Abstract
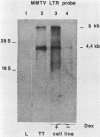
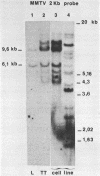
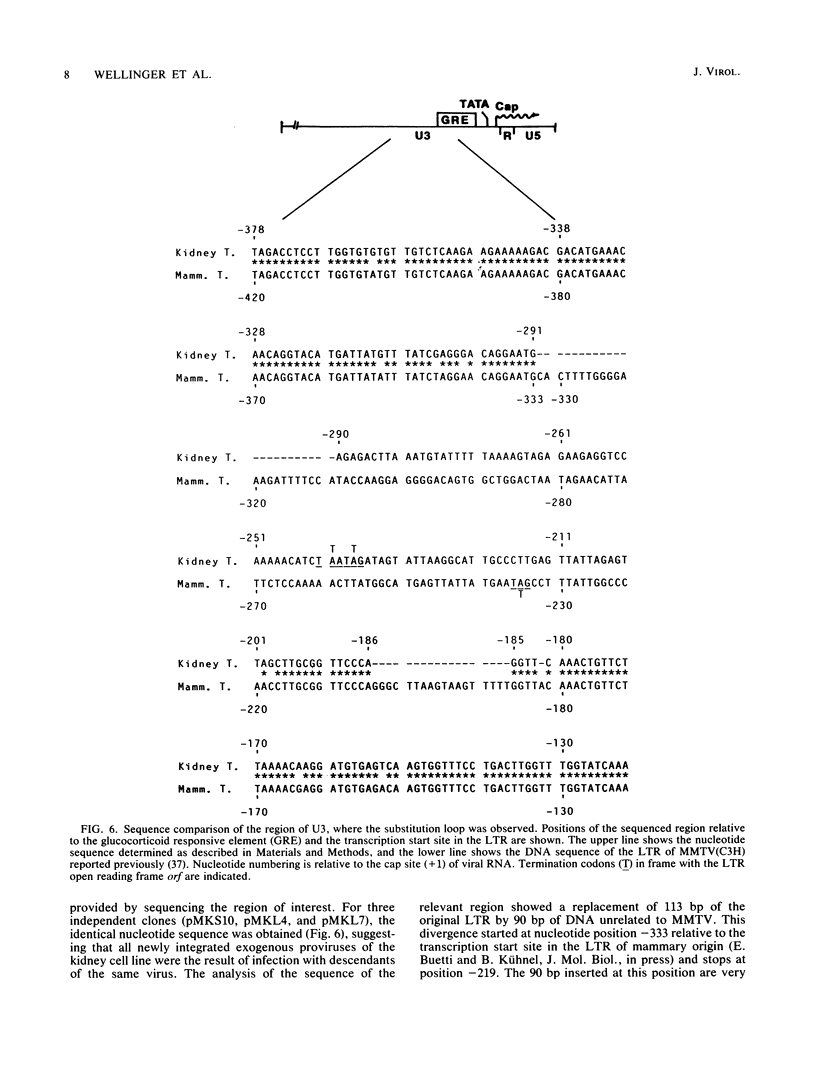
Mouse mammary tumor virus (MMTV) is a B-type retrovirus which induces predominantly mammary carcinomas after a relatively long latency period. To date, very little is known about the reasons for the strict tissue specificity of MMTV. The BALB/cf/Cd strain of mice, which was infected with milk-borne MMTV (C3H), shows a high incidence of kidney adenocarcinomas, and our data suggest that MMTV might be involved in the formation of these tumors. Newly integrated exogenous MMTV proviruses were found in the genome of transplanted tumor cells as well as in the DNA of a cell line derived from one tumor, but not in normal cells of BALB/cf/Cd mice. The MMTV DNA in these tumor cells was transcribed and viral RNA synthesis was strongly stimulated by glucocorticoid hormones. Viral structural polypeptides, comparable in size and antigenicity to MMTV polypeptides of infected mammary tumor cells were synthesized and processed normally in the cell line and were organized correctly into intracytoplasmic particles. Heteroduplex analysis of the molecularly cloned MMTV proviral DNAs of kidney and mammary tumor origin revealed a high degree of homology in the gag, pol, and env genes. A striking difference, however, was observed in the U3 region of the two LTRs that might relate to the different tissue specificity of the two viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Avian oncovirus proteins expressed on the surface of infected cells. Virology. 1980 Apr 30;102(2):251–261. doi: 10.1016/0042-6822(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Cloned mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Cell. 1981 Feb;23(2):335–345. doi: 10.1016/0092-8674(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Dekaban G. A., Ball J. K. Integration of type B retroviral DNA in virus-induced primary murine thymic lymphomas. J Virol. 1984 Dec;52(3):784–792. doi: 10.1128/jvi.52.3.784-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Structure and processing of the mouse mammary tumor virus glycoprotein precursor pr73env. J Virol. 1980 Aug;35(2):349–361. doi: 10.1128/jvi.35.2.349-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dudley J. P., Butel J. S. Effect of dexamethasone on expression of endogenous mouse mammary tumor virus sequences in BALB/c tumor cell lines. Virology. 1979 Jul 30;96(2):453–462. doi: 10.1016/0042-6822(79)90103-x. [DOI] [PubMed] [Google Scholar]
- Dudley J., Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984 Jan;49(1):92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felluga B., Claude A., Mrena E. Electron microscope observations on virus particles associated with a transplantable renal adenocarcinoma in BALB-cf-Cd mice. J Natl Cancer Inst. 1969 Aug;43(2):319–333. [PubMed] [Google Scholar]
- Garcia M., Wellinger R., Vessaz A., Diggelmann H. A new site of integration for mouse mammary tumor virus proviral DNA common to BALB/cf(C3H) mammary and kidney adenocarcinomas. EMBO J. 1986 Jan;5(1):127–134. doi: 10.1002/j.1460-2075.1986.tb04186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J., Haverman J., Nusse R., van Blitterswijk W. J., Cleton F. J., Hageman P. C., van Nie P., Calafat J. Immunologic, virologic, and genetic aspects of mammary tumor virus-induced cell-surface antigens: presence of these antigens and the Thy 1.2 antigen on murine mammary gland and tumor cells. J Natl Cancer Inst. 1975 Jun;54(6):1323–1333. doi: 10.1093/jnci/54.6.1323. [DOI] [PubMed] [Google Scholar]
- Hilkens J., van der Zeijst B., Buijs F., Kroezen V., Bleumink N., Hilgers J. Identification of a cellular receptor for mouse mammary tumor virus and mapping of its gene to chromosome 16. J Virol. 1983 Jan;45(1):140–147. doi: 10.1128/jvi.45.1.140-147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle R. H. Mapping of a human genetic regulator element by somatic cell genetic analysis. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1220–1227. doi: 10.1073/pnas.66.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. Mouse mammary tumor virus-related sequences in mouse lymphocytes are inducible by 12-O-tetradecanoyl phorbol-13-acetate. J Virol. 1984 Dec;52(3):1000–1004. doi: 10.1128/jvi.52.3.1000-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Links J., Calafat J., Buijs F., Tol O. Simultaneous chemical induction of MTV and MLV in vitro. Eur J Cancer. 1977 Jun;13(6):557–587. [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Hilkens J., Hilgers J., Groner B., Hynes N. E. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982 Sep;43(3):819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Weijers P. Rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in T-cell leukemias of mouse strain GR result in a novel enhancer-like structure. Mol Cell Biol. 1985 Apr;5(4):823–830. doi: 10.1128/mcb.5.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Sarkar N. H., Old L. J., Moore D. H., Scheer D. I., Hilgers J. Characteristics of the structural components of the mouse mammary tumor virus. II. Viral proteins and antigens. Virology. 1971 Oct;46(1):21–38. doi: 10.1016/0042-6822(71)90003-1. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van der Ploeg L., van Duijn L., Michalides R., Hilgers J. Impaired maturation of mouse mammary tumor virus precursor polypeptides in lymphoid leukemia cells, producing intracytoplasmic A particles and no extracellular B-type virions. J Virol. 1979 Oct;32(1):251–258. doi: 10.1128/jvi.32.1.251-258.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Prakash O. Proteins encoded by the long terminal repeat region of mouse mammary tumor virus: identification by hybrid-selected translation. J Virol. 1984 Sep;51(3):604–610. doi: 10.1128/jvi.51.3.604-610.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli R., McMaster G. K., Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985 May 24;13(10):3617–3633. doi: 10.1093/nar/13.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Whittington E. S. Identification of the structural proteins of the murine mammary tumor virus that are serologically related to the antigens of intracytoplasmic type-A particles. Virology. 1977 Aug;81(1):91–106. doi: 10.1016/0042-6822(77)90061-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Kufe D., Schlom J. Type-specific antigenic determinants on the major external glycoprotein of high- and low-oncogenic murine mammary tumor viruses. J Virol. 1977 Nov;24(2):525–533. doi: 10.1128/jvi.24.2.525-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Long C. A., Sheffield J. B., Tamura A., Tanaka H. Murine mammary tumor virus deficient in the major glycoprotein: biochemical and biological studies on virions produced by a lymphoma cell line. Virology. 1980 Jul 30;104(2):279–293. doi: 10.1016/0042-6822(80)90333-5. [DOI] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Enhancer activity correlates with the oncogenic potential of avian retroviruses. EMBO J. 1985 Apr;4(4):949–956. doi: 10.1002/j.1460-2075.1985.tb03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]