Abstract
Transcellular Cl− and HCO3− transport is a vital function of secretory epithelia and exit across the luminal membrane is mediated by members of the SLC26 transporters in conjunction with cystic fibrosis transmembrane conductance regulator (CFTR) channel. Typically, secretory epithelia express several SLC26 transporters in the same tissue; however, how their specific function is determined in vivo is not known. In the present work we used the parotid gland duct which expressed Slc26a4 and Slc26a6 and the model systems of Slc26a4−/− and Slc26a6−/− mice to study the role and regulation of these SLC26 transporters. We examined the transport modes of SLC26A4 expressed in Xenopus oocytes and report that SLC26A4 functions as a coupled, electroneutral I−/Cl−, I−/HCO3− and Cl−/HCO3− exchanger with 1: 1 stoichiometry, with I− as the preferred anion. In the duct, Slc26a4 is expressed in the luminal membrane and mainly mediates I− secretion with minimal role in luminal HCO3− transport. By contrast, Slc26a6 mediates luminal Cl−/HCO3− exchange activity with minimal role in I− secretion. Furthermore, silencing of CFTR altered Cl−/HCO3− exchange by Slc26a6, but had no effect on I− secretion by Slc26a4. Accordingly, deletion of Slc26a6, but not deletion of Slc26a4, results in dysregulation of CFTR. These findings provide the first evidence for a selective role of the SLC26 transporters expressed in the same tissue in epithelial anion transport and suggest that transport specificity is achieved by both the properties of the transporters and the composition of the complexes they form.
Secretion of HCO3− and absorption of Cl− are observed in many epithelia, including the pancreas (Steward et al. 2005) and salivary glands (Melvin et al. 2005), and is mediated by CFTR and members of the SLC26 Cl− and HCO3− transporters that are expressed on the luminal membrane of many epithelia (Dorwart et al. 2008). Many CFTR-expressing cells express more than one member of the SLC26 family. Several members of the family interact with CFTR to exert mutual activation, thereby modulating Cl− and HCO3− transport (Ko et al. 2002, 2004). Elimination of Cl− absorption and HCO3− secretion are important elements in the pathogenesis of diseases of epithelia such as in cystic fibrosis (Kopelman et al. 1988) and pancreatitis (Petersen & Forsmark, 2002).
Members of the SLC26 family can transport physiologically relevant anions other than Cl− and HCO3−. For example, in addition to Cl− and HCO3−, SLC26A4 transports I− (Scott et al. 1999; Soleimani et al. 2001) and Slc26a6 transports oxalate and formate (Jiang et al. 2002; Ko et al. 2002; Xie et al. 2002; Shcheynikov et al. 2006). The transport properties of the SLC26 family are remarkably diverse (Dorwart et al. 2008). SLC26A1 and SLC26A2 are SO4− transporters (Markovich & Aronson, 2007), Slc26a3 and Slc26a6 are electrogenic Cl−/HCO3− exchangers (Ko et al. 2002; Xie et al. 2002), while SLC26A7 (Kim et al. 2005) and SLC26A9 (Dorwart et al. 2007) are selective Cl− channels. However, the transport properties of many SLC26 transporters are not known. While SLC26A4 transports I−, Cl− and HCO3−, the information available in the literature is otherwise incomplete and confusing. When expressed in HEK cells, SLC26A4 was noted to function as an I− and Cl− channel (Yoshida et al. 2004). However, others have not detected SLC26A4-mediated Cl− or I− current, but found that expression of SLC26A4 activated a K+ current (Dossena et al. 2005). Cl−/HCO3− exchange-like activity was reported in HEK cells stably expressing SLC26A4 (Soleimani et al. 2001). However, the stoichiometry and kinetics of this transporter are not known. Clearly, a detailed characterization of I−, Cl− and HCO3− transport by SLC26A4 and the relationship between them is needed to understand its function in I−, Cl− and HCO3− transport. In the present work, we expressed SLC26A4 in Xenopus oocytes and measured net I−, Cl− and HCO3− transport and observed that SLC26A4 functions as a coupled, electroneutral I−/Cl−/HCO3− exchanger with preference for I− over Cl−.
Mutations in SLC26A4 cause Pendred syndrome, which is characterized by deafness and goiter (Everett et al. 1997). Goiter results from the absence of pendrin-mediated I− transport within thyroid follicular cells (Scott et al. 1999), whereas hearing loss occurs from malformation of the inner ear, and possibly from the acidic endolymphatic pH (Wangemann et al. 2007). In addition, Slc26a4 is expressed in the apical regions of subsets of intercalated cells in several segments of the renal tubule (Wall, 2005), where it mediates Cl− absorption and HCO3− secretion (Wall, 2005). Thus, Slc26a4 functions as a Cl− and HCO3− transporter in many tissues (Soleimani et al. 2001; Ko et al. 2002; Wall, 2005).
Expression of SLC26A4 in tissues other than the thyroid, inner ear and kidney and its role in I− transport and Cl−-dependent HCO3− secretion have not been examined. This is particularly relevant to salivary gland since large numbers of patients undergoing 131I− therapy to treat thyroid cancer develop dry mouth disease due to damage to their salivary glands (Mandel & Mandel, 2003). Salivary glands are unique among secretory epithelia with respect to I− transport and express the 2Na+–I− symporter (NIS) in the basolateral membrane of the duct (Josefsson et al. 2002). This suggests that salivary gland ducts can actively uptake I− and are damaged by the incorporated 131I− during therapy. This raises the question whether the salivary duct also expresses luminal SLC26A4 and is capable of active I− secretion. In a previous work we showed that the salivary gland duct expresses Slc26a6 (Ko et al. 2004) and that both Slc26a4 and Slc26a6 are regulated by CFTR when expressed in HEK cells (Ko et al. 2002). However, while Slc26a6 has a C-terminal PDZ ligand, SLC26A4 does not and thus it is not clear whether it is regulated by CFTR in vivo. In addition, expression of multiple SLC26 transporters in the same tissue raises the question of their specific role in I−, Cl− and HCO3− transport.
Here, we used the Slc26a4−/− and Slc26a6−/− mice as model systems and report that the parotid duct expresses Slc26a4 in the luminal membrane and mediates active secretion of I− which is not regulated by CFTR. On the other hand, ductal luminal HCO3− transport is primarily mediated by Slc26a6 and is regulated by CFTR. Accordingly, deletion of Slc26a4 has no effect on CFTR function, whereas deletion of Slc26a6 results in dysregulation of CFTR activity in vivo. These findings highlight the selective role of the SLC26 transporters in vivo and suggest that stimulation of SLC26A4 should be considered as target for treatment of patients undergoing 131I− therapy.
Methods
Solutions
The Hepes-buffered solution for ducts contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes (pH 7.4 with NaOH) and 10 glucose. Cl−-free solutions were prepared by replacing Cl− with gluconate. I−-containing solutions were prepared by substituting Cl− with I−. HCO3−-buffered solutions were prepared by replacing 25 mm Na+ anion with 25 mm Na+–HCO3− and reducing Hepes to 2.5 mm. HCO3−-buffered solutions were gassed with 5% CO2 and 95% O2. Oocytes were in ND96 (mm): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2 and 5 Hepes, pH 7.5. The HCO3−-buffered solution contained (mm): 71 NaCl, 25 NaHCO3, 2 KCl, 1.8 CaCl2, 1 MgCl2 and 5 Hepes-Na, pH 7.5. Cl−-free medium was prepared by replacing Cl− with gluconate.
Expression of SLC26A4 in Xenopus oocytes
All procedures for maintaining the frogs and for preparation of oocytes followed NIH guidelines and were approved by the Animal Care and Use Committee of UT Southwestern medical centre. SLC26A4 in the pGEMHE vector was linearized with Nhe I and used to transcribe cRNA. Oocytes were obtained by partial ovariectomy of anaesthetized female Xenopus as detailed before (Kim et al. 2005). Oocytes in stages V–VI were injected with 5 ng cRNA in a final volume of 50 nl and incubated at 18°C in ND96 supplemented with 2.5 mm pyruvate and antibiotics. Oocytes were studied 48–96 h after injection.
Measurement of pHi, Cl−i and I−i in Xenopus oocytes
pHi, Cl− and I− were measured with ion-sensitive microelectrodes. Electrode preparation and the detailed measurement of pHi, Cl−i and voltage, and calibrations were as detailed before (Shcheynikov et al. 2006). In brief, pH was measured with a H+ exchanger resin (hydrogen ionophore I, cocktail B; Fluka) using the FD-233 electrometer. The bath was earthed via a 150 mm KCl agar bridge connected to an Ag–AgCl wire. The signals from the voltage and pH electrodes were used to extract changes in pH that were used to calculate the rates of HCO3− transport using the buffering capacity of 39.6 ± 1.3 mm (pH unit)−1 (n = 52) (Shcheynikov et al. 2006). Cl− and I− were measured with a Cl−-sensitive liquid ion exchanger (477913m, Corning). The slope of the Cl− microelectrode was about 56 mV for a 10-fold change in Cl− or I− concentrations. Cl−i activity was calculated as detailed before (Shcheynikov et al. 2006). The Cl− resin was used as an I− sensor since it is more sensitive to I− than to Cl−. The relative sensitivity of the electrodes for Cl− and I− was calculated from eqn (2) of Baumgarten & Fozzard (1981) for Cl− and I− concentrations between 3 and 100 mm and was determined to be 67 ± 3 times more sensitive to I− than to Cl−. Simultaneously measuring of pH and Cl− or pH and I− with a three-electrode setup was with the OC-725C amplifier and the FD-223 electrometer with a common reference electrode for both amplifiers. The dependence of I− fluxes on extracellular Cl− and I− concentration curves in Fig. 4 were fitted with a Hill equation of the type V =Vmax(Sn/Kn+ Sn) were S is the concentrations of Cl− or I−, K is the K0.5 and n is the Hill coefficient.
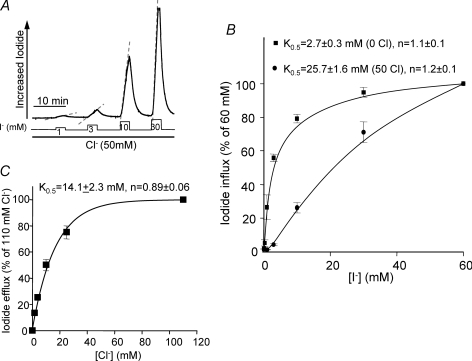
Figure 4. Cl−o and I−o dependence of SLC26A4.
A shows the protocol used to measure I− influx in the presence of 50 mm Cl−o. Osmolarity was maintained by replacing Na+-gluconate with NaI. The rates of I− influx measured in the presence (•) and absence (▪) of 50 mm Cl−o are plotted in B as a function of I− concentration (n = 6). The rates are expressed as percentage of the rate measured with 60 mm I−o. In C, the rates of I− efflux as a function of [Cl−]o were measured by incubating the oocytes in Cl−-free media containing 10 mm I− to load the oocytes with I− as in Fig. 2A and then the oocytes were incubated with the Cl−o concentrations indicated in C. The rates are plotted as percentage of the rate measured with 110 mm Cl− (n = 4).
Preparation of sealed ducts
All procedures for maintaining the mice and for isolation of parotid and pancreatic ducts followed NIH guidelines and were approved by the Animal Care and Use Committee of UT Southwestern medical centre. Mouse parotid and pancreatic ducts were microdissected and cultured as detailed elsewhere (Wang et al. 2006). In brief, the parotid glands and pancreas were removed from mice and injected with 50 U ml−1 collagenase, 400 U ml−1 hyaluronidase, 0.2 mg ml−1 soybean trypsin inhibitor and 2 mg ml−1 BSA, minced and incubated at 37°C for 30 min. The media was replaced and incubation continued for an additional 30 min. The tissue was washed with DMEM (Sigma-Aldrich) and dissected intralobular ducts were placed in DMEM and cultured at 37°C for 24–48 h.
Analysis of Slc26a6, Slc26a4 and NIS gene expression by RT-PCR
Total RNA was extracted from microdissected parotid and pancreatic ducts and used for RT-PCR with 40 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min.
Treatment of sealed ducts with CFTR dicer siRNA
Ducts were treated with scrambled (CUUCCUCU-CUUUCUCUCCCUUGUGA) or CFTR-specific dicer siRNA (GUGCAAAUUCAGAGCUUUGUGGAACAG) exactly as described before (Wang et al. 2006).
Measurement of pHi in sealed ducts
pHi of the sealed duct cells was measured using the pH indicator BCECF as described before (Wang et al. 2006). BCECF fluorescence was measured at excitation and wavelengths of 440 and 490 nm and at emission wavelengths above 530 nm.
Measurement of intraluminal I− in the sealed ducts
Intraluminal I− was measured using the same setup as for oocytes. Ducts with inner diameters of 40–60 μm were immobilized with large-bore suction pipettes. The lumen was impaled under stereomicroscopic guidance using recording pipettes with 1–2 μm tips filled with the Cl−-sensitive exchanger (see Fig. 5). Four criteria are used to verify that the electrodes are in the lumen: (1) visual inspection by the experimentalist, (2) maintenance of lumen potential between −20 to −30 mV, (3) lower Cl− concentration in the duct lumen (91 ± 5 mm, n = 36) than in the bath (150 mm), and (4) slow response of the electrodes to changes in bath Cl− and I− concentrations.
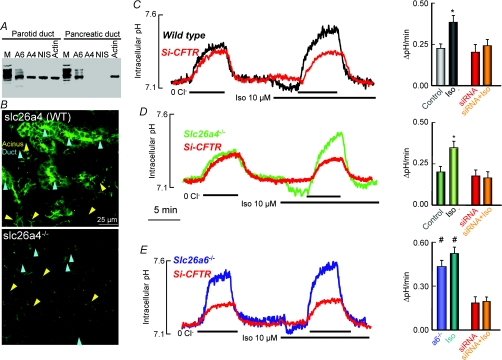
Figure 5. Slc26a6, but not Slc26a4, mediates luminal Cl−/HCO3− exchange in the parotid duct.
A shows RT-PCR analysis of Slc26a6 (A6), Slc26a4 (A4), and Na+–I− symporter (NIS) in parotid and pancreatic ducts. Pancreatic duct is used as a control for Slc26a4 and NIS and actin is loading control. M stands for markers. B shows immunolocalization of Slc26a4 in submandibular section. The lower image is with a submandibular gland from Slc26a4−/− mice. Acini and ducts are marked by yellow and cyan arrowheads, respectively. Note that Slc26a4 is expressed in the luminal membrane of the duct and is not expressed in the acini. In C–E, Cl−/HCO3− exchange activity was measured in sealed ducts by equilibrating the ducts in HCO3−-buffered media for at least 15 min before alternately incubating them in Cl−-containing and Cl−-free medium before and after stimulation with 10 μm isoproterenol (Iso). The ducts were cultured for 36–48 h and treated with scrambled (control) or CFTR siRNA (red traces). Ducts were microdissected from wild-type (C), Slc26a4 (D) or Slc26a6 (E) deficient mice. The columns are the mean ±s.e.m. of 6–8 ducts from 3 or 4 mice of each line. Note that deletion of Slc26a4 has no effect on basal or stimulated Cl−/HCO3− exchange activity, while deletion of Slc26a6 enhances basal activity that is not further stimulated by Iso. KD of CFTR inhibited only the stimulated Cl−/HCO3− exchange activity in wild-type and Slc26a4−/− ducts, whereas KD of CFTR inhibited the enhanced basal Cl−/HCO3− exchange activity in Slc26a6−/− ducts.
Immunolocalization
Immunolocalization of Slc26a4 in salivary glands employed procedures previously described (Wang et al. 2006). Briefly, frozen sections were permeabilized with 0.5 ml of cold methanol. Non-specific sites were blocked and the sections were incubated with a 1: 100 dilution of anti-Slc26a4 antibodies (a gift from Dr Bidart, University of Paris) overnight at 4°C and were detected with goat anti-rabbit IgG tagged with FITC. Images were recorded with a Bio-Rad 1024 confocal microscope.
Preparation of single parotid duct cells
Single parotid duct cells were prepared by our enzymatic procedure (Zeng et al. 1997). The mouse parotid glands were removed, minced and treated with 0.025% trypsin for 7 min at 37°C. Trypsin digestion was stopped with 1.5 mg ml−1 soybean trypsin inhibitor and the tissue was digested for 20 min with 70 U ml−1 chromatographically purified collagenase. The liberated single cells were collected by centrifugation and kept on ice until use.
Current measurement in parotid duct cells
CFTR Cl− current was measured by recording the whole-cell Cl− current as detailed before (Zeng et al. 1997). To isolate the Cl− current, the pipette solutions contained 150 mm NMDG-Cl, 1 mm MgCl2, 1 mm EGTA, 0.5 mm ATP and 10 mm Hepes at pH 7.3. The same solution without ATP was used as the bath. Currents were recorded using the Axopatch 200B patch-clamp amplifier at a holding potential of −60 mV and results were collected at 5 kHz and filtered at 1 kHz. Current density was normalized to cell capacitance. After establishing the whole-cell mode, the cell capacitance was obtained by delivering 20 mV, 20 ms pulses and eliminating the transient currents by compensation for the serial resistance and the capacitance. The compensated capacitance is taken as the whole-cell capacitance.
Statistical analysis
Results in all experiments are given as the mean ±s.e.m. and analysed by ANOVA with a P < 0.05 indicating significance.
Results
SLC26A4 is a coupled electroneutral Cl−/HCO3− exchanger
SLC26A4 transport properties were examined in Xenopus oocytes in order to measure SLC26A4-mediated I−, Cl− and HCO3− flux and current simultaneously. Moreover, this system enables measurement of ion flux at constant membrane potential. Figure 1A demonstrates that the anion-selective resin 477913 is ∼60 times more sensitive to I− than Cl−, making it a suitable I− sensor. In contrast, the Cl− and I− sensitivity of this resin is not affected by HCO3− or pH (Shcheynikov et al. 2006).
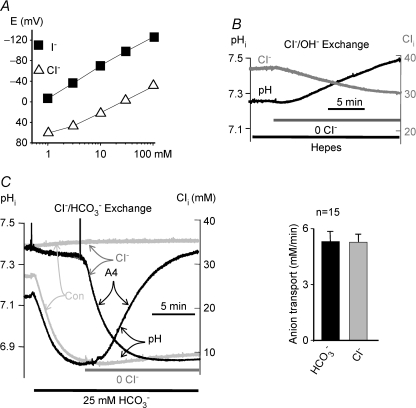
Figure 1. Stoichiometry of SLC26A4 mediates Cl−/HCO3− exchange.
A shows the response of the anion-sensitive liquid ion exchange resin 477913 to Cl− (▵) and I− (▪). In B and C, oocytes injected with SLC26A4 cRNA were used to simultaneously measure pHi (dark traces) and Cl−i (grey traces) in Hepes (B) or HCO3−-buffered media (C). The control (light grey traces in C) is with water-injected oocytes. The changes in pHi and Cl−i were used to calculate the rates of HCO3− and Cl− transport in mm min−1 and the mean ±s.e.m. of 15 experiments are given in the histogram in C.
To measure Cl−/OH− exchange, Cl− and pH were measured simultaneously in oocytes bathed in Hepes-buffered solutions (Fig. 1B). Following extracellular Cl− (Cl−o) removal, intracellular Cl− (Cl−i) falls, whereas intracellular pH (pHi) rises, indicating that SLC26A4 can mediate Cl−/OH− exchange. Then we measured the influence of HCO3− on SLC26A4 activity. Exposing oocytes to HCO3−-buffered media resulted in rapid cytoplasmic acidification due to hydration of CO2. The change in Cl−i and pHi in response to Cl−o removal was greatly accelerated in HCO3−-buffered solutions (Fig. 1C), indicating that Cl− and HCO3− transport by SLC26A4 are coupled. The rates of Cl− and HCO3− fluxes derived from the changes in Cl−i and pHi yielded SLC26A4 Cl−/HCO3− exchange stoichiometry of 1.1 ± 0.1 (n = 15).
Properties of I− transport by SLC26A4
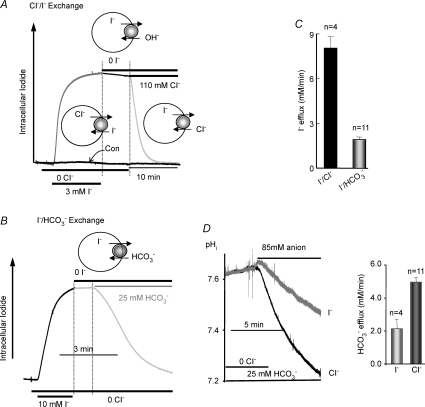
While the anion-sensitive resin has greater sensitivity to I− than Cl−, because oocyte Cl−i is ∼35 mm it was not possible to specifically determine intracellular I− (I−i) concentration. Therefore, we report relative changes in [I−]i. Exposing oocytes bathed in HCO3−- and Cl−-free media to 3 mm I− rapidly increased [I−]i due to I−o/Cl−i exchange. Following removal of I−o a slow decline in [I−]i was observed, consistent with slow SLC26A4-mediated I−i/OH−o exchange. Addition of either 110 mm Cl− (Fig. 2A) or 25 mm HCO3− (Fig. 2B) to the media resulted in a rapid fall in [I−]i due to SLC26A4-mediated I−i/Cl−o and I−i/HCO3−o exchange, respectively. The relative rates of I−/Cl− and I−/HCO3− exchange are given in Fig. 2C. Finally, we compared the rates of Cl−o/HCO3−i and I−o/HCO3−i exchange (Fig. 2D). The oocytes were incubated in HCO3−-buffered media and were depleted of Cl−i by incubation in Cl−-free medium as in Fig. 1C. The oocytes were then exposed to either 85 mm Cl− or 85 mm I− to measure the rates of HCO3− efflux. Figure 2D shows that SLC26A4-mediated I−o/HCO3−i exchange was about 50% slower than Cl−o/HCO3−i exchange.
Figure 2. SLC26A4 mediates Cl−/I− and I−/HCO3− exchange.
In A and B, oocytes expressing SLC26A4 were incubated in Hepes-buffered, Cl−-free media and then exposed to Cl−-free media containing 3 (A) or 10 mm I− (B) to measure Cl−i/I−o exchange. The black trace in A is control with water-injected oocytes. At the time period bordered by dashed lines the oocytes were incubated with Cl−- and I−-free media to test for I−i/OH−o exchange. Oocyte in A was then exposed to Cl−o to measure I−i/Cl−o exchange and the oocyte in B was exposed to Cl−-free, HCO3−-buffered media to measure I−i/HCO3−o exchange (light grey portion of traces). C shows the mean ±s.e.m. of the indicated number of experiments. D compares the rates of HCO3−i/Cl−o (dark trace) and HCO3−i/I−o exchange (grey trace). Oocytes in HCO3−-buffered media were incubated in Cl−-free media to deplete the oocytes of Cl− and load them with HCO3− (see Fig. 1C). Then, the oocytes were exposed to HCO3−-buffered media containing either 85 mm Cl− or 85 mm I−. The histogram shows the mean ±s.e.m. of the indicated number of experiments.
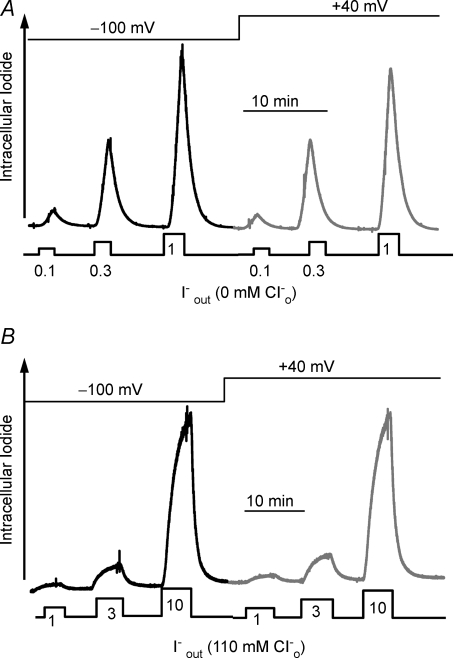
Figure 3A and B shows that SLC26A4-mediated Cl−/I− exchange is not affected by holding the membrane potential at −100 or +40 mV, whether the exchange was measured in the absence of Cl−o (Fig. 3A) or in the presence of 110 mm Cl−o (Fig. 3B). This indicates that the Cl−/I− exchange is electroneutral, consistent with Cl−/I− exchange stoichiometry of 1: 1.
Figure 3. Lack of effect of the membrane potential on anion transport by SLC26A4.
In A and B, I− influx was measured while holding the membrane potential at −100 mV or at +40 mV. In A, I− influx was measured in Cl−-free medium at the indicated I− concentrations. Efflux was measured by incubating the oocytes in standard oocyte media containing 110 mm Cl−. In B, I− influx was measured at the indicated I− concentrations while Cl− was kept constant at 110 mm.
Figure 3A shows that SLC26A4 mediates I−o/Cl−i exchange even when the ratio of extracellular Cl− to I− exceeds 100: 1. We explored the dependence of I− influx, vis-à-vis Cl−i/I−o exchange, on [I−]o, in both the presence and absence of Cl−o using the protocol of Fig. 4A. Oocytes expressing SLC26A4 were incubated in Cl−-free medium or media containing 50 mm Cl− and alternately exposed to increasing [I−]o. When I− influx was measured in the absence of Cl−o, the oocytes were incubated with 50 mm Cl−o between changes in [I−] to allow recovery of Cl−i and removal of I−i. Figure 4B shows that the K0.5 for I−o in the absence of Cl−o is 2.7 ± 0.3 mm with a Hill coefficient of 1.1 ± 0.1 and in the presence of 50 mm Cl− 25.7 ± 1.6 mm with a Hill coefficient of 1.2 ± 0.1.
To measure the Cl−o dependence of I− efflux, the oocytes were incubated in Cl−-free medium containing 10 mm I−o, and I− efflux was measured at different [Cl−]o. In the periods between changes in [Cl−]o the oocytes were incubated in Cl−-free medium containing 10 mm I− to restore [I−]i. The K0.5 of SLC26A4 for Cl−o is 14.1 ± 2.3 mm with a Hill coefficient of 0.89 ± 0.06 (Fig. 4C). Hence, the SLC26A4 K0.5 for I−o (2.7 mm) is about 5 times lower than that for Cl−o (14.1 mm).
Slc26a6 and Slc26a4 in ductal luminal HCO3− transport
To study the role of Slc26a4 in vivo we examined the expression of Slc26a4 in mouse salivary glands. Figure 5A shows that Slc26a6, Slc26a4 and Na+–I− symporter (NIS) mRNA are expressed in the parotid duct. Previous studies demonstrated Slc26a6 protein expression in the apical membrane of both the parotid and the pancreatic ducts (Ko et al. 2004). Whereas NIS is expressed in the basolateral membrane (Josefsson et al. 2002), Slc26a4 is expressed in the apical membrane of salivary duct cells (Fig. 5B).
Since Slc26a4 and Slc26a6 transport Cl− and HCO3− and are both expressed in the parotid duct, we examined their role in parotid duct luminal HCO3− transport by measuring Cl−/HCO3− exchange in wild-type, Slc26a4−/− and Slc26a6−/− ducts. Moreover, since CFTR activates both Slc26a4 and Slc26a6 in vitro (Ko et al. 2002), using dicer siRNA, we examined the effect of ‘knocking down’ (KD) CFTR in sealed parotid ducts in primary culture. KD of CFTR not only eliminates CFTR activity but also regulatory effects that are independent of CFTR activity. We found that microdissected intralobular parotid ducts seal spontaneously after 12–24 h in primary culture, similar to observations in the pancreatic duct (Ashton et al. 1990; Wang et al. 2006).
Figure 5C shows the Cl−/HCO3− exchange activity in sealed parotid ducts from wild-type mice. Following the application of 10 μm isoproterenol (isoprenaline) (Iso) pHi fell by 0.09 ± 0.02 pH units (n = 11). Removal and re-addition of Cl−o showed that Iso increased Cl−/HCO3− exchange activity by about 75% (Fig. 5C). Figure 5C shows that with CFTR KD Iso-induced stimulation of Cl−/HCO3− exchange is attenuated, while basal Cl−/HCO3− exchange activity is unaffected. These findings are similar to those reported before for secretin-stimulated pancreatic duct (Wang et al. 2006). Notably, Fig. 5D shows that the basal and stimulated Cl−/HCO3− exchange activity and its dependence on CFTR was the same in Slc26a4−/− and wild-type ducts. Therefore, we conclude that Slc26a4 contribution to Iso-stimulated Cl−/HCO3− exchange is minimal.
By contrast to the results with deletion of Slc26a4, deletion of Slc26a6 affected both basal and stimulated Cl−/HCO3− exchange activity (Fig. 5E). Genetic disruption of Slc26a6 increased basal Cl−/HCO3− exchange activity in the parotid duct that was not stimulated further with Iso stimulation. Notably, KD of CFTR markedly attenuated the enhanced basal Cl−/HCO3− exchange activity. Thus, Slc26a6 and CFTR act in tandem to facilitate luminal HCO3− transport in the parotid gland, similar to previous observations in the pancreatic duct (Ko et al. 2004; Wang et al. 2006). Hence, Slc26a6 has a similar role in the pancreatic and parotid ducts, whereas Slc26a4 has a minor role in luminal HCO3− transport in the parotid duct. These data provide the first evidence that SLC26 transporters have selective roles in vivo.
Slc26a4, but not Slc26a6, mediates I− secretion
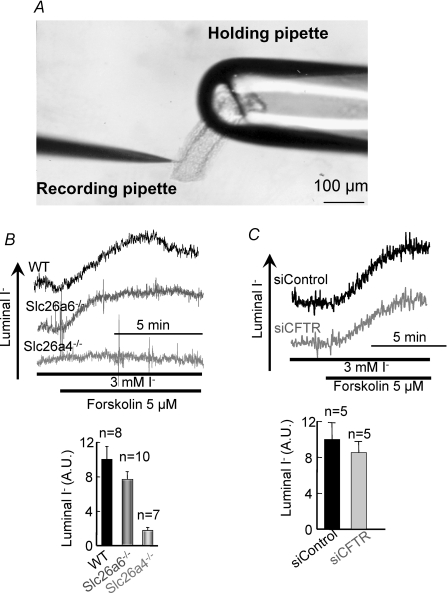
To determine the roles of Slc26a4 and Slc26a6 in parotid duct I− secretion we measured luminal I− in the sealed ducts. The experimental system is shown in Fig. 6A. To obtain sufficient signal/noise, ducts were incubated with 3 mm I− and after stabilization of the signal they were stimulated with forskolin. Figure 6B shows that stimulation of wild-type ducts with forskolin increased luminal [I−]. I− secretion was not affected by deletion of Slc26a6. By contrast, disruption of Slc26a4 almost completely abolished active I− secretion. These findings further demonstrate the selective role of the SLC26 transporters in vivo. While Slc26a6 dominates luminal HCO3− transport but has no role in I− secretion, Slc26a4 dominates I− secretion and has a minor role in luminal HCO3− transport.
Figure 6. Slc26a4, but not Slc26a6, mediates luminal I− secretion in the parotid duct.
A shows an image of sealed ducts held by a large-bore holding pipette and penetrated with a recording pipette. In B, wild-type (dark trace and columns), Slc26a6−/− (grey trace and columns), and Slc26a4−/− (light grey trace and columns) sealed parotid ducts were incubated with 3 mm I− for 15 min before stimulation of I− secretion with 5 μm forskolin. The columns are the mean ±s.e.m. of the indicated number of experiments. Note that activation of I− secretion is observed with wild-type and Slc26a6−/− ducts, but not with Slc26a4−/− ducts. In C, wild-type ducts treated with scrambled (dark trace and columns) or CFTR siRNA (grey trace and columns) were used to measure stimulated I− secretion. The columns show the mean ±s.e.m. of 5 experiments. AU, arbitrary units. Note that KD of CFTR that reduced Cl−/HCO3− exchange activity in these ducts (Fig. 5C) had no effect on I− secretion.
Selective communication of the SLC26 transporters with CFTR
CFTR and Slc26a4 are expressed in the parotid duct and CFTR activates Slc26a4 when both proteins are over-expressed in HEK cells (Ko et al. 2002). Therefore, we explored whether CFTR regulates Slc26a4 and whether Slc26a4 affects CFTR activity in vivo. While KD of CFTR markedly reduces parotid duct luminal HCO3− transport (Fig. 5), Fig. 6C shows that following CFTR KD I− secretion is unchanged. Hence, although CFTR can conduct I− (Ketchum et al. 2004) it does not transport I− and does not affect Slc26a4 function under physiological conditions.
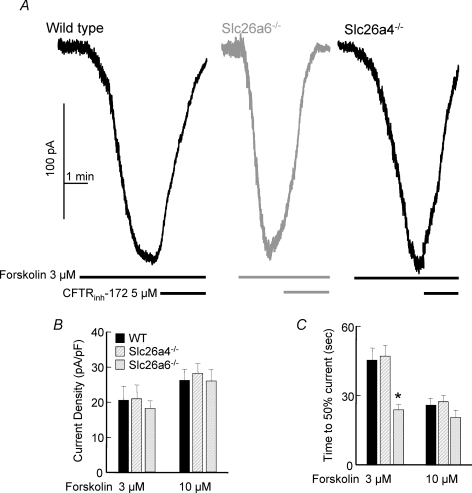
Previous work showed that CFTR and Slc26a6 interact within the pancreatic duct, that Slc26a6 regulates resting CFTR activity and deletion of Slc26a6 facilitates the rate of activation of CFTR (Wang et al. 2006). These findings are confirmed in Fig. 7A–C, which show that deletion of Slc26a6 facilitates activation of CFTR by submaximal stimulus strength in parotid duct cells. To verify that the cAMP-stimulated Cl− current measured under the conditions of Fig. 7 is mediated by CFTR, it is shown that the current is inhibited by the inhibitor of CFTR channel activity CFTRinh-172. Figure 7B and C show that this was not due to a change in current density and thus expression of CFTR. Significantly, Fig. 7A–C show that deletion of Slc26a4 has no effect on ductal CFTR activity.
Figure 7. Differential regulation of CFTR by Slc26a4 and Slc26a6.
In A–C, single duct cells were isolated from the parotid duct of wild-type, Slc26a6−/− and Slc26a4−/− mice, as indicated in the figure, and used to measure CFTR Cl− current. The identity of the Cl− current was ascertained by its activation by forskolin stimulation and inhibition by CFTRinh-172. The cells were stimulated with the submaximal and maximal forskolin concentrations of 3 and 10 μm. Current density (pA pF−1) is given in B and the time for 50% activation of the current is given in C for the two forskolin concentrations. Note that deletion of Slc26a4 has no effect on CFTR current, whereas deletion of Slc26a6 facilitated activation of CFTR, indicative of the tonic inhibition of CFTR activity by Slc26a6 reported before (Wang et al. 2006).
Discussion
The present work examined the transport properties and the physiological role of Slc26a4 in the parotid gland. We also explored the selective function of SLC26 transporters expressed in the same duct. Simultaneous measurement of Cl−, I− and HCO3− transport revealed that Slc26a4 functions as a coupled Cl−/HCO3−, Cl−/I− and I−/HCO3− exchanger with 1: 1 stoichiometry. This conclusion is strongly supported by the findings that in SLC26A4-expressing oocytes, changes in Cl−o have no effect on the membrane potential (not shown) and the Cl−/I− exchange is not affected by a 140 mV shift in the membrane potential. Furthermore, the Cl−o and I−o dependence of SLC26A4-mediated I− fluxes occurred with a Hill coefficient close to 1, which further supports electroneutral transport of these ions by SLC26A4 and suggests a single external anion-interacting site for SLC26A4. The Hill coefficient for I−o was not altered by Cl−o. Cl−o only changed the K0.5 for I−o. This behaviour suggests that Slc26A4 transports both I− and Cl− and that Cl− and I− compete for interaction with the same external site.
The results in Figs 1–4 show that SLC26A4 functions as a coupled electroneutral Cl−/HCO3−/I− exchanger. The preferred SLC26A4 substrate is I− and SLC26A4 transports I− in the presence of high Cl−. This ensures that SLC26A4 will transport I− when the cells uptake I− by NIS. The systemic I− concentration is between 10 and 100 μm (Kogai et al. 2006). With NIS stoichiometry of 2Na+/1I− and a membrane potential of −60 mV, the estimated [I−]i in thyroid follicular and salivary gland duct cells is 1–10 mm. With [Cl−]i in duct cells of about 10 mm (Zeng et al. 1997), SLC26A4 should mainly engage in luminal I−i/Cl−o and I−i/HCO3−o exchange. Since the rate of I−/HCO3− exchange in the presence of 25 mm HCO3− is about 50% of the I−/Cl− exchange observed with 110 Cl−, and since the duct absorbs the Cl− and secretes HCO3− to generate a Cl−-poor, HCO3−-rich fluid, probably a large fraction of I− efflux into the duct lumen in the intralobular duct and the bulk of the I− efflux in the main duct is mediated by I−/HCO3− exchange.
The preference of Slc26a4 for I− over Cl− ensures that Slc26a4 mediates mostly I− secretion in both salivary ducts and thyroid follicular cells. While Slc26a4-mediated I− secretion occurs in exchange for Cl− or HCO3−, in the parotid duct Slc26a4 has a minor role in luminal HCO3− transport. Dedication of SLC26A4 to I− efflux in the salivary glands, is also evident from the effect of 131I therapy on salivary gland function. Large numbers of patients undergoing 131I− therapy to treat thyroid cancer develop dry mouth disease due to damage to their salivary glands (Mandel & Mandel, 2003). This suggests that SLC26A4 is particularly susceptible to damage by 131I. This probably leads to inhibition of I− efflux and accumulation of 131I in the glands and, consequently, their damage. This suggests that stimulation of SLC26A4 during 131I therapy should be useful in protecting the salivary glands and preventing their damage.
Regulation of SLC26A4 activity is poorly understood. A mechanism we described for several SLC26 transporters, including for expressed SLC26A4, is regulation by CFTR (Ko et al. 2002, 2004). However, in the present work, we found that in vivo Slc26a4 in not regulated by CFTR, whereas Slc26a6 is. In retrospect, this is not surprising. Slc26a6, but not Slc26a4, has a C terminus PDZ ligand and the PDZ ligands of both Slc26a6 and CFTR are required for their assembly into a HCO3− transporting complex and their mutual regulation (Ko et al. 2004). It is likely that when CFTR and Slc26a4 are over-expressed, they are forced to interact, where CFTR activates Slc26a4. However, in vivo the two proteins do not interact and thus CFTR does not affect I− secretion and Slc26a4 does not affect CFTR activity. In this respect, when PDZ ligands of CFTR and Slc26a6 are deleted, the activation of Slc26a6 by CFTR is attenuated and can be rescued by over-expression of these mutants (Ko et al. 2004).
Like many other epithelia, the salivary gland duct absorbs Cl− and secretes HCO3− (Melvin et al. 2005). The present study demonstrates that in the parotid duct, Slc26a6 is the major luminal Cl−/HCO3− exchanger (Figs 5–7), which functions in conjunction with CFTR. The Cl− and HCO3− transport properties of the parotid (present work) and of the pancreatic duct in Slc26a6−/− mice (Wang et al. 2006) are similar. In both ducts, stimulation with agonists that increase cAMP activates Cl−/HCO3− exchange activity. Moreover, stimulated, but not basal, Cl−/HCO3− exchange activity requires CFTR and in both ducts deletion of Slc26a6 augmented Cl−-dependent HCO3− fluxes that are inhibited by silencing of CFTR. These findings suggest that Slc26a6 has a similar role in Cl− absorption and HCO3− secretion by the pancreatic and parotid ducts.
The present findings suggest that a contributing factor to the dedicated function of the SLC26 transporters is the transporting complexes that they form. A surprising observation is that Slc26a4 does not appreciably contribute to ductal Cl−/HCO3− exchange activity even in the absence of I− (Fig. 5). The two most likely reasons are the 1: 1 and 1: 2 Cl−/HCO3− exchange stoichiometry of Slc26a4 and Slc26a6, respectively, and the lack of regulation of Slc26a4 by CFTR in vivo. With a 1Cl−: 2HCO3− stoichiometry Slc26a6 dominates Cl−/HCO3− exchange activity in the presence of Slc26a4. In addition, Slc26a6 interacts with CFTR to increase the activity of Slc26a6 and CFTR, whereas Slc26a4 does not interact with CFTR in vivo (Fig. 7). Segregating the Slc26a4 and Slc26a6–CFTR complexes may serve to independently regulate ductal I− secretion and Cl− absorption and HCO3− secretion and gain specificity of the secretory process.
Acknowledgments
We thank Dr Alan Verkman (UC, San Francisco) for a gift of CFTRinh-172. This work was supported by NIH grants DE12309, DK38938 and the Ruth Harrell Professorship to S.M. and by NIH grant DK061521to S.M.W.
Conflict of interest
All authors declare that they do not have any conflict of interest to disclose.
References
- Ashton N, Argent BE, Green R. Effect of vasoactive intestinal peptide, bombesin and substance P on fluid secretion by isolated rat pancreatic ducts. J Physiol. 1990;427:471–482. doi: 10.1113/jphysiol.1990.sp018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten CM, Fozzard HA. Intracellular chloride activity in mammalian ventricular muscle. Am J Physiol Cell Physiol. 1981;241:C121–C129. doi: 10.1152/ajpcell.1981.241.3.C121. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Dossena S, Maccagni A, Vezzoli V, Bazzini C, Garavaglia ML, Meyer G, Furst J, Ritter M, Fugazzola L, Persani L, Zorowka P, Storelli C, Beck-Peccoz P, Botta G, Paulmichl M. The expression of wild-type pendrin (SLC26A4) in human embryonic kidney (HEK 293 Phoenix) cells leads to the activation of cationic currents. Eur J Endocrinol. 2005;153:693–699. doi: 10.1530/eje.1.02018. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- Josefsson M, Grunditz T, Ohlsson T, Ekblad E. Sodium/iodide-symporter: distribution in different mammals and role in entero-thyroid circulation of iodide. Acta Physiol Scand. 2002;175:129–137. doi: 10.1046/j.1365-201X.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- Ketchum CJ, Rajendrakumar GV, Maloney PC. Characterization of the adenosinetriphosphatase and transport activities of purified cystic fibrosis transmembrane conductance regulator. Biochemistry. 2004;43:1045–1053. doi: 10.1021/bi035382a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006;13:797–826. doi: 10.1677/erc.1.01143. [DOI] [PubMed] [Google Scholar]
- Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95:349–355. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13:265–271. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]
- Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol. 2007;69:361–375. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Forsmark CE. Chronic pancreatitis and maldigestion. Semin Gastrointest Dis. 2002;13:191–199. [PubMed] [Google Scholar]
- Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens. 2005;14:480–484. doi: 10.1097/01.mnh.0000168390.04520.06. [DOI] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K. Mechanism of iodide/chloride exchange by pendrin. Endocrinology. 2004;145:4301–4308. doi: 10.1210/en.2004-0048. [DOI] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Muallem S. Membrane-specific regulation of Cl− channels by purinergic receptors in rat submandibular gland acinar and duct cells. J Biol Chem. 1997;272:32956–32965. doi: 10.1074/jbc.272.52.32956. [DOI] [PubMed] [Google Scholar]