Abstract
Serotonin (5-hydroxytryptamine, 5-HT) receptors (5-HTRs) play critical roles in brain and cardiovascular functions. In the vasculature, 5-HT induces potent vasoconstrictions, which in aorta are mainly mediated by activation of the 5-HT2AR subtype. We previously proposed that one signalling mechanism of 5-HT-induced vasoconstriction could be c-Src, a member of the Src tyrosine kinase family. We now provide evidence for a central role of c-Src in 5-HT2AR-mediated contraction. Inhibition of Src kinase activity with 10 μm 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) prior to contraction resulted in ∼90–99% inhibition of contractions induced by 5-HT or by α-methyl-5-HT (5-HT2R agonist). In contrast, PP2 pretreatment only partly inhibited contractions induced by angiotensin II and the thromboxane A2 mimetic, U46619, and had no significant action on phenylephrine-induced contractions. 5-Hydroxytryptamine increased Src kinase activity and PP2-sensitive tyrosine-phosphorylated proteins. As expected for c-Src identity, PP2 pretreatment inhibited 5-HT-induced contraction with an IC50 of ∼1 μm. Ketanserin (10 nm), a 5-HT2A antagonist, but not antagonists of 5-HT2BR (100 nm SB204741) or 5-HT2CR (20 nm RS102221), prevented 5-HT-induced contractions, mimicking PP2 and implicating 5-HT2AR as the major receptor subtype coupled to c-Src. In HEK 293T cells, c-Src and 5-HT2AR were reciprocally co-immunoprecipitated and co-localized at the cell periphery. Finally, 5-HT-induced Src activity was unaffected by inhibition of Rho kinase, supporting a role of c-Src upstream of Rho kinase. Together, the results highlight c-Src activation as one of the early and pivotal mechanisms in 5-HT2AR contractile signalling in aorta.
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter with potent vasoconstricting properties that regulates a variety of functions in the nervous and cardiovascular systems (Watts, 2005). The primary targets of 5-HT in the vasculature are 5-HT2A receptors (5-HT2AR). This class of receptors are expressed abundantly in brain cortical regions where they play important roles in normal and pathological states (Watts et al. 2001; Watts, 2002; Villazon et al. 2002; Nagatomo et al. 2004; Gavarini et al. 2004; Weisstaub et al. 2006). Classical kinase pathways of 5-HT2AR-mediated vasoconstriction are protein kinase C (Banes et al. 1999; cf. De Witt et al. 2001), Rho kinase (RhoK; Kandabashi et al. 2002) and extracellular signal-regulated kinases (Watts et al. 2001; Ishihata et al. 2002).
Src protein tyrosine kinases are potential regulators of various cellular functions aside from cellular differentiation and proliferation (Thomas & Brugge, 1997). For example, in the brain they participate in synaptic plasticity (Salter & Kalia, 2004) and in the immune system they transmit T-cell receptor-mediated survival signals (Zamoyska et al. 2003). Src tyrosine kinases are a family of at least nine members with partial tissue specificity, with c-Src being abundant in vascular tissues (Oda et al. 1999).
In vascular smooth muscle, Src tyrosine kinases have been implicated in the contractile signalling pathway triggered by the G-protein-coupled receptor (GPCR) agonist, 5-HT (Banes et al. 1999; Alioua et al. 2002). In addition, they may also participate in contractions induced by angiotensin II (Ang II) and the α1-adrenergic agonist phenylephrine (PE; Touyz et al. 2001; Alioua et al. 2002). In particular, a role for c-Src is supported by c-Src gene ablation, which produces reduced Ang II-induced Ca2+ transients of cultured mesenteric myocytes (Touyz et al. 2001). Also, a role for Src is substantiated by inhibitors of its kinase activity [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2) and 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP1)] that bind to the Src catalytic domain in the ATP-binding site and contiguous hydrophobic pocket (Schindler et al. 1999; Zhu et al. 1999; Bishop et al. 2001). Based on the pharmacology of PP2 and its ability to reverse agonist-induced contractions with an IC50 concurring with c-Src inhibition (Hanke et al. 1996; Waltenberger et al. 1999), our previous work proposed c-Src tyrosine kinase as a candidate Src family member to participate in vasoconstriction, including that triggered by 5-HT (Alioua et al. 2002). We now show that c-Src activation is an early mechanism crucial for 5-HT-induced contractions via 5-HT2AR, and that c-Src preferential coupling to contractile agonists follows the sequence 5-HT > AngII > thromboxane A2 (TXA2) >>>> phenylephrine (PE). We also show that in coexpressing HEK 293T cells, c-Src and 5-HT2AR co-localize and can associate with each other.
Methods
Animals
Sprague–Dawley or Fisher 344 male rats (3 months old) were killed with an overdose of inhaled isofluorane. Protocols received institutional approval and followed guidelines consistent with the recommendations of the American Veterinary Medical Association (AVMA) Panel on Euthanasia and of USDA Animal Care Policy.
Materials
4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP2), 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3) and Y27632 were from Calbiochem. The thromboxane A2 mimetic, U46619, was from Cayman Chemical. Ketanserin and α-methyl-5-HT were from Sigma; SB204741 and RS102221 were purchased from Tocris Cookson Inc. Mouse anti-phosphotyrosine (anti-pY) monoclonal antibody (mAb; clone 4G10) and anti-Src mAb (clone GD11) were from Upstate. Anti-Src mAb was raised against Rous sarcoma virus (Schmidt-Ruppin D strain) Src (v-Src) recombinant protein (Parsons et al. 1984) and is referred to here as anti-c-Src mAb because it recognizes purified c-Src but not the closely related Src family members, Fyn, Yes or Lyn, recombinant proteins in immunoblots (our unpublished observations). Furthermore, a pre-adsorption test confirming its specificity was performed using purified recombinant human c-Src (SRC N1, CalBiochem; see Fig. 2C). Anti-c-Src polyclonal antibody (pAb; sc-19) and antigenic peptide were from Santa Cruz Biotechnology (see Fig. 6 for specificity tests). Src kinase activity kit was from Upstate. Anti-c-Myc mAb and pAb were from Sigma. Secondary antibodies were, for immunoblots, Alexa Fluor 680 goat anti-rabbit immunoglobulin G (IgG) and IRDye800 goat anti-mouse IgG (Li-Cor); and for immunocytochemistry, Alexa Fluor 568 goat anti-mouse IgG and Alexa-Fluor 488 goat anti-rabbit IgG (Molecular Probes).
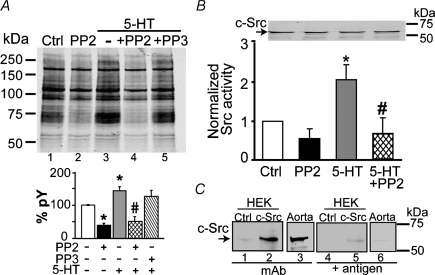
Figure 2. 5-Hydroxytryptamine increases total tyrosine-phosphorylated proteins and Src activity.
A, top panel shows a typical immunoblot of total tyrosine-phosphorylated proteins in aortic lysates, showing that 10 μm 5-HT alone (−; lane 3) increases, 10 μm PP2 pre-incubation decreases (lane 4) and 10 μm PP3 (lane 4) has no effect on protein tyrosine phosphorylation (pY). Signals of non-stimulated samples (control, Ctrl; lane 1) were also reduced by PP2 (lane 2). A, bottom panel shows mean values of total pY signals normalized to total protein signal (Ponceau S staining) and expressed as a percentage of the control, which was set as 100%. The pY signals and protein staining with Ponceau S were measured as the integrated intensity of each lane. Open bar, constitutive pY (Ctrl); black bar, PP2 inhibition of constitutive pY, indicating that a significant component is Src related; grey bar, 5-HT stimulated pY; cross-hatched bar, PP2 inhibition of 5-HT-induced pY; reverse-hatched bar, lack of PP3 effect. n = 4 independent experiments; *P < 0.05 versus Ctrl and #P < 0.05 versus 5-HT. B, Src kinase activity measured in aortic tissue lysates after stimulation or not (Ctrl) with 5-HT (10 μm) and with or without pre-incubation with PP2. Src kinase activity was measured by [γ-32P]-ATP radioactive incorporation to a Src optimal peptide and normalized to the radioactive phosphate [32P] incorporated per minute per milligram protein of the control sample. The immunoblot shows an equal amount of c-Src total protein in all samples (50 μg protein per lane) using anti-c-Src mAb (2 μg ml−1) and 0.06 μg ml−1 secondary Ab. A similar result was obtained with a generic Src tyrosine kinase Ab (not shown). n = 4 independent experiments for Src activity measurement and immunoblot; *P < 0.05 versus Ctrl and #P < 0.05 versus 5-HT. C, pre-adsorption test. The blot was pre-incubated (overnight, 4°C) with 0.3 μg ml−1 anti-c-Src mAb (lanes 1–3) or with a mixture of 0.3 μg ml−1 anti-c-Src mAb and 1 μg ml−1 of antigenic protein (recombinant full-length c-Src; lanes 4–6) prior to treatment with secondary Ab (0.04 μg ml−1, 1 h, at room temperature). The blot was then washed for 2 days before scanning. Samples were lysates from mock-transfected HEK 293T cells (Ctrl; 10 μg protein), from HEK 293T cells expressing c-Src (10 μg protein) and from aortic tissue (50 μg protein). In A and B, statistical analysis was performed using ANOVA followed by the multiple comparison Newman–Keuls test.
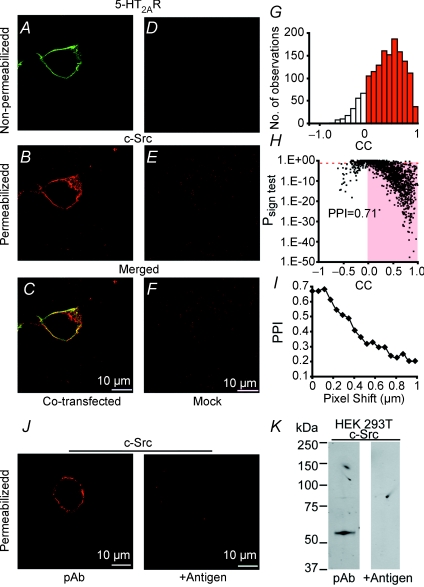
Figure 6. Co-localization of 5-HT2AR and c-Src at the surface membrane of HEK 293T cells.
A–C, cell cotransfected with 5-HT2AR and c-Src. D–F, Mock-transfected cell. Labelling of 5-HT2AR (green) was in live cells (non-permeabilized); the same cells were then fixed, permeabilized and labelled for c-Src (red). Merged images (yellow) show both proteins expressed at the cell surface. G, corresponding correlation coefficient (CC) histogram of 5-HT2AR to c-Src signals, but analysing all 16 confocal planes taken every 0.2 μm at 0.0575 μm per pixel. H, Psign test value of each data point as a function of its CC; PPI = 0.71. I, PPI of a single confocal plane when 5-HT2AR image was shifted every 57.5 nm in the x-axis. J, left panel, cell expressing c-Src, permeabilized and labelled with anti-c-Src pAb as in B; right panel, lack of signal with pre-incubation with antigen. K, left panel, immunoblot of lysates from HEK 293T cells expressing c-Src show a band of the expected molecular size of ∼60 kDa; right panel, signal is absent by pre-adsorbing the antigenic peptide with the pAb. Scale bars represent 10 μm.
Isometric contraction
Sprague–Dawley or Fisher 344 male rats (3 months old) were used. Data showed no substantial difference between the two strains. Aortic rings (endothelium denuded with a cotton thread) were mounted in modified Krebs buffer (mm): 119 NaCl, 4.7 KCl, 1.6 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 22 NaHCO3, 8 Hepes, 5 creatine, 20 taurine, 5 pyruvate and 5 glucose (pH 7.4, adjusted with NaOH) with 95% O2–5% CO2 at 37°C and connected to an isometric force transducer (World Precision Instruments). Tension was recorded with WINDAQ/200 (DATAQ Instrument). Rings were equilibrated for 60 min at the optimal resting tension (1 g) before experimentation. Several sets of experiments were performed, as follows:
Evaluation of the 5-HT2AR contribution to 5-HT-induced contraction by use of antagonists targeting different receptor subtypes: for 5-HT2AR, 10 nm ketanserin (a concentration ∼10 or 400 times lower than the IC50 for 5-HT2CR and 5-HT2BR, respectively); for 5-HT2BR, 0.1 μm SB204741 (a concentration that would only inhibit 5-HT2BR); and for 5-HT2CR, 20 nm RS102221 (a concentration that only inhibits 5-HT2CR). Aortic rings were incubated with each one of the antagonists for 20–30 min and then exposed to cumulative additions of 5-HT.
Comparison of the effectiveness of the Src inhibitor, PP2, in preventing contraction by different agonists. Aortic rings were pre-incubated with PP2 (10 μm), or its inactive analogue, PP3, for 20 min prior to agonist stimulation. Vasoconstrictors were used at a concentration producing 75% of their maximal effect (EC75), which were: 5.6 μm for 5-HT; 1.2 μm for α-methyl-5-HT (5-HT2R agonist); 2.5 nm for the TXA2 analogue, U46619; 20 nm for Ang II; and 18 nm for PE.
PP2 inhibitory dose–response curves of agonist-induced contraction. Aortic rings were incubated with single doses of PP2 (0.01–10 μm) for 20 min before addition of 5.6 μm 5-HT or 2.5 nm U46619.
Effectiveness of RhoK inhibitor in 5-HT-induced contraction. Aortic rings were incubated with single doses of the RhoK inhibitor, Y27632 (0.01–10 μm), for 20 min before 5-HT addition.
Contraction was measured as the integral of the force at constant time intervals or as maximal contraction. The percentage contraction was given by 100 × (agonist-induced contraction in the absence or presence of drugs/80 mm KCl-induced contraction). Effective concentration values (EC50) were calculated using a Hill function:
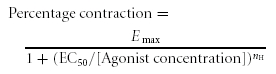 |
where Emax is maximal contraction, EC50 the concentration needed for 50% contraction and nH the Hill coefficient. Percentage inhibition by PP2 was calculated according to:
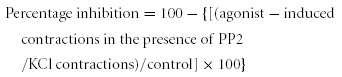 |
where control is the ratio of agonist-induced contraction in the absence of PP2 to the KCl-induced contraction. Mean data points were also fitted to a Hill function.
Tissue lysates, immunoblots and Src tyrosine kinase activity
Five aortas were used for each experiment. Each thoracic aorta was rubbed with a cotton swab from the endothelial face, and cut into five pieces that were sequentially assigned to each experimental condition. This assured equal representation of distal, median and proximal regions. Aortic segments were pre-incubated for 20 min with PP2 (10 μm), PP3 (10 μm) or Y27632 (10 μm), followed by stimulation with 5-HT (10 μm) or not. Ten minutes after stimulation, aortas were snap-frozen in liquid nitrogen. Tissue lysates were obtained by powdering the tissue on dry ice, followed by homogenization (4°C) in lysis buffer containing (mm): 50 Tris, 150 NaCl and 5 EDTA (pH 7.4, adjusted with HCl) supplemented with 2 Na3VO4, 50 NaF, 1 phenylmethylsulfonyl fluoride (PMSF), 0.1% nonylphenylpolyethylene glycol (NP-40 alternative), 0.25% sodium-deoxycholate and protease inhibitors (1/5th tablet Roche Diagnostics PROTEASE inhibitor per 10 ml). The lysate was precleared at 1000g for 10 min, followed by 30 min at 10 000g. Proteins were boiled in SDS loading buffer, separated on 7.5% SDS–polyacrylamide gels, and transferred to nitrocellulose membranes for immunoblot with anti-pY mAb (0.5 μg ml−1) or anti-c-Src mAb (2 μg ml−1). Signals were visualized using an infrared fluorescence system (Odyssey Imaging System, Li-Cor). The density of bands was measured with AutoQuant software (AutoQuant Imaging Inc.). Src tyrosine kinase activity in aortic lysates was quantified by [γ-32P]-ATP radioactive incorporation to a Src optimal peptide (KVEKIGEGTYYGVVYK, amino acids 6–20 of p34cdc2) according to the manufacturer's instructions (Upstate).
Preparation of 5-HT2AR expression construct
The wild-type human 5-HT2AR cDNA (NM_000621) cloned into pc-NMV6 was purchased from OriGene Technologies. To create an N-terminal epitope-tagged receptor, the 5-HT2AR coding sequence was PCR-amplified with a forward primer encoding a BamHI site and the epitope sequence of c-Myc (AEEQKLISEEDL), and a reverse primer including a NotI site. The PCR product was purified by agarose gel electrophoresis and Qiaex II (Qiagen), digested with BamHI/NotI and subcloned into pcDNA3 vector (Invitrogen). The predicted protein sequence is MVAEEQKLISEEDLV, followed by methionine of the 5-HT2AR sequence. To enhance membrane incorporation, a signal sequence (MKTIIALSYIFCLVFA; Guan et al. 1992) was added at the 5′-end using a similar cloning strategy. Cloned inserts were checked by sequence analysis.
Transient transfections
HEK 293T cells were grown to 70% confluence in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 50 units ml−1 penicillin (Invitrogen), 50 μg ml−1 streptomycin (Invitrogen) and 10% fetal bovine serum (Hyclone). Before transfection, medium was changed to DMEM without serum and antibiotics; the cells were then transfected with combinations of plasmids for 5-HT2AR and c-Src (1:1 molar ratio) using Lipofectamine™ 2000 (Invitrogen). Plasmid DNA to Lipofectamine™ 2000 ratio was 1:2 (w/v). Transfected cells were used 48 h after transfection. Control experiments included mock-transfected cells and cells transfected with 5-HT2AR or with c-Src alone. The c-Src (GenBank Accession No. V00402) construct used has been previously described (Alioua et al. 2002).
Non-permeabilized (live) and permeabilized immunostaining of HEK 293T cells
Cells were seeded on 12 mm diameter coverslips (placed on 12-well plates) 24 h before transfection. The transfection steps were the same as described in the subsection above. After 48 h, cells that were mock-transfected or cotransfected with 5-HT2AR and c-Src were incubated with anti-c-Myc mAb (6 μg ml−1 in DMEM; to label the extracellular c-Myc epitope of 5-HT2AR) for 1 h on an ice chamber placed in a 5% CO2: 95% air incubator at 37°C. This procedure prevented significant Ab endocytosis. The cells were then washed with phosphate-buffered saline (PBS), fixed, permeabilized and immunolabelled with anti-c-Src pAb (0.6 μg ml−1) in PBS containing 1% normal goat serum and 0.2% Triton X-100 followed by incubation with secondary antibodies (2 μg ml−1; 1 h, room temperature). Cells were mounted using Prolong (Molecular Probes) for imaging. Confocal images were taken every 0.2 μm at 0.0575 μm per pixel with a confocal microscope (Olympus Fluoview). After three-dimensional blind deconvolution (AutoQuant Imaging Inc.), pixel intensities were measured with Metamorph (Universal Imaging Corporation) and analysed with home-made software to obtain correlation coefficients (CC) and protein proximity index (PPI). Briefly, the images were divided with a grid of equal squares (2 μm2), and the CC and the statistical significance using the normal approximation of the sign test (Psign test) were calculated for each square in the grid. The CC between arrays A and B of images A and B was calculated from:
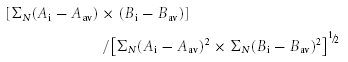 |
where Ai and Bi are the intensities of pixel i in image A and B, respectively; Aav and Bav their average values; and N is the number of pixels. To quantify the degree of protein proximity or PPI, we divided the number of squares with positive CC values and Psign test < 0.05 by the total number of squares as follows:
where N(CC > 0; P < 0.05) is the number of squares with CC > 0 and Psign test < 0.05 and Ntotal is the total number of squares (Lu et al. 2006; Ropero et al. 2006).
Immunoprecipitation (IP) and co-immunoprecipitation (co-IP)
At 48 h after transfection, the cells from different groups were harvested and lysed with lysis buffer, as mentioned above, supplemented with 2 mm Na3VO4, 1 mm NaF, 1 mm PMSF, 0.1% NP-40 alternative, 0.25% sodium-deoxycholate and protease inhibitors (1/5th tablet Roche Diagnostics PROTEASE inhibitor™ per 10 ml). Lysates were kept on ice for 30 min with shaking and centrifuged at 1200g for 10 min. The supernatant (cell lysate) was precleared with rProtein A beads (Amersham) before IP. Immunoprecipitation was performed with rProtein A beads (50 μl) pre-incubated with anti-c-Myc (2 μg) or anti-c-Src (2 μg) mAbs for 3 h at 4°C. The prebound beads were washed and incubated with 500 μl cell lysate (1 mg protein) overnight at 4°C with shaking. Immunoprecipitated products were washed extensively and eluted from the beads by resuspension in 15 μl 3 × SDS sample loading buffer and heating for 5 min at 67°C (Xia et al. 2003). Boiling was avoided because it induced aggregation of the immunoprecipitated 5-HT2AR proteins. The whole IP products were separated in SDS gels and immunoblotted simultaneously with anti-c-Myc pAb (0.4 μg ml−1) or mAb (1.2 μg ml−1) and anti-c-Src mAb (0.5 μg ml−1) or pAb (0.2 μg ml−1). Signals were detected with infrared fluorescence that allowed simultaneous membrane staining of 5-HT2AR and c-Src using Alexa Fluor 680- and IRDye800-conjugated secondary Abs, respectively.
Statistics
Results are expressed as means ±s.e.m. In contraction experiments, n is the number of rats. Unless otherwise stated, data were analysed by Student's unpaired t test or by ANOVA followed by the multiple comparison Newman–Keuls test. Values of P < 0.05 were considered significantly different.
Results
Src tyrosine kinase and 5-HT2AR show a strong functional coupling in native aorta
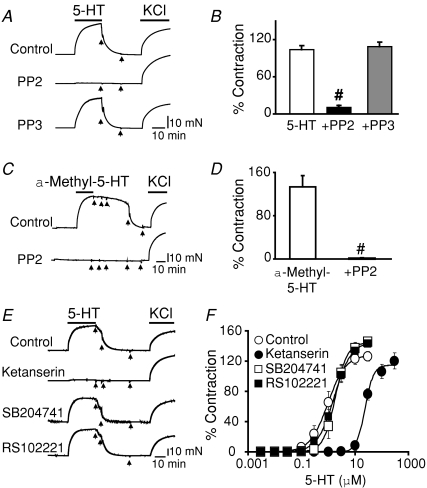
We first investigated the degree of functional coupling between Src and 5-HT receptors by pre-incubating aortic rings with 10 μm PP2 prior to stimulation with 5.6 μm 5-HT. Figure 1A shows that pre-incubation with PP2 completely abolished 5-HT-induced contraction (middle trace). In contrast, the inactive analogue, PP3 (bottom trace), had no effect, since the 5-HT-induced contraction was similar to the control trace (top trace). After washout (arrows), 80 mm KCl was able to elicit contractions of similar strength to those of 5-HT in all cases. Data analysis demonstrates that Src inhibition with PP2, prior to 5-HT stimulation, causes an almost complete inhibition of the 5-HT contractile response (90 ± 2.5% inhibition; n = 4–12) with respect to 5-HT alone (Fig. 14B). The same result was obtained if the 5-HT2R agonist α-methyl-5-HT was used. Figure 1C illustrates the contractile response to 1.2 μmα-methyl-5-HT stimulation (control) and its complete prevention by pre-incubation with 10 μm PP2. Mean values in Fig. 1D show a 99%± 0.8 (n = 3) PP2 inhibition of 5-HT2R-mediated contractions. Consistent with a major role of 5-HT2AR in 5-HT- and α-methyl-5-HT-induced contractions in aorta (Watts et al. 2001) and in Src coupling, 10 nm ketanserin (a concentration that only inhibits 5-HT2AR, see Methods) was able to mimic the PP2 response and fully prevent the contraction induced by 10 μm 5-HT. In contrast, antagonists of 5-HT2BR (100 nm SB204741) and 5-HT2CR (20 nm RS102221) had no effect on 5-HT-induced contractions (Fig. 1E and F). Together, the results demonstrate that the main receptor involved in 5-HT-induced contraction coupled to Src tyrosine kinase is the 5-HT2AR. In addition, they implicate Src tyrosine kinase activity as a key factor in mediating 5-HT-induced contraction to the extent that Src pre-inhibition by PP2 practically abolished 5-HT-induced contraction.
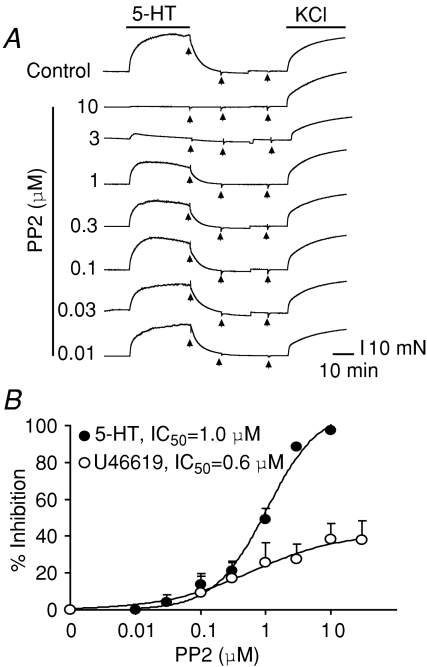
Figure 1. The Src tyrosine kinase inhibitor, PP2, prevents contraction triggered by 5-HT or the 5-HT2R agonist, α-methyl-5-HT.
A, contractile traces of aortic rings without endothelium induced by 5.6 μm 5-HT (EC75) and followed by 80 mm KCl. Rings were pre-incubated for 20 min or not (control) with 10 μm PP2 or with 10 μm of its inactive analogue, PP3. 5-Hydroxytrypamine-induced contractions were as potent as those induced by KCl and were prevented by PP2. B, mean contraction expressed as a percentage of the response to 80 mm KCl (n = 4–12). C, similar contractile responses were obtained using 1.2 μmα-methyl-5-HT (EC75) stimulation, and these were prevented by pre-incubation with 10 μm PP2. D, mean contraction expressed as a percentage of the response to 80 mm KCl (n = 3). E, contractile traces induced by 10 μm 5-HT recorded after 30 min pre-incubation without (control) or with 5-HT2AR, 5-HT2BR or 5-HT2CR antagonists at concentrations that target specific receptor types (see Methods): 10 nm ketanserin (5-HT2AR), 100 nm SB204741 (5-HT2BR) and 20 nm RS102221 (5-HT2CR). After washout, 80 mm KCl induced contractions in all cases. F, 5-HT dose–response curves in the absence or presence of antagonists. Mean data points were fitted to a Hill function. Only 10 nm ketanserin shifted the dose–response curve to the right (control EC50= 1 μmversus ketanserin EC50= 24 μm; n = 3). Arrows indicate washout. Values are expressed as means ±s.e.m.; #P < 0.05 versus 5-HT or versusα-methyl-5-HT.
Src tyrosine kinase activity is increased by 5-HT
To test whether 5-HT induces Src activity as predicted by the inhibition of 5-HT-induced contraction by PP2, we directly measured changes in tyrosine phosphorylated proteins and in Src activity after 5-HT stimulation for 10 min. The amount of tyrosine-phosphorylated proteins was evaluated with anti-pY mAb in lysates of aortas stimulated or not with 5-HT. The immunoblot in Fig. 2A shows that the overall protein tyrosine phosphorylation was increased with 10 μm 5-HT stimulation (10 min), decreased with 10 μm PP2 pre-incubation (20 min) and unaffected by 10 μm PP3. In addition, PP2 reduced basal tyrosine phosphorylation (control, Ctrl). The bar graph in Fig. 2A summarizes the mean percentage values of pY signals due to constitutive (lanes 1, 2) and 5-HT-stimulated phosphorylation (lanes 3–5), where PP2 but not PP3 fully inhibited 5-HT-induced increase in pY signals, indicating that 5-HT promotes Src activation (n = 4 independent experiments). Furthermore, measurement of Src kinase activity using the same tissue lysates as in Fig. 2A revealed that 5-HT promotes an increase in Src kinase activity of about twofold, and that this increase is completely reversed by PP2 (Fig. 2B). Although statistically insignificant, PP2 consistently diminished Src activity in non-stimulated conditions, indicating that aortic tissues possess a modest basal Src activity (n = 4 independent experiments). As expected for a short-term signalling process, the total amount of c-Src protein remained the same after 5-HT stimulation as shown by immunoblot with anti-c-Src mAb (Fig. 2B, top). To confirm the specificity of the mAb, we performed a pre-adsorption test (Fig. 2C). The mAb recognizes an ∼60 kDa band that is almost undetectable in mock-transfected (Ctrl) HEK 293T cells (endogenous c-Src, lane 1) but clearly detected in HEK 293T cells transfected with c-Src (Fig. 2C, lane 2) and in aorta (Fig. 2C, lane 3; Fig. 2B, top). As expected, the signal was significantly reduced by pre-incubation of the mAb with recombinant c-Src (+ antigen; Fig. 2C, lanes 4–6). In summary, the results demonstrate that 5-HT induces Src phosphorylating activity that is fully prevented by PP2, which supports Src activation as a key mechanism in 5-HT-induced contraction via 5-HT2AR.
Role of Src tyrosine kinase in contraction induced by Ang II, the TXA2 analogue, U46619, and the α1-adrenergic agonist, PE
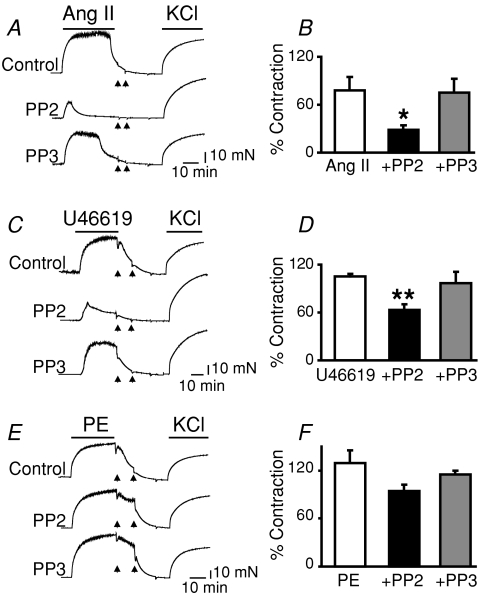
A systematic study of several GPCR agonists was performed to investigate the role of Src in their contractile responses. Contrary to the strong coupling of Src to 5-HT-induced contractions, the degree of Src coupling to Ang II- and U46619-induced contractions was weaker, and was null for PE-induced contractions as measured by PP2-mediated inhibition of contraction (Fig. 3). Angiotensin II contractile responses were inhibited by 64 ± 4.6% (Fig. 3A and B; n = 5), and those for U46619 were inhibited only by 40 ± 5.6% (Fig. 3C and D; n = 3–11) by PP2, which allowed in these cases transient and small responses (Fig. 3A and C, middle traces). Pre-incubation with PP2 had no significant effect on contractions induced by PE (Fig. 3E and F; n = 4) or KCl (data not shown). The absence or diminished contraction in response to 5-HT induced by PP2 (Fig. 1A), Ang II and the TXA2 analogue, U46619 (Fig. 3A and C), were not due to loss of tissue integrity, since the aortic rings were readily stimulated with 80 mm KCl after washout of the agonists (arrows). As expected for a specific effect of PP2, pretreatment with its inactive analogue, PP3, showed no significant effect on the ability of the tested agonists to induce contraction (lower traces, Fig. 3A, C and E). This analysis uncovers aortic 5-HT2AR as a preferred GPCR that couples to Src, being in the other side of the spectrum α1-adrenergic GPCRs.
Figure 3. Role of Src tyrosine kinase in contractions induced by Ang II, the TXA2 analogue, U46619, and PE in rat aorta.
Endothelium-denuded aortic rings were pretreated with 10 μm PP2 or with its inactive analogue, PP3 (10 μm), for 20 min before stimulation with Ang II (20 nm), U46619 (2.5 nm) or PE (18 nm) at their EC75. A, C and E show contractile traces of Ang II (A), U46619 (C) and PE stimulation (E). After washing out the drugs (arrows), all of the rings were contracted with 80 mm KCl. B, D and F show mean contraction expressed as a percentage of the response to 80 mm KCl, showing PP2 inhibitory effectiveness Ang II > U46619 (B and D) and lack thereof for PE-induced contraction (F). Pretreatment with PP3 had no significant effect in all cases. n = 5 (Ang II), n = 3–11 (U46619) and n = 4 (PE); *P < 0.05 and **P < 0.01 versus respective agonists. Statistical analysis was performed using ANOVA followed by the multiple comparison Newman–Keuls test.
Pharmacological evidence for a role of c-Src in 5-HT-induced contraction
The pyrazolopyrimidines, PP1 and PP2, inhibit several members of the Src tyrosine kinase family with similar affinities (Hanke et al. 1996; Waltenberger et al. 1999). Both drugs can differentiate Src-family kinase members Lck, Fyn and Hck, which have IC50 values of ∼5–20 nm (Hanke et al. 1996), from c-Src, which has IC50 values of ∼0.3 μmin vitro and ∼1 μm in c-Src-transformed cells (Waltenberger et al. 1999). c-Src is abundant in smooth muscle (Oda et al. 1999) and is therefore a good candidate to mediate 5-HT-induced contraction. We reasoned that if this were the case, the PP2 IC50 for 5-HT-induced contraction would be in the high nanomolar to low micromolar range. Figure 4A shows traces of a typical dose–response curve using PP2 concentrations ranging from 10 nm to 10 μm. As in Fig. 1, PP2 was pre-incubated for ∼20 min prior to stimulation with 5-HT and therefore different aortic rings were used to test each inhibitor concentration. It is evident that low nanomolar concentrations were unable to prevent 5-HT-induced contractions and that low micromolar concentrations were needed, supporting a role for c-Src. Figure 4B shows the mean dose–response curves fitted to a Hill function (continuous line) with an IC50 of 1.0 ± 0.1 μm and a maximal inhibition (Imax) of 109 ± 6% (n = 3; ±s.e.m. of the fit). For comparison, we also obtained the PP2 dose–response curve for the TXA2 analogue, U46619, which showed a similar IC50 of 0.6 ± 0.09 μm (P > 0.05) but much less efficacy than 5-HT, with an Imax of 42 ± 1.4% (n = 6–10; ±s.e.m. of the fit). These results indicate that most of aortic 5-HT2ARs are coupled to c-Src to mediate contraction, whereas only ∼60% of TXA2 receptors have this property.
Figure 4. Role of c-Src in 5-HT- and U46619-induced contraction.
A, tension of aortic rings was recorded without (control) or after 20 min pre-incubation with different doses of PP2 (0.01–10 μm) followed by addition of 5.6 μm 5-HT. At the end of each experiment, drugs were washed out (arrows) and the rings were stimulated with 80 mm KCl. B, percentage inhibition was calculated as described in the Methods. Mean data points were fitted (continuous line) to a Hill function. Fitted curves are for 5-HT (•), IC50= 1.0 ± 0.1 μm PP2, nH= 1.0 ± 0.1, Imax= 109 ± 6% (n = 3); and for U46619 (^), IC50= 0.6 ± 0.09 μm PP2, Imax= 42 ± 1.4%, nH= 0.6 ± 0.05 (n = 6–10). The PP2 IC50 values for both 5-HT and U46619 contractions indicate a role for c-Src (see text).
Association of 5-HT2AR with c-Src in HEK 293T cells
We next examined whether 5-HT2AR and c-Src can associate with each other in HEK 293T cells cotransfected with 5-HT2AR tagged with c-Myc epitope at its NH2-terminus (Fig. 5A). Experiments in native tissues were not possible owing to the lack of appropriate 5-HT2AR antibodies (see Discussion). Each pair of images in Fig. 5B–D corresponds to the same immunoblot that was simultaneously labelled for 5-HT2AR (with anti-c-Myc Ab) and c-Src. Figure 5B illustrates expression in lysates of cells transfected or not with c-Src ± 5-HT2AR that served as reference for the co-IP experiments. Signals for c-Src were, as expected, at ∼60 kDa, with a very faint signal of endogenous c-Src detected in lysates from mock-transfected cells. The main immunosignals for cells transfected with 5-HT2AR showed an apparent molecular mass of ∼60–66 kDa and a faint band that was sometimes difficult to detect at ∼51–55 kDa, close to the calculated molecular mass of c-Myc-tagged 5-HT2AR (∼54 kDa); the two c-Myc immunoreactive protein species could correspond to the mature and immature forms of the receptor, respectively, as observed for other receptor subtypes (Weiss et al. 1998; Monk et al. 2004).
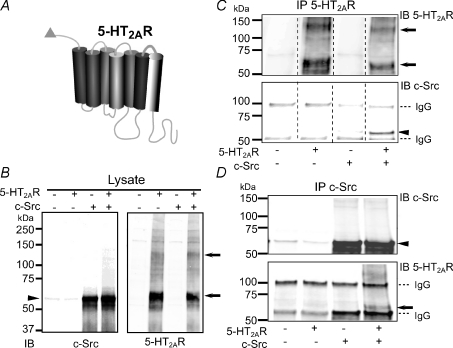
Figure 5. Association of 5-HT2AR and c-Src in cotransfected HEK 293T cells.
A, diagram of 5-HT2AR with c-Myc tag (triangle) at the N-terminus. B, immunoblot (IB) showing the expression of c-Src (left panel, anti-c-Src mAb) and 5-HT2AR (right panel, anti-c-Myc pAb) in lysates of cells transfected or not with 5-HT2AR ± c-Src. Thirty micrograms of total protein was loaded in each lane. C, IP of 5-HT2AR (with anti-c-Myc mAb) immunoblotted (IB) for 5-HT2AR (with anti-c-Myc pAb; top) and for c-Src (with anti-c-Src mAb; bottom). Only when both proteins were coexpressed, IP of 5-HT2AR pulled down c-Src (bottom panel, last lane). D, reverse co-IP using anti-c-Src mAb for IP. Immunoblots were probed with anti-c-Src pAb (top) and anti-c-Myc mAb for 5-HT2AR (bottom). The 5-HT2AR co-immunoprecipitates with c-Src only when both proteins are coexpressed (bottom panel, last lane). In all panels, arrows mark 5-HT2AR immunosignals with apparent molecular masses of ∼60–62 and ∼125–128 kDa that would correspond to mature monomeric and dimeric forms of the receptor. A faint band at ∼51–55 kDa (B and C) could be the immature form of the 5-HT2AR. Arrowheads mark c-Src signal at ∼60 kDa. Immunoglobulin G is marked with a dashed line. Three independent experiments were performed in each set of conditions. Concentrations of Abs are given in the Methods.
Figure 5C and D exemplifies the reciprocal co-IP of c-Src with 5-HT2AR. When anti-c-Myc mAb was used to immunoprecipitate 5-HT2AR (Fig. 5C, top panel; immunoprecipitated 5-HT2AR was labelled with anti-c-Myc pAb), c-Src was clearly co-immunoprecipitated only when both proteins were coexpressed (Fig. 5C, bottom panel, last lane, arrowhead); we also observed that 5-HT2AR immunosignals matched with monomeric and dimeric forms (arrows, Fig. 5B and C). Likewise, when anti-c-Src mAb was used to immunoprecipitate c-Src (Fig. 5D, top panel, arrowhead), 5-HT2AR was pulled down (Fig. 5D, bottom panel, arrow) only when both proteins were coexpressed. Also, the endogenous c-Src was immunoprecipitated by anti-c-Src mAb (Fig. 5D, top panel, faint bands in first two lanes). The specificity of the co-IP assay is supported by the facts that each of the co-IP experiments was dependent on the expression of both c-Src and 5-HT2AR proteins (Fig. 5C and D) and that IP with no Ab gave no signals (not shown). Similar co-IP of c-Src was obtained if N-terminal c-Myc-, C-terminal FLAG-tagged 5-HT2AR was used. In this case, the immunoprecipitated 5-HT2AR (with anti-c-Myc mAb) was detected with anti-FLAG pAb (not shown). The experiments demonstrate that 5-HT2AR and c-Src can associate with each other and indicate that they may share similar microenvironments, forming a signalling complex.
Co-localization of 5-HT2AR with c-Src tyrosine kinase in HEK 293T cells
The possibility that 5-HT2AR and c-Src proteins share similar microenvironments was tested using fluorescence confocal microscopy in HEK 293T cells coexpressing both proteins. In particular, we were interested to determine whether they could co-localize at the plasma membrane, since this geography would facilitate their functional coupling. The results are illustrated in Fig. 6, which shows examples of single confocal sections at the middle of the cell. Live labelling of 5-HT2AR (Fig. 6A; non-permeabilized, with anti-c-Myc mAb) and of c-Src after permeabilization (Fig. 6B; with anti-c-Src pAb) shows that both proteins are mainly compartmentalized at the plasmalemmal surface. When images are overlapped (Fig. 6C), fluorescence signals show a striking ‘co-localization’. Mock-transfected cells showed practically no signals (Fig. 6D–F). Figure 6G–I shows the corresponding analysis of protein proximity index (PPI), which for this cell was 0.71. The correlation coefficient (CC) histogram of all confocal planes demonstrated that the majority of signals were positively correlated (Fig. 6G) and with a Psign test < 0.05 (Fig. 6H, shaded box). The localization precision of the method in the x–y plane was evaluated by shifting one of the planes every 57.5 nm in the x-axis, which demonstrated that the PPI decays to about half of its initial value by a shift of only ∼400 nm (Fig. 6I). Image analysis of several preparations demonstrated a high degree of protein proximity at the plasma membrane, with a mean PPI value of 0.7 ± 0.02 (n = 23 cells, 3 transfections). The fidelity of the analysis was evaluated by labelling the c-Src protein with two different antibodies (pAb and mAb) which, as expected, gave a higher PPI of 0.84 ± 0.01 (n = 18 cells, 3 transfections). The specificity of the anti-c-Src pAb was defined by the lack of signals when the pAb was pre-incubated with the antigenic peptide in immunocytochemistry (0.6 μg ml−1 pAb, 6 μg ml−1 antigen; Fig. 6J, right panel) and immunoblot analysis (0.4 μg ml−1 pAb, 4 μg ml−1 antigen; Fig. 6K, right panel) of HEK 293T cells expressing c-Src. In addition, the anti-c-Src pAb recognized a band of the expected molecular size for c-Src at ∼60 kDa (Fig. 6K, left panel). In summary, the visualization of 5-HT2AR and c-Src in close proximity (at least 400 nm apart) in HEK 293T cells indicates that the two proteins have the ability to share a similar subcellular distribution that could be important for effectively responding to 5-HT stimulation.
Contractile signalling of 5-HT2AR to c-Src, an early mechanism upstream of RhoK
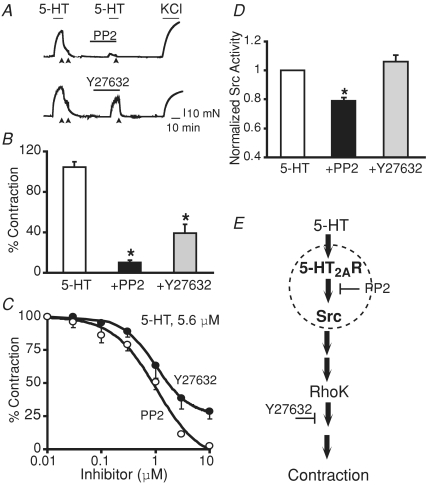
The ability of 5-HT2AR and c-Src to form a complex in HEK 293T cells would predict a functional coupling in the early stages of the contractile signalling cascade. In support of this hypothesis, Src activation was found to occur upstream of RhoK a well-established signalling pathway linked to the 5-HT cascade (Kandabashi et al. 2002). First, we performed contraction experiments to compare the inhibitory action of the Src kinase activity inhibitor, PP2, at a concentration ∼10 times the IC50 value for c-Src, with that of RhoK activity inhibitor, Y27632, at ∼10 times its IC50 value (Uehata et al. 1997; Waltenberger et al. 1999). Figure 7A shows contraction traces of aortic rings in response to 5-HT application followed by washout (arrows) and pre-incubation with 10 μm PP2 or 10 μm Y27632 for 20 min prior to application of 5-HT. As expected, both the inhibitors, PP2 and Y27632, significantly blocked 5-HT-induced contraction. However, the inhibitory action of PP2 was more pronounced than that of Y27632. At 10 μm 5-HT, which elicits maximal contraction, 10 μm PP2 produced ∼92% inhibition versus∼68% inhibition by 10 μm Y27632 (n = 3; Fig. 7B). Dose–response curves confirmed that PP2 has a higher efficacy than Y27632 at all concentrations tested in inhibiting 5.6 μm (EC75) 5-HT-induced contraction (Fig. 7C), highlighting the functional relevance of Src. Next, Src kinase activity was measured in lysates of aortic tissue stimulated with 5-HT (10 μm) in the presence or absence of 10 μm PP2 or 10 μm Y27632. We expected that if Src were upstream of RhoK, blockade of RhoK should not affect Src kinase activity. In fact, Src kinase activity was unaffected by Y27632 but significantly inhibited by PP2 (Fig. 7D). These results support the notion that Src (probably c-Src) activation precedes RhoK activity in the 5-HT–5-HT2AR–c-Src contractile signalling pathway (Fig. 7E).
Figure 7. Rho kinase inhibitor, Y27632, represses 5-HT-induced contraction but has no effect on 5-HT-induced Src kinase activity in rat aorta.
A, examples of PP2 and Y27632 effect on 5-HT-induced contraction in aortic rings without endothelium. After the first 5-HT (10 μm) challenge, rings were washed out (arrows) and incubated with either 10 μm of PP2 or Y27632 for 20 min prior to challenging again with 5-HT. Rings were then washed out (arrows) and stimulated with KCl (80 mm). B, mean values of contraction (expressed as a percentage of KCl response) elicited by 10 μm 5-HT alone or when strips were pre-incubated with 10 μm of either PP2 or Y27632. The Rhok inhibitor Y27632 can diminish 5-HT-induced contraction (∼68%) but is less potent when compared with the Src inhibitor, PP2 (∼92%); n = 3, *P < 0.05 versus 5-HT. C, percentage 5-HT (5.6 μm)-induced contraction (normalized to 80 mm KCl-induced contraction) as a function of Y27632 and PP2 concentration; n = 3–4. D, Src kinase activity measured in aortic lysates of tissues pre-incubated or not with 10 μm PP2 or 10 μm Y27632 prior to stimulation with 10 μm 5-HT. Radioactive [32P] incorporation was normalized to values obtained with 5-HT alone; n = 4 independent experiments, *P < 0.05 versus 5-HT. E, Diagram of 5-HT2AR–c-Src–RhoK contractile cascade induced by 5-HT. Since Y27632 is unable to inhibit Src activity, RhoK signalling must occur downstream of Src to regulate contraction. The dashed circle represents the 5-HT2AR and c-Src macromolecular complex. Statistical analysis was performed using ANOVA followed by the multiple comparison Newman–Keuls test.
Discussion
Central role of c-Src in 5-HT2AR contractile signalling
We found that Src activation is a critical and early mechanism of 5-HT contractile responses in vascular smooth muscle (Figs 1 and 7). The central role of c-Src in rat aorta is supported by the robust quasi-full blockade of 5-HT- or α-methyl-5-HT-induced contraction by the Src kinase inhibitor, PP2, with an IC50 of ∼1 μm, reminiscent of PP1 and PP2 IC50 values for c-Src (Waltenberger et al. 1999; Wijetunge et al. 2000; Fig. 4). Furthermore, the increase in Src kinase activity and phosphorylated proteins by 5-HT and their inhibition by PP2 (Fig. 2) confirm the activation of this tyrosine kinase in 5-HT contraction signalling. Similar to our findings with PP2, it was recently shown that the analogue PP1 is also able to produce a large inhibition of 5-HT-induced contraction when used at a single dose of 10 μm, although in this case the IC50 value was not determined (Ogden et al. 2006). 5-Hydroxytryptamine–c-Src coupling that leads to contraction mainly involves 5-HT2AR in aorta, since activation of 5-HT2R with α-methyl-5-HT was abolished by PP2 by nearly 100%, and 5-HT2AR antagonist (10 nm ketanserin), but not antagonists of 5-HT2BR or 5-HT2CR, prevented 5-HT-induced contractions (Fig. 1). These results agree with the concept that 5-HT2AR is the major contractile receptor subtype in rat and mouse aorta, although mRNAs of 5-HT1B/1D, 5-HT1F, 5-HT2A/2B and 5-HT7 receptor are found (Ullmer et al. 1995; Watts et al. 2001; McKune & Watts, 2001).
Src activation as an early aortic mechanism upstream of RhoK in the 5-HT contractile pathway is supported by the lack of inhibition of Src kinase activity by the RhoK inhibitor, Y27632 (Fig. 7) and goes along with the localization of c-Src at the myocyte plasmalemma (Fig. 8) and its striking co-localization with 5-HT2AR at the plasma membrane of HEK 293T cells (Fig. 6). Interestingly, in coronary smooth muscle, sphingosylphosphorylcholine-dependent regulation of contraction also appears to be linked to Src upstream of RhoK, because Src autophosphorylation is resistant to Y27632; however, in this case, a role of Fyn Src kinase was supported (Nakao et al. 2002). Since in aorta, the pharmacological profile of Src inhibition matches that for c-Src (IC50∼1 μm) and not of Fyn (IC50∼5–25 nm; Hanke et al. 1996; Waltenberger et al. 1999; Wijetunge et al. 2000) and c-Src can co-localize with 5-HT2AR in cotransfected cells (Fig. 6), it is safe to propose that in aorta c-Src is the main Src family player in 5-HT contractile signalling. Further, distinct RhoK-linked signalling pathways may be triggered by specific Src family members in an agonist-dependent manner.
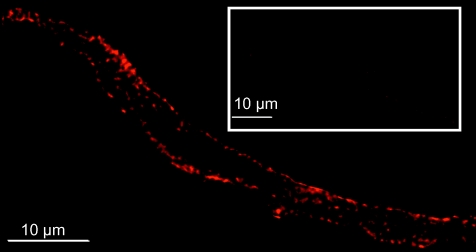
Figure 8. c-Src expression in single aortic myocytes.
Example of an aortic myocyte labelled with anti-c-Src mAb. Fluorescence signal at the periphery of the cell is observed. No labelling was observed with secondary Ab alone (inset). See Fig. 2C for blocking of mAb signal in aortic tissue. Enzymatic dissociation of aortic myocytes has been previously described (Alioua et al. 2008).
In addition to RhoK, potential downstream networks of Src activation in 5-HT2AR-dependent regulation of aortic contraction include c-Src-dependent inhibition of large conductance voltage and Ca2+-activated K+ (MaxiK) channels (Alioua et al. 2002) and Src–Crk-associated substrate (CAS) interactions (Ogden et al. 2006). A functional association of Src with phospholipase C γ1 has been demonstrated in hypothalamic GT1-7 cells upon 5-HT stimulation (Kim et al. 2006), but participation of this complex in vascular contraction is yet to be established.
c-Src selectivity in agonist-induced contraction
The experiments illustrated in Figs 1 and 3 highlight the differential role of c-Src when aortic smooth muscle is challenged with the distinct constricting agonists, 5-HT, Ang II, U46619 (TXA2 analogue) and PE. In contrast to the quasi-full prevention of 5-HT-induced contraction by c-Src inhibition using PP2 prior agonist stimulation, the PE-induced contraction was unaffected, while U46619- and Ang II-induced contrations were partly blocked. The role of c-Src in TXA2-induced contraction is shown for the first time here and is supported by our pharmacological experiments demonstrating an IC50 for PP2 of ∼0.6 μm (Fig. 4) which, as mentioned before, is within the range of c-Src inhibition (Waltenberger et al. 1999). With respect to the role of c-Src in Ang II-induced aortic contraction, several indirect lines of evidence support the role of this kinase in cultured myocytes, as follows: (1) studies in cultured aortic myocytes where PP1 or c-Src Ab almost or completely abolished events [e.g. phosphatidylinositol (3,4,5)-trisphosphate (PIP3) production] that would ultimately lead to contraction (Marrero et al. 1995; Venema et al. 1998); (2) inhibition (∼62%) of Ang II-induced Ca2+ transients in cultured mesenteric smooth muscle of c-Src null mice (Touyz et al. 2001); and (3) full inhibition of Ang II-induced c-Src activity by 10 μm PP2 in cultured human vascular myocytes (Touyz et al. 2001). In native tissues, we showed evidence that in aorta Ang II contractile signalling is also linked to Src kinase, since PP2 (10 μm) inhibited ∼65% contraction (Fig. 3A and B). These results are supported by experiments in mesenteric arteries also showing that 10 μm PP2 inhibits ∼80% of Ang II-induced contraction (Touyz et al. 2001). In summary, in native aorta both Ang II and TXA2 use Src (probably c-Src)-dependent pathways, at least partly.
Our previous results showed that vessels precontracted with PE (α1-adrenergic receptor agonist) were relaxed by postexposure to PP2, implicating Src as part of the PE contractile cascade (Alioua et al. 2002). However, in the present study, pre-exposure to PP2 could not prevent PE-induced contraction. A simple explanation is that the PE contractile cascade does not require Src tyrosine kinase activation in the early steps of excitation but rather in steps that sustain contraction. In contrast to our findings using the Src tyrosine kinase inhibitor, PP2, PE-induced contractions were significantly inhibited by pre-incubation with inhibitors of broad action like geldanomysin and tyrphostin in aorta, carotid and mesenteric arteries (Di Salvo et al. 1993; Carter & Kanagy, 2002) and by genistein in pulmonary arteries (Janssen et al. 2001), suggesting that tyrosine kinases other than Src play key roles in α1-adrenergic induced contraction, especially in aorta. Differences in other vessels may also represent distinct tyrosine kinase coupling to contraction in the vascular tree. Other adrenergic receptors that work via c-Src-independent contractile pathways in aorta are α2-adrenergic receptors (Carter & Kanagy, 2002).
G-protein-coupled receptors and c-Src macromolecular complexes
In line with our results in HEK 293T cells showing that 5-HT2AR can associate with c-Src in a macromolecular complex (Figs 5 and 6), growing evidence indicates that GPCRs, in addition to interacting with classical G-proteins, interact with a variety of either soluble or transmembrane proteins (Hall et al. 1999; Bockaert et al. 2004). Furthermore, recombinant studies have shown a direct interaction between c-Src and β3-adrenergic receptors (Cao et al. 2000), and between c-Src and Gαs and Gαi (Ma et al. 2000). An indirect interaction via β-arrestin has been reported for c-Src and β2-adrenergic receptors (Luttrell et al. 1999). The physical association between 5-HT2AR and c-Src could be direct or via a scaffolding protein.
Owing to the limitation of specific 5-HT2AR Abs (commercial or home-made), we could not develop immunocytochemistry or co-IP experiments to directly confirm that these two proteins associate with each other in native aortic tissues. In particular, the mAb from Pharmingen (Wu et al. 1998), which gave fine plasma membrane labelling in aortic myocytes and a band close to the expected molecular size in aortic cell lysates and lysates of the A7r5 aortic cell line (that could be blocked by its antigenic epitope peptide), raised concern because it could not label in immunoblots or immunocytochemistry HEK 293T or COS M6 cells transfected with 5-HT2AR tagged with c-Myc (N-terminus) or FLAG (C-terminus) epitope for positive recognition. Moreover, this mAb yielded similar immunoblot signals in brain and aortic lysates of wild-type and 5-HT2AR knockout animals (not shown; Weisstaub et al. 2006), thus precluding its usage. As an alternative approach, we used HEK 293T cells coexpressing c-Myc-tagged 5-HT2AR and c-Src proteins, asserting first that their ectopic expression leads to a distribution pattern (expressing at the surface membrane) resembling that of c-Src (Fig. 8) or expected for 5-HT2AR in aortic myocytes; this reassured their usage as a model system. The results in coexpressing cells highlighted the finding that 5-HT2AR and c-Src can distribute closely and interact with each other and, although the results cannot be fully extrapolated to the native tissue, they hint at the possibility that, by forming a macromolecular complex, c-Src and 5-HT2AR would facilitate contractile signal transduction in native aorta. Direct proof of this concept in native tissues awaits development of new molecular tools.
Functional significance
In resistance vessels such as the mesenteric arteries, as in the aorta, 5-HT2AR and c-Src are present (Watts, 2002; Luo et al. 2004; Yogi et al. 2007), and our unpublished results indicate that they are also functionally coupled (pretreatment with 10 μm PP2 prevents by ∼93% the 5-HT-induced contraction of mesenteric rings elicited at 5-HT EC75 of 1 μm, n = 3). Since c-Src contributes to normal Ca2+ signalling (Touyz et al. 2001) and to inhibition of MaxiK channel activity (Alioua et al. 2002), it is possible that c-Src coupling to 5-HT2AR activation regulates vascular tone via these mechanisms and in situations where enough serotonin is present locally to activate 5-HT2AR. Under normal conditions, plasma 5-HT levels are ∼100 nm or less (Crawford, 1965; Somerville, 1976; Engbaek & Voldby, 1982), which would produce ∼5% of the maximal contraction in aorta (Fig.1) and ∼10% in mesenteric arteries (Watts, 2002). This level of 5-HT2AR activity could contribute to the maintenance of normal vascular tone. In line with this view, 5-HT2AR expression seems to increase in mesenteric arteries of hypertensive rats (Li et al. 2007). In addition, 5-HT2AR and c-Src contractile coupling may be related to pathophysiological situations where severe aortic atherosclerosis increases the risk for thromboembolism (Rauch et al. 2001) and release of 5-HT from platelets that would cause acute vasoconstriction. The coupling between c-Src and 5-HT2AR may also serve other functions mediated by 5-HT2AR, such as cell growth and differentiation.
Acknowledgments
This study was supported by National Institutes of Health grants HL77705 (L.T.), HD046510 and HL088640 (E.S.) and American Heart Association National Center Scientist Development Awards 043508N (A.A.), 0830145N (P.K.) and 0435116N (M.E.).
References
- Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo1 caveolin-binding motif, a mechanism of caveolin-1–Slo1 interaction regulating Slo1 surface expression. J Biol Chem. 2008;283:4808–4817. doi: 10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes A, Florian JA, Watts SW. Mechanisms of 5-hydroxytryptamine2A receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J Pharmacol Exp Ther. 1999;291:1179–1187. [PubMed] [Google Scholar]
- Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. Direct binding of activated c-Src to the β3-adrenergic receptor is required for MAP kinase activation. J Biol Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- Carter RW, Kanagy NL. Tyrosine kinases regulate intracellular calcium during α2-adrenergic contraction in rat aorta. Am J Physiol Heart Circ Physiol. 2002;283:H1673–H1680. doi: 10.1152/ajpheart.01034.2001. [DOI] [PubMed] [Google Scholar]
- Crawford N. Systemic venous platelet-bound and plasma free serotonin levels in non-carcinoid malignancy. Clinica Chimica Acta. 1965;12:274–281. [Google Scholar]
- De Witt BJ, Kaye AD, Ibrahim IN, Bivalacqua TJ, D'Souza FM, Banister RE, Arif AS, Nossaman BD. Effects of PKC isozyme inhibitors on constrictor responses in the feline pulmonary vascular bed. Am J Physiol Lung Cell Mol Physiol. 2001;280:L50–L57. doi: 10.1152/ajplung.2001.280.1.L50. [DOI] [PubMed] [Google Scholar]
- Di Salvo J, Steusloff A, Semenchuk L, Satoh S, Kolquist K, Pfitzer G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun. 1993;190:968–974. doi: 10.1006/bbrc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Engbaek F, Voldby B. Radioimmunoassay of serotonin (5-hydroxytryptamine) in cerebrospinal fluid, plasma, and serum. Clin Chem. 1982;28:624–628. [PubMed] [Google Scholar]
- Gavarini S, Becamel C, Chanrion B, Bockaert J, Marin P. Molecular and functional characterization of proteins interacting with the C-terminal domains of 5-HT2 receptors: emergence of 5-HT2 ‘receptosomes’. Biol Cell. 2004;96:373–381. doi: 10.1016/j.biolcel.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Ishihata A, Tasaki K, Katano Y. Involvement of p44/42 mitogen-activated protein kinases in regulating angiotensin II- and endothelin-1-induced contraction of rat thoracic aorta. Eur J Pharmacol. 2002;445:247–256. doi: 10.1016/s0014-2999(02)01790-9. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Lu-Chao H, Netherton S. Excitation-contraction coupling in pulmonary vascular smooth muscle involves tyrosine kinase and Rho kinase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L666–L674. doi: 10.1152/ajplung.2001.280.4.L666. [DOI] [PubMed] [Google Scholar]
- Kandabashi T, Shimokawa H, Mukai Y, Matoba T, Kunihiro I, Morikawa K, Ito M, Takahashi S, Kaibuchi K, Takeshita A. Involvement of Rho-kinase in agonists-induced contractions of arteriosclerotic human arteries. Arterioscler Thromb Vasc Biol. 2002;22:243–248. doi: 10.1161/hq0202.104274. [DOI] [PubMed] [Google Scholar]
- Kim HS, Yumkham S, Choi JH, Son GH, Kim K, Ryu SH, Suh PG. Serotonin stimulates GnRH secretion through the c-Src-PLCγ1 pathway in GT1–7 hypothalamic cells. J Endocrinol. 2006;190:581–591. doi: 10.1677/joe.1.06727. [DOI] [PubMed] [Google Scholar]
- Li J, Cao YX, Liu H, Xu CB. Enhanced G-protein coupled receptors-mediated contraction and reduced endothelium-dependent relaxation in hypertension. Eur J Pharmacol. 2007;557:186–194. doi: 10.1016/j.ejphar.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Lu G, Kang YJ, Han J, Herschman HR, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281:6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- Luo G, Xu CB, Cao YX, Edvinsson L. Transcriptional up-regulation in expression of 5-hydroxytryptamine2A and transcriptional down-regulation of angiotensin II type 1 receptors during organ culture of rat mesenteric artery. Basic Clin Pharmacol Toxicol. 2004;95:280–287. doi: 10.1111/j.1742-7843.2004.t01-1-pto950506.x. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- McKune CM, Watts SW. Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling. J Pharmacol Exp Ther. 2001;297:88–95. [PubMed] [Google Scholar]
- Marrero MB, Schieffer B, Paxton WG, Schieffer E, Bernstein KE. Electroporation of pp60c-src antibodies inhibits the angiotensin II activation of phospholipase C-γ1 in rat aortic smooth muscle cells. J Biol Chem. 1995;270:15734–15738. doi: 10.1074/jbc.270.26.15734. [DOI] [PubMed] [Google Scholar]
- Monk SA, Williams JM, Hope AG, Barnes NM. Identification and importance of N-glycosylation of the human 5-hydroxytryptamine3A receptor subunit. Biochem Pharmacol. 2004;68:1787–1796. doi: 10.1016/j.bcp.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Rashid M, Muntasir HA, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther. 2004;104:59–81. doi: 10.1016/j.pharmthera.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Nakao F, Kobayashi S, Mogami K, Mizukami Y, Shirao S, Miwa S, Todoroki-Ikeda N, Ito M, Matsuzaki M. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol. 1999;77:606–617. [PubMed] [Google Scholar]
- Ogden K, Thompson JM, Hickner Z, Huang T, Tang DD, Watts SW. A new signaling paradigm for serotonin: use of Crk-associated substrate in arterial contraction. Am J Physiol Heart Circ Physiol. 2006;291:H2857–H2863. doi: 10.1152/ajpheart.00229.2006. [DOI] [PubMed] [Google Scholar]
- Parsons SJ, McCarley DJ, Ely CM, Benjamin DC, Parsons JT. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984;51:272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U, Osende JI, Fuster V, Badimon JJ, Fayad Z, Chesebro JH. Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences. Ann Intern Med. 2001;134:224–238. doi: 10.7326/0003-4819-134-3-200102060-00014. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Eghbali M, Minosyan TY, Tang G, Toro L, Stefani E. Heart estrogen receptor α: distinct membrane and nuclear distribution patterns and regulation by estrogen. J Mol Cell Cardiol. 2006;41:496–510. doi: 10.1016/j.yjmcc.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- Somerville BW. Platelet-bound and free serotonin levels in jugular and forearm venous blood during migraine. Neurology. 1976;26:41–45. doi: 10.1212/wnl.26.1.41. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19:441–449. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- Venema RC, Ju H, Venema VJ, Schieffer B, Harp JB, Ling BN, Eaton DC, Marrero MB. Angiotensin II-induced association of phospholipase Cγ1 with the G-protein-coupled AT1 receptor. J Biol Chem. 1998;273:7703–7708. doi: 10.1074/jbc.273.13.7703. [DOI] [PubMed] [Google Scholar]
- Villazon M, Padin JF, Cadavid MI, Enguix MJ, Tristan H, Orallo F, Loza MI. Functional characterization of serotonin receptors in rat isolated aorta. Biol Pharm Bull. 2002;25:584–590. doi: 10.1248/bpb.25.584. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Uecker A, Kroll J, Frank H, Mayr U, Bjorge JD, Fujita D, Gazit A, Hombach V, Levitzki A, Bohmer FD. A dual inhibitor of platelet-derived growth factor β-receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells. A novel candidate for prevention of vascular remodeling. Circ Res. 1999;85:12–22. doi: 10.1161/01.res.85.1.12. [DOI] [PubMed] [Google Scholar]
- Watts SW. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in deoxycorticosterone acetate-salt hypertension. Hypertension. 2002;39:825–829. doi: 10.1161/hy0302.104668. [DOI] [PubMed] [Google Scholar]
- Watts SW. 5-HT in systemic hypertension: foe, friend or fantasy. Clin Sci (Lond) 2005;108:399–412. doi: 10.1042/CS20040364. [DOI] [PubMed] [Google Scholar]
- Watts SW, Yang P, Banes AK, Baez M. Activation of Erk mitogen-activated protein kinase proteins by vascular serotonin receptors. J Cardiovasc Pharmacol. 2001;38:539–551. doi: 10.1097/00005344-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Weiss HM, Haase W, Michel H, Reilander H. Comparative biochemical and pharmacological characterization of the mouse 5HT5A 5-hydroxytryptamine receptor and the human β2-adrenergic receptor produced in the methylotrophic yeast Pichia pastoris. Biochem J. 1998;330:1137–1147. doi: 10.1042/bj3301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wijetunge S, Lymn JS, Hughes AD. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60c-src kinase activity. Br J Pharmacol. 2000;129:1347–1354. doi: 10.1038/sj.bjp.0703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Yoder EJ, Shih J, Chen K, Dias P, Shi L, Ji XD, Wei J, Conner JM, Kumar S, Ellisman MH, Singh SK. Development and characterization of monoclonal antibodies specific to the serotonin 5-HT2A receptor. J Histochem Cytochem. 1998;46:811–824. doi: 10.1177/002215549804600704. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, Touyz RM. Endothelin-1, but not Ang II, activates MAP kinases through c-Src-independent Ras-Raf-dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1960–1967. doi: 10.1161/ATVBAHA.107.146746. [DOI] [PubMed] [Google Scholar]
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev. 2003;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Kim JL, Newcomb JR, Rose PE, Stover DR, Toledo LM, Zhao H, Morgenstern KA. Structural analysis of the lymphocyte-specific kinase Lck in complex with non-selective and Src family selective kinase inhibitors. Structure Fold Des. 1999;7:651–661. doi: 10.1016/s0969-2126(99)80086-0. [DOI] [PubMed] [Google Scholar]