Abstract
It is known that cerebral blood flow declines with age in sedentary adults, although previous studies have involved small sample sizes, making the exact estimate of decline imprecise and the effects of possible moderator variables unknown. Animal studies indicate that aerobic exercise can elevate cerebral blood flow; however, this possibility has not been examined in humans. We examined how regular aerobic exercise affects the age-related decline in blood flow velocity in the middle cerebral artery (MCAv) in healthy humans. Maximal oxygen consumption, body mass index (BMI), blood pressure and MCAv were measured in healthy sedentary (n = 153) and endurance-trained (n = 154) men aged between 18 and 79 years. The relationships between age, training status, BMI and MCAv were examined using analysis of covariance methods. Mean ±s.e.m. estimates of regression coefficients and 95% confidence intervals (95% CI) were calculated. The age-related decline in MCAv was −0.76 ± 0.04 cm s−1 year−1 (95% CI =−0.69 to −0.83, r2= 0.66, P < 0.0005) and was independent of training status (P = 0.65). Nevertheless, MCAv was consistently elevated by 9.1 ± 3.3 cm s−1 (CI = 2.7–15.6, P = 0.006) in endurance-trained men throughout the age range. This ∼17% difference between trained and sedentary men amounted to an approximate 10 year reduction in MCAv ‘age’ and was robust to between-group differences in BMI and blood pressure. Regular aerobic-endurance exercise is associated with higher MCAv in men aged 18–79 years. The persistence of this finding in older endurance-trained men may therefore help explain why there is a lower risk of cerebrovascular disease in this population.
Normal ageing is associated with marked structural and functional alterations in the cardiovascular and cerebrovascular systems, which are linked to neurophysiological and psychological changes (Matteis et al. 1998; Niehaus et al. 2001). Given the rapid increase in ageing and life expectancy, and the related medical costs associated with treatment of age-related disorders, identifying effective interventions to ameliorate the normal decline in cardiovascular and cerebrovascular function are critical. Engagement in regular aerobic-endurance exercise is associated with enhanced systemic arterial endothelial function, reduced large elastic artery stiffness, and a reduced risk of arterial atherosclerotic clinical disease in middle-aged and older adults (Clarkson et al. 1999; DeSouza et al. 2000; Taddei et al. 2000). Furthermore, the results of longitudinal studies indicate that improvements in cardiovascular fitness can provide a positive effect on human cognitive abilities (Kramer et al. 1999), potentially offsetting declines in cerebral tissue density (Colcombe et al. 2003) and increasing brain volume in ageing humans (Colcombe et al. 2006). Animal studies have clearly identified that voluntary physical exercise improves long-term stroke outcome by mechanisms related to improved angiogenesis and elevations in cerebral blood flow (CBF) (Endres et al. 2003; Gertz et al. 2006). It therefore seems possible that habitual exercise favourably maintains cerebral perfusion during otherwise healthy ageing. The aim of this study was to provide the first large-scale systematic examination of the association between physical fitness and cerebral blood flow, as estimated using transcranial Doppler ultrasound. The study used rigorously screened healthy humans to avoid known effects of some prevalent age-related diseases on cerebral blood flow.
Methods
Subjects
A total of 307 healthy men aged 18–79 years were studied. For at least the previous 2 years (range 2–31 years), participants were either sedentary (no regular physical activity; n = 153) or were endurance exercise-trained (vigorous aerobic-endurance exercise more than 4 times per week and competing in local road running or cycling races; n = 154). The endurance-trained group and sedentary group were specifically recruited on the basis of life-long physical activity or inactivity, respectively. The training history information revealed that, in the sedentary group, there were no reports of progressive training prior to a 2 year period of inactivity. The higher aerobic capacity throughout ageing in the trained group was confirmed by means of an incremental exercise test to volitional exhaustion. Across the full age range in our study, the mean (s.e.m.) values for 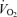 peak were 34.9 (0.4) ml kg−1 min−1 for the sedentary group compared with 52.4 (0.4) ml kg−1 min−1 for the active group (P < 0.0005). Participants were included if they were normotensive (< 140/90 mmHg), non-smokers, non-obese and free of overt chronic diseases, as assessed by detailed medical history, physical examination and 12-lead ECG. None of the participants were taking any medication. The study was approved by the Local Ethical Committee, conformed to the standards set by the Declaration of Helsinki, and written informed consent was obtained.
peak were 34.9 (0.4) ml kg−1 min−1 for the sedentary group compared with 52.4 (0.4) ml kg−1 min−1 for the active group (P < 0.0005). Participants were included if they were normotensive (< 140/90 mmHg), non-smokers, non-obese and free of overt chronic diseases, as assessed by detailed medical history, physical examination and 12-lead ECG. None of the participants were taking any medication. The study was approved by the Local Ethical Committee, conformed to the standards set by the Declaration of Helsinki, and written informed consent was obtained.
Experimental design
Following familiarization of each subject, participants arrived at the laboratory having abstained from exercise and alcohol for 24 h, and having not consumed a heavy meal or items containing caffeine for 4 h. To ascertain the reproducibility of the transcranial Doppler measurements, 71 subjects returned to the laboratory (>1 week) at the same time of day following the first monitoring period.
Measurements
Measurements were performed with subjects in the supine position following a minimum of 15 min of rest. Blood flow velocity in the right or left middle cerebral artery was measured using a 2 MHz pulsed Doppler ultrasound system (DWL Doppler, Sterling VA, USA) using search techniques described elsewhere (Aaslid et al. 1982). The Doppler probe was secured with a headband device (Spencer Technologies, Nicolet Instruments, Madison, WI, USA) to maintain optimal insonation position and angle throughout the protocol. End diastolic, peak systolic and mean cerebral blood flow velocities were recorded automatically. The mean MCAv was defined as: 1/3 (peak systolic flow velocity + 2 × end diastolic flow velocity). In 89 subjects, across the age ranges, bilateral MCAv was obtained. As there were no significant differences between right and left MCAv, the average value was used. Beat-to-beat arterial blood pressure and heart rate were measured using finger photoplethysmography (Finometer, TPD Biomedical Instrumentation, the Netherlands) and ECG, respectively. Manual blood pressure recordings were intermittently used to confirm the accuracy of the finger photoplethysmography measurements. End-tidal CO2 was sampled from a leak-free mask and measured by a gas analyser (model CD-3A CO2 analyser, AEI Technologies, Pittsburgh, PA, USA). All data were acquired continuously at 200 Hz using an analog-to-digital converter (Powerlab/16SP ML795; ADInstruments, Colorado Springs, CO, USA) interfaced with a computer, and were subsequently analysed using commercially available software (Chart version 5.02, ADInstruments). Cerebrovascular resistance index (CVRi) was calculated as MAP/MCAv.
Maximal oxygen consumption
On a different day, subjects performed incremental treadmill (n = 211) or cycling (n = 96) exercise to exhaustion for the direct determination of maximal oxygen consumption (aerobic fitness) from continuous measurement of their respiratory gas exchanges. The exercise was considered to be maximal when three of the following criteria were obtained: no change in oxygen consumption while increasing workload (levelling off criterion), respiratory exchange ratio > 1.1, heart rate (HR) within 10% of maximal predicted value, and maximal rating of perceived exertion or the inability of the subjects to maintain the pedalling or running frequency despite maximum effort and verbal encouragement. The maximal oxygen consumption rates obtained in cycling tests were multiplied by 1.06 to adjust for the local fatigue effects and lower maximal values typically obtained from cycle tests (J. C. Cotter, unpublished findings). Following this adjustment, mean (s.e.m.) maximal oxygen consumption differed by only 0.6 (1.3) ml kg−1 min−1 between the participants who performed the cycling and treadmill tests. This difference was not statistically significant (P = 0.65) and was unaffected by the age of participants (P = 0.48).
Data analysis
After permitting a 5–10 min period to achieve steady-state respiratory gas exchange following placement of the facemask, all data were averaged over the subsequent 3–5 min. The relationships between age, training status, maximal oxygen consumption and MCAv were examined with linear least-squares regression analysis. Beta coefficients for regression slopes and intercepts were calculated along with the associated 95% confidence intervals (CI). In the first stage of our analysis, sample estimates of the slope and intercept for the age–MCAv and age–CVRi relationships were compared between physically active and inactive cohorts using analysis of covariance (Zar, 1999). Using the NQuery Advisor software (Statistical Solutions Ltd, Cork), statistical power was estimated for our primary outcome of MCAv and a regression model with two covariates (age and training status). For a multiple linear regression model with a ‘moderate’ squared multiple correlation of 0.3, it was estimated that a sample size of 300 would have 99% power to detect a ‘small’ increase in R2 of 0.1 due to including the additional covariate of training status (alpha = 0.05). In the second stage of our analysis, we examined the relationships between age, maximal oxygen consumption and MCAv using multiple regression analysis. Body mass index and mean arterial pressure were also entered as possible predictors of MCAv. A step-wise multiple regression was applied using the backward method of predictor entry (Field, 2005). Multicollinearity analysis was performed on all the statistically significant predictor variables in the model (Field, 2005). All data analysis was performed using the Statistical Package for the Social Sciences (version 14).
Results
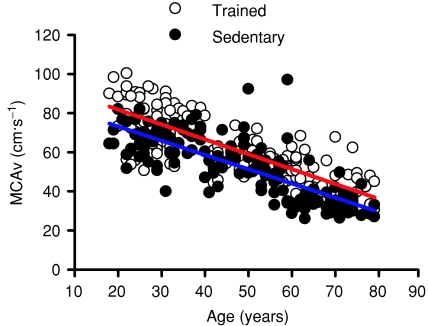
The day-to-day reproducibility of MCAv had a coefficient of variation of 4.5%. The 95% repeatability coefficient (Bland & Altman, 1999) was 6.8 cm s−1. This small magnitude of measurement error was consistent across the measurement range (30–80 cm s−1). The relationships between age, training status and MCAv are presented in Fig. 1. There was a progressive decline in MCAv of 0.76 ± 0.04 cm s−1 year−1 (CI = 0.69–0.83, r2= 0.66, P < 0.0005 (∼1% year−1)), which was independent of training status (P = 0.65). Nevertheless, mean MCAv was consistently elevated by 9.1 ± 3.3 cm s−1 (CI = 2.7–15.6, P = 0.006 (∼17%)) in endurance-trained men throughout the age range (Fig. 1). The regression equations, in cm s−1, were:
| (1) |
| (2) |
By solving the above regression equations for age and interpolating a typical mean MCAv of 50 cm s−1, it was calculated that the difference between trained and sedentary men amounted to an approximate 10 year reduction in MCAv equivalent ‘age’.
Figure 1. Relationship between age, cerebral blood flow velocity and physical fitness.
The red line represents linear regression for the endurance-trained group. The blue line represents linear regression for the sedentary group. MCAv was consistently elevated by 9.1 ± 3.3 cm s−1 [CI = 2.7–15.6, P = 0.006 (∼17%)] in endurance-trained men throughout ageing.
The age-related increase in CVRi was found to be 0.034 ± 0.002 mmHg cms−1 year−1 (CI = 0.030–0.038) for the untrained participants compared with 0.024 ± 0.001 mmHg cms−1 year−1 (CI = 0.022–0.027) for the trained participants. The difference between these slopes was statistically significant (P < 0.0005). No significant difference was found between the intercepts of these two slopes (P = 0.203).
The multiple regression analysis model was statistically significant (P < 0.0005). Age and maximal oxygen consumption were found to be the only statistically significant (P < 0.05) predictors of MCAv, with slopes of −0.56 (CI =−0.48 to −0.63) and 0.45 (CI = 0.35 to 0.56), respectively. The partial correlation between age and MCAv was −0.64 when all other variables were controlled statistically. The low variance inflation factors (< 1.8) and high tolerance statistics (> 0.5) indicated that the multicollinearity between age and maximal oxygen consumption was not a cause for concern. The coefficient of determination (r2) for the multiple regression model was 0.70 and the prediction equation was:
| (3) |
Discussion
Our findings provide the first evidence in humans that MCAv is elevated by habitual aerobic-endurance exercise, independent of possible confounding variables. Importantly, our reported decline in MCAv with age is consistent with previous reports of reductions in total CBF, as assessed using a variety of imaging techniques (Kashimada et al. 1994; Buijs et al. 1998; Scheel et al. 2000; Beason-Held et al. 2007; Stoquart-ElSankari et al. 2007). These studies collectively indicate that, because ageing is associated with global cerebral atrophy, the observed decreases in CBF reflect a global decrease in cerebral perfusion, without any disturbance of regional perfusion or oxygen consumption. Whilst acknowledging that different mechanisms might underlie the comparable training benefit in MCAv in the younger age group compared to that in the old age group, the findings from the present study strongly indicate that habitual physical exercise may potentially offset some of the ‘normal’ age-associated process of global cerebral atrophy.
We can only speculate on the mechanisms underlying these fitness-related changes in MCAv during healthy ageing. Physical training has beneficial effects on multiple cardiovascular risk factors such as dyslipidaemia, hypertension, diabetes and cardiovascular events (Clarkson et al. 1999). The effect of exercise on clinical outcome could also be partially related to a direct and independent positive effect of physical training on endothelial dysfunction in the conduit arteries (DeSouza et al. 2000) or in the peripheral microcirculation (Higashi et al. 1999; Shephard & Balady, 1999; Rinder et al. 2000; Franzoni et al. 2004). Thus, regular physical activity is associated with increased endothelium-dependent vasodilatation (Taddei et al. 2000) and NO availability. Interestingly, a link between endothelial function and cerebrovascular function has been reported (Lavi et al. 2006; Ainslie et al. 2007; Hoth et al. 2007), indicating a common pathway between these responses. Our results in human subjects are the first to confirm findings of highly controlled animal studies which have reported that physical exercise can elevate CBF (Endres et al. 2003; Gertz et al. 2006). These animal studies also provide evidence that such voluntary physical exercise not only improves long-term stroke outcome but also provides a prophylactic treatment strategy for increasing angiogenesis, CBF and reducing brain injury during cerebral ischaemia (Endres et al. 2003; Gertz et al. 2006). Moreover, voluntary exercise has also been demonstrated to enhance axonal regeneration and neurogenesis, long-term potentiation and learning (Carro et al. 2000; Vaynman et al. 2004). Although an up-regulation of endothelial NO synthase activity has been implicated as the key mechanism to increase CBF and reduce brain injury during cerebral ischaemia (Endres et al. 2003, 2004; Gertz et al. 2006), other factors such as insulin-like growth factor-1 and brain-derived neurotrophic factor have also been implicated as downstream mediators of the neuroprotective actions of exercise (Carro et al. 2000; Vaynman et al. 2004). In humans, the extent to which physical exercise could be used as a treatment strategy to improve CBF and reduce brain injury during cerebral ischaemia is unknown, but appears worthy of further investigation.
A noteworthy observation was that the age-related increase in CVRi was greater in the untrained participants compared with the trained participants. These training-induced differences occurred independently of any between-group differences in end-tidal 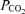 . Although sympathetic neural control of the cerebral circulation is controversial, it is known that cerebral arteries are richly innervated with sympathetic nerve fibres (Nielsen & Owman, 1967; Nelson & Rennels, 1970). Since there is progressive sympathoexcitation with human ageing (reviewed in Seals & Dinenno, 2004), it seems plausible that an exaggerated sympathetically mediated cerebral vasoconstriction might underlie the age-related increases in CVRi. It should be noted, however, that the extent to which sympathetically mediated cerebral vasoconstriction occurs with human ageing – or might be attenuated with physical training – is unknown. Likewise, whether this apparent elevation in CVRi during sedentary ageing was the cause of the reductions in MCAv or merely the consequence of cerebral atrophy and related cerebral hypoperfusion remains to be established.
. Although sympathetic neural control of the cerebral circulation is controversial, it is known that cerebral arteries are richly innervated with sympathetic nerve fibres (Nielsen & Owman, 1967; Nelson & Rennels, 1970). Since there is progressive sympathoexcitation with human ageing (reviewed in Seals & Dinenno, 2004), it seems plausible that an exaggerated sympathetically mediated cerebral vasoconstriction might underlie the age-related increases in CVRi. It should be noted, however, that the extent to which sympathetically mediated cerebral vasoconstriction occurs with human ageing – or might be attenuated with physical training – is unknown. Likewise, whether this apparent elevation in CVRi during sedentary ageing was the cause of the reductions in MCAv or merely the consequence of cerebral atrophy and related cerebral hypoperfusion remains to be established.
Methodological considerations
Although we used Doppler ultrasound to measure flow velocity, rather than blood flow, in the MCA, previous reports indicate that MCAv is a reliable index of cerebral blood flow (Kirkham et al. 1986; Giller et al. 1993; Valdueza et al. 1997; Serrador et al. 2000; Peebles et al. 2007). With transcranial Doppler, we monitored blood flow velocity in the MCA, an area that transports blood to large brain volumes including both grey and white matter. Thus, we cannot distinguish between any regional differences within these areas. Our study included only healthy non-smoking male adults without evidence of overt chronic diseases; as such, our results can only be generalized to this population. The possibility that habitual exercise could have even greater beneficial effects on cerebral function in smokers, adults with other risk factors, and patients with chronic disease (especially cardiovascular disease, atherosclerosis and type 2 diabetes) warrants further research. Due to our rigorous inclusion criteria, it is possible that even our sedentary group may be genetically protected from developing atherosclerosis; if so, we may be underestimating the impact of vigorous exercise on MCAv. It should be noted that we were not able to assess the possibility of any atherosclerosis of the intracranial vessels; however, the presence of any early undetected atherosclerosis of the intracranial cerebral vessels would tend to elevate, rather than reduce, MCAv and therefore would seem unlikely to explain the clear between fitness-group differences in MCAv.
In summary, the main findings of the present study indicate that as little as 2 years of regular aerobic-endurance exercise can improve MCAv throughout otherwise healthy human ageing. These results may serve as the point of reference for ‘normal’ responses for comparison against pathological disorders, and indicate how active living can potentially delay cerebrovascular related brain disease from occurring with ageing.
Acknowledgments
Funding from the Health Research Council of New Zealand; Sport and Recreational Council of New Zealand; University of Otago Research Grant; Department of Physiology, University of Otago; British Council. G.A. was supported by travel grants from The Physiological Society and The British Association of Sport and Exercise Sciences during the preparation of this manuscript.
References
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Murrell C, Peebles K, Swart M, Skinner MA, Williams MJ, Taylor RD. Early morning impairment in cerebral autoregulation and cerebrovascular CO2 reactivity in healthy humans: relation to endothelial function. Exp Physiol. 2007;92:769–777. doi: 10.1113/expphysiol.2006.036814. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Meth Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology. 1998;209:667–674. doi: 10.1148/radiology.209.3.9844657. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using SPSS. 2. London: Sage Publications; 2005. [Google Scholar]
- Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quinones-Galvan A. Effects of age and physical fitness on microcirculatory function. Clin Sci (Lond) 2004;106:329–335. doi: 10.1042/CS20030229. [DOI] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, Paul RH, Jefferson AL, Haley AP, Cohen RA. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimada A, Machida K, Honda N, Mamiya T, Takahashi T, Kamano T, Inoue Y, Osada H. [Measurement of cerebral blood flow in normal subjects by phase contrast MR imaging] (in Japanese) Nippon Igaku Hoshasen Gakkai Zasshi. 1994;54:1116–1125. [PubMed] [Google Scholar]
- Kirkham FJ, Padayachee TS, Parsons S, Seargeant LS, House FR, Gosling RG. Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: velocity as an index of flow. Ultrasound Med Biol. 1986;12:15–21. doi: 10.1016/0301-5629(86)90139-0. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke. 1998;29:963–967. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- Nelson E, Rennels M. Innervation of intracranial arteries. Brain. 1970;93:475–490. doi: 10.1093/brain/93.3.475. [DOI] [PubMed] [Google Scholar]
- Niehaus L, Lehmann R, Roricht S, Meyer BU. Age-related reduction in visually evoked cerebral blood flow responses. Neurobiol Aging. 2001;22:35–38. doi: 10.1016/s0197-4580(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967;6:773–776. doi: 10.1016/0006-8993(67)90134-5. [DOI] [PubMed] [Google Scholar]
-
Peebles K, Celi L, McGrattan K, Murrell C, Thomas K, Ainslie PN. Human cerebrovascular and ventilatory CO2 reactivity to end-tidal, arterial and internal jugular vein
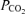 . J Physiol. 2007;584:347–357. doi: 10.1113/jphysiol.2007.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
. J Physiol. 2007;584:347–357. doi: 10.1113/jphysiol.2007.137075. [DOI] [PMC free article] [PubMed] [Google Scholar] - Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- Scheel P, Ruge C, Petruch UR, Schoning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31:147–150. doi: 10.1161/01.str.31.1.147. [DOI] [PubMed] [Google Scholar]
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequnces of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–972. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhaupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol. 1997;18:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice Hall; 1999. [Google Scholar]