditions, MIP-1α is capable of acting to a large extent through its alternative receptor, CCR1, which is also expressed in myoblasts. Indeed, we have previously reported that CCR1 is expressed by mouse muscle fibres in vivo, and that CCR1 mRNA transcript levels are significantly up-regulated in the context of pro-inflammatory stimulation by cytokines as well as in dystrophic skeletal muscle (Demoule et al. 2005). Interestingly, it has been reported that human CCR2 and CCR5 can heterodimerize and activate Gq and/or G11 rather than Gαi-dependent signalling pathways (Mellado et al. 2001). Whether such alternative signalling pathways, involving heterodimers of different receptors and alternate G protein subunits, also exist in skeletal muscle cells under certain physiological conditions, remains to be determined.
Several different signalling pathways have been implicated in the proliferative responses of myoblasts to various growth factors, including JAK2-STAT3 (Spangenburg & Booth, 2002), PI3K/Akt (Milasincic et al. 1996), and the MAPKs (Wu et al. 2000; Tortorella et al. 2001; Penn et al. 2001). The ERK1/2 MAPK pathway is most classically triggered by growth factor binding, and is activated in skeletal muscle in response to physical exercise or injury (Nakamura et al. 2005; Sakamoto & Goodyear, 2002). Most studies have found that ERK1/2 activation promotes myoblast proliferation (Bennett & Tonks, 1997; Coolican et al. 1997; Wu et al. 2000), and this effect appears to be dependent upon the timing of ERK1/2 activation relative to induction of the myogenic program (Penn et al. 2001). For example, bFGF (FGF2), a potent myoblast mitogen and inhibitor of differentiation, induces peak activation of ERKs within 2–10 min, and blocking this effect with a MEK inhibitor prevents FGF-induced myoblast proliferation (Milasincic et al. 1996; Tortorella et al. 2001; Jones et al. 2001). Similarly, while IGF-1 and -2 are able to stimulate either proliferation or differentiation of myoblasts depending upon experimental conditions, it appears that ERKs are primarily involved in IGF-mediated proliferative effects on myoblasts (Coolican et al. 1997; Jones et al. 2001; Adi et al. 2002). Our data indicate that ERK1/2 activation is absolutely required to allow for the observed proliferative responses of myoblasts to CC chemokine stimulation. In addition, previous work has implicated the ERK1/2 MAPK pathway in the myoblast migratory response to cell wounding (Yeow et al. 2002). Indeed, the accelerated wound space closure observed in our study after chemokine treatment was probably due in large part to increases in the migratory behaviour of myoblasts, since the differences occurred at a much earlier time point than was observed for chemokine-induced increases in myoblast cell number.
After experimental muscle injury in vivo, there is an early up-regulation of MIP-1α, MIP-β and MCP-1, which corresponds temporally to the proliferative behaviour of myoblasts within the injured muscles (Grounds & McGeachie, 1992; Hirata et al. 2003). However, due to the fact that cardiotoxin-induced injury also triggers the widespread proliferation of several other cell types within the muscle in vivo, we found that this high background noise level precluded accurate quantification of chemokine-induced changes in standard proliferation markers within the specific myoblast population of interest. Therefore, we employed myogenin as an alternative marker, since its expression reflects declining myoblast proliferation and transition toward the post-mitotic differentiated muscle fibre. In this regard, prior studies have shown that other growth factors which induce myoblast proliferation in an ERK-dependent manner also transiently suppress the expression of myogenin (Yablonka-Reuveni & Rivera, 1997), and that the onset of myogenin expression temporally follows the decline in cells positive for proliferating cell nuclear antigen (Yablonka-Reuveni & Rivera, 1994). Accordingly, our in vivo studies are also consistent with a CC chemokine-induced mitogenic effect on myoblasts, since we demonstrate that myogenin expression is suppressed to a greater degree during the early phases of regeneration when the injured muscles are treated by intermittent injections of MCP-1. However, while this experiment demonstrates that MCP-1 can modulate myogenesis in vivo, we cannot rule out the possibility that MCP-1 influenced the interaction between myoblasts and leucocytes under these conditions, since both cell types are present within the in vivo environment after injury.
Hereditary muscle pathologies such as muscular dystrophies, as well as traumatic or exercise-induced muscle injuries, are associated with a significant inflammatory response within the muscle (Tidball, 2005). In models of chronic muscle disease such as the muscular dystrophies and other myopathies, the presence of inflammatory cells and/or their mediators within the muscle has been associated with an aggravation of muscle pathology (Wehling et al. 2001; Gosselin & McCormick, 2004; Acharyya et al. 2007). Both neutrophils and macrophages also have the capacity to kill muscle cells (Nguyen & Tidball, 2003). On the other hand, macrophages recruited to areas of acutely damaged muscle have been shown to promote more effective muscle repair (Lescaudron et al. 1999; Summan et al. 2006; Tidball & Wehling-Henricks, 2007). This dichotomy is probably related to the presence of different subpopulations of macrophages, which express specialized and polarized functions under the influence of different environmental cues (Arnold et al. 2007; Mantovani et al. 2007). Chemokines are excellent candidate molecules for playing a central role in regulating the proportions of different subpopulations of macrophages and other leucocytes within damaged muscles, as well as the chronicity of inflammation and efficacy of the subsequent muscle remodelling response. In principle, the chemokines released from damaged muscles under these conditions could originate from multiple sources including non-muscle cell types (e.g. resident macrophages, endothelial cells, etc.) and infiltrating leucocytes, as well as the muscle cells themselves.
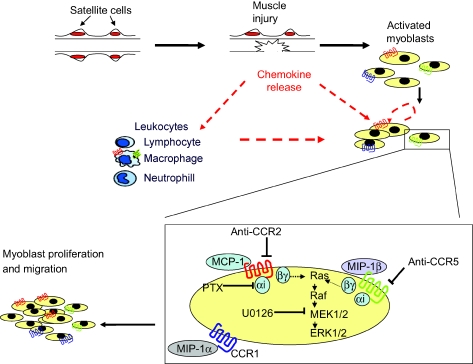
Based upon our findings, a dual role for the CC chemokines expressed by injured muscle is thus suggested (see Fig. 9): one being a direct local effect on myoblasts to stimulate their proliferation, and the other consisting of a more distant effect on leucocytes to induce their trafficking to sites of muscle damage. Achieving the appropriate equilibrium between these two functions of chemokines is likely to be a critically important determinant of the ultimate success of the muscle repair process. Therefore, interventions aimed at modulating the balance between these distinct roles of CC chemokines in injured muscle could represent a novel strategy for the treatment of destructive muscle pathologies.
Figure 9. Proposed mechanisms by which CC chemokines can participate in the muscle repair process.
Chemokines can potentially be released from damaged muscle fibres, other resident cell types within the muscle, infiltrating leucocytes or myoblasts themselves. After binding to their receptors (CCR1, CCR2, CCR5) on myoblasts, the chemokines induce phosphorylation of ERK1/2 MAPK through a Gαi and MEK-dependent signalling pathway. This leads to myoblast proliferation and most probably enhanced migration, which is later followed by differentiation and eventual reconstitution of the mature muscle fibre.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Fonds de la recherche en sante du Quebec.
References
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, et al. Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adi S, Bin-Abbas B, Wu NY, Rosenthal SM. Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology. 2002;143:511–516. doi: 10.1210/endo.143.2.8648. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer YRN, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, IκB kinase, and nuclear factor-κB and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279:3188–3196. doi: 10.1074/jbc.M311660200. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Bernasconi P, Megna P, Galbiati S, Cornelio F, Mantegazza R. Increased expression of β-chemokines in muscle of patients with inflammatory myopathies. J Neuropathol Exp Neurol. 2000;59:164–169. doi: 10.1093/jnen/59.2.164. [DOI] [PubMed] [Google Scholar]
- Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol. 2006;292:C953–967. doi: 10.1152/ajpcell.00154.2006. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- De Rossi M, Bernasconi P, Baggi F, de Waal MR, Mantegazza R. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol. 2000;12:1329–1335. doi: 10.1093/intimm/12.9.1329. [DOI] [PubMed] [Google Scholar]
- Demoule A, Divangahi M, Danialou G, Gvozdic D, Larkin G, Bao W, Petrof BJ. Expression and regulation of CC class chemokines in the dystrophic (mdx) diaphragm. Am J Respir Cell Mol Biol. 2005;33:178–185. doi: 10.1165/rcmb.2004-0347OC. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Tuana BS. Identification of low molecular weight GTP-binding proteins and their sites of interaction in subcellular fractions from skeletal muscle. J Biol Chem. 1991;266:17613–17620. [PubMed] [Google Scholar]
- Fedorov YV, Jones NC, Olwin BB. Regulation of myogenesis by fibroblast growth factors requires βγ subunits of pertussis toxin-sensitive G proteins. Mol Cell Biol. 1998;18:5780–5787. doi: 10.1128/mcb.18.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, Diaz-Gonzalez F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for β-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1025–C1032. doi: 10.1152/ajpcell.00096.2002. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, McCormick KM. Targeting the immune system to improve ventilatory function in muscular dystrophy. Med Sci Sports Exerc. 2004;36:44–51. doi: 10.1249/01.MSS.0000106185.22349.2C. [DOI] [PubMed] [Google Scholar]
- Grounds MD, McGeachie JK. Skeletal muscle regeneration after crush injury in dystrophic mdx mice: an autoradiographic study. Muscle Nerve. 1992;15:580–586. doi: 10.1002/mus.880150508. [DOI] [PubMed] [Google Scholar]
- Harfouche R, Hussain SN. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol. 2006;291:H1635–H1645. doi: 10.1152/ajpheart.01318.2005. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, Motoyoshi K, Kamakura K, Miyagoe-Suzuki Y, Takeda S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003;163:203–215. doi: 10.1016/S0002-9440(10)63644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Fedorov YV, Rosenthal RS, Olwin BB. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J Cell Physiol. 2001;186:104–115. doi: 10.1002/1097-4652(200101)186:1<104::AID-JCP1015>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Locati M, Torre YM, Galliera E, Bonecchi R, Bodduluri H, Vago G, Vecchi A, Mantovani A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005;16:679–686. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Machida S, Spangenburg EE, Booth FW. Forkhead transcription factor FoxO1 transduces insulin-like growth factor's signal to p27Kip1 in primary skeletal muscle satellite cells. J Cell Physiol. 2003;196:523–531. doi: 10.1002/jcp.10339. [DOI] [PubMed] [Google Scholar]
- Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, Jones DR, Toran JL, Martinez A. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve. 1999;22:724–732. doi: 10.1002/(sici)1097-4598(199906)22:6<724::aid-mus9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Milasincic DJ, Calera MR, Farmer SR, Pilch PF. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol Cell Biol. 1996;16:5964–5973. doi: 10.1128/mcb.16.11.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 2000;113:407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Yoshida K, Ueda H, Takeda S, Ikeda S. Up-regulation of mitogen activated protein kinases in mdx skeletal muscle following chronic treadmill exercise. Biochim Biophys Acta. 2005;1740:326–331. doi: 10.1016/j.bbadis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J Physiol. 2003;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn BH, Berkes CA, Bergstrom DA, Tapscott SJ. How to MEK muscle. Mol Cell. 2001;8:245–246. doi: 10.1016/s1097-2765(01)00331-8. [DOI] [PubMed] [Google Scholar]
- Porter JD, Guo W, Merriam AP, Khanna S, Cheng G, Zhou X, Andrade FH, Richmonds C, Kaminski HJ. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscul Disord. 2003;13:223–235. doi: 10.1016/s0960-8966(02)00242-0. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyna S, Krolick KA. Chemokine production by rat myocytes exposed to interferon-γ. Clin Immunol. 2000;94:105–113. doi: 10.1006/clim.1999.4828. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyna S, Stegall T, Krolick KA. Muscle responds to an antibody reactive with the acetylcholine receptor by up-regulating monocyte chemoattractant protein 1: a chemokine with the potential to influence the severity and course of experimental myasthenia gravis. J Immunol. 2002;169:1579–1586. doi: 10.4049/jimmunol.169.3.1579. [DOI] [PubMed] [Google Scholar]
- Robertson TA, Maley MA, Grounds MD, Papadimitriou JM. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993;207:321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Schecter AD, Berman AB, Yi L, Ma H, Daly CM, Soejima K, Rollins BJ, Charo IF, Taubman MB. MCP-1-dependent signaling in CCR2−/− aortic smooth muscle cells. J Leukoc Biol. 2004;75:1079–1085. doi: 10.1189/jlb.0903421. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol. 2002;283:C204–C211. doi: 10.1152/ajpcell.00574.2001. [DOI] [PubMed] [Google Scholar]
- Summan M, Warren GL, Mercer RR, Chapman R, Van Hulderman T RN, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- Vandenburgh HH, Shansky J, Solerssi R, Chromiak J. Mechanical stimulation of skeletal muscle increases prostaglandin F2 alpha production, cyclooxygenase activity, and cell growth by a pertussis toxin sensitive mechanism. J Cell Physiol. 1995;163:285–294. doi: 10.1002/jcp.1041630209. [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19:2187–2198. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- Warren GL, O'Farrell L, Summan M, Hulderman T, Mishra D, Luster MI, Kuziel WA, Simeonova PP. Role of CC chemokines in skeletal muscle functional restoration after injury. Am J Physiol Cell Physiol. 2004;286:C1031–C1036. doi: 10.1152/ajpcell.00467.2003. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Karasinski J, Moss J, England MA. Migration of muscle cells. Nature. 1994;368:406–407. doi: 10.1038/368406a0. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Morgan JE, Clifford MA, Partridge TA. The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol (Berl) 1987;175:527–536. doi: 10.1007/BF00309688. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 1997;15:1–27. doi: 10.3109/08977199709002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeow K, Cabane C, Turchi L, Ponzio G, Derijard B. Increased MAPK signaling during in vitro muscle wounding. Biochem Biophys Res Commun. 2002;293:112–119. doi: 10.1016/S0006-291X(02)00190-0. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]