Abstract
BACKGROUND
There are limited data on the treatment and long-term outcome of patients with renal dysfunction across the broad spectrum of acute coronary syndromes (ACS) in Canada.
OBJECTIVES
To examine the treatment patterns and outcome of ACS patients with renal dysfunction.
METHODS
In the prospective, multicentre, Canadian ACS Registry, 3510 patients hospitalized for ACS (including unstable angina, ST and non-ST elevation myocardial infarction) were categorized into four groups: normal renal function (creatinine clearance [CrCl] 90 mL/min or greater; n=1152), mild renal dysfunction (CrCl 60 mL/min to 89 mL/min; n=1253), moderate renal dysfunction (CrCl 30 mL/min to 59 mL/min; n=944) and severe renal dysfunction (CrCl less than 30 mL/min; n=161). Multivariable logistic regression analysis was performed to examine the independent prognostic value of renal dysfunction, and the association of various therapies with one-year survival.
RESULTS
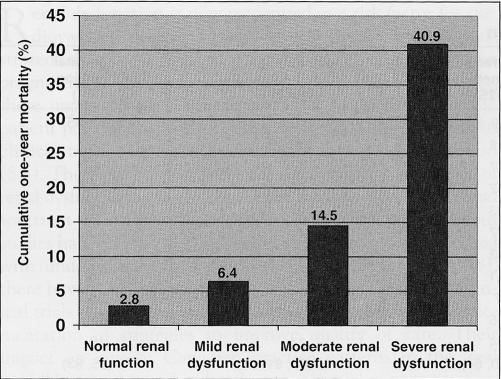
All-cause mortality at one year was 2.8%, 6.4%, 14.5% and 40.9% in patients with normal renal function, and mild, moderate and severe renal dysfunction, respectively (P for trend <0.001). After adjusting for other prognosticators, moderate (OR 1.82, 95% CI 1.08 to 3.08) and severe (OR 6.29, 95% CI 3.37 to 11.77) renal dysfunction remained independent predictors of one-year death. Patients with renal dysfunction were less likely to receive fibrinolytic therapy, to undergo coronary angiography and revascularization in hospital, and to be treated with acetylsalicylic acid, beta-blockers and lipid-lowering therapy at discharge and at one-year follow-up. The association of in-hospital revascularization, and discharge use of acetylsalicylic acid and beta-blockers with better one-year survival was similar among patients with normal and impaired renal function.
CONCLUSIONS
Renal dysfunction is prevalent and independently predicts higher mortality in patients with ACS. The current underutilization of effective therapies may contribute to the poor outcome. There remains an important opportunity to improve care in this high-risk population.
Keywords: Coronary disease, Kidney, Prognosis, Registries
Abstract
HISTORIQUE
Les données sur le traitement et l’issue à long terme des patients atteints d’une dysfonction rénale dans le vaste spectre des syndromes coronariens aigus (SCA) au Canada sont limitées.
OBJECTIFS
Examiner les tableaux thérapeutiques et l’issue des personnes atteintes d’un SCA et d’une dysfonction rénale.
MÉTHODOLOGIE
Dans le registre canadien multicentre et prospectif des SCA, 3 510 patients hospitalisés en raison d’un SCA (y compris l’angine instable et l’infarctus du myocarde avec ou sans surélévation du segment ST) ont été classés en quatre groupes : fonction rénale normale (clairance de la créatinine [ClCr] 90 mL/min ou plus; n=1 152), dysfonction rénale bénigne (ClCr 60 mL/min à 89 mL/min, n = 1 253), dysfonction rénale modérée (ClCr 30 mL/min à 59 mL/min, n=944) et grave dysfonction rénale (ClCr inférieure à 30 mL/min, n=161). Une analyse de régression logistique multivariable a été exécutée pour examiner la valeur pronostique indépendante de la dysfonction rénale et l’association de diverses thérapies avec la survie au bout d’un an.
RÉSULTATS
Les taux de mortalité toutes causes confondues au bout d’un an s’élevaient à 2,8 %, à 6,4 %, à 14,5 % et à 40,9 % chez les patients présentant une fonction rénale normale et une dysfonction rénale bénigne, modérée ou grave, respectivement (P pour la tendance < 0,001). Après rajustement compte tenu d’autres facteurs pronostiques, les dysfonctions rénales modérée (RR 1,82, 95 % IC 1,08 à 3,08) et grave (RR 6,29, 95 % IC 3,37 à 11,77) demeuraient des prédicteurs indépendants de décès au bout d’un an. Les patients atteints d’une dysfonction rénale étaient moins susceptibles de recevoir un traitement fibrinolytique, de subir une angiographie coronarienne et une revascularisation en milieu hospitalier et d’être traités à l’acide acétylsalicylique, aux béta-bloquants et aux hypolipémiants au congé et au suivi au bout d’un an. La revascularisation en milieu hospitalier et l’utilisation d’acide acétylsalicylique et de béta-bloquants au congé combinées à un taux de survie plus favorable au bout d’un an étaient similaires chez les patients présentant une fonction rénale normale ou altérée.
CONCLUSIONS
La dysfonction rénale est prévalent et prédit en soi un taux de mortalité plus élevé chez les patients atteints d’un SCA. La sous-utilisation actuelle de thérapies efficaces peut contribuer aux sombres issues. Il reste d’importantes occasions d’améliorer les soins à cette population très vulnérable.
Renal dysfunction is now recognized as a risk factor for cardiovascular disease (1), and several studies have demonstrated that it is also a powerful independent adverse prognosticator in acute coronary syndromes (ACS). However, these important studies were restricted to relatively specific patient populations, such as the elderly (2), those enrolled in clinical trials (3,4) or diagnosed with myocardial infarction (5,6). There are limited data on the prognostic significance of renal dysfunction among less selected patients across the broad spectrum of ACS in Canada. Moreover, although previous studies have shown that impaired renal function was associated with underutilization of standard medical therapies (5,7,8), there have been major advances in evidence from recent clinical trials to guide the management of ACS, and in the implementation of strategies to improve quality of care. Their impact on current Canadian practice patterns in the ‘real world’ has not been elucidated.
Accordingly, the objectives of the present study were to examine the prognostic importance of renal dysfunction across the wide spectrum of ACS, the contemporary use of pharmacological and interventional therapies, and the association of treatment with survival at one year among patients with renal dysfunction.
METHODS
Canadian ACS Registry
Details of the Canadian ACS Registry rationale and methods have been previously described (9). Briefly, patients were eligible for enrollment in this prospective, observational study if they were 18 years or older on presentation; admitted to hospital with a suspected ACS (defined by symptoms consistent with acute cardiac ischemia within 24 h of onset); and the qualifying ACS was not precipitated or accompanied by a serious comorbidity, such as trauma or gastrointestinal bleeding. To reduce selection bias, there were no other specific exclusion criteria and consecutive patient enrollment was encouraged at all sites, which included 51 academic and community hospitals across Canada. In each participating hospital, the designated physician or study coordinator recorded patient demographic and clinical data, laboratory results, in-hospital treatment, outcome and discharge diagnosis and medications on standardized case report forms, which were then centrally scanned into an electronic database. Based on the discharge electrocardiogram and definitions on the case report form, the final diagnosis was made by the attending physician according to one of the following categories: unstable angina, Q-wave myocardial infarction and non-Q wave myocardial infarction. Standardized definitions of adverse events and outcomes were used, and central data checks were performed with queries sent for correction. The local hospital research ethics committees approved the study, and all patients followed after discharge gave informed consent.
Between September 1999 and June 2001, 4627 patients with ACS were recruited into the Canadian ACS Registry. Creatinine clearance (CrCl) (mL/min) was estimated by the Cockcroft-Gault formula (10): (140 – age [years]) × weight (kg)/serum creatinine (μmol/L) × 1.2 (× 0.85 for women). In accordance with the National Kidney Foundation practice guidelines (11), renal function was defined as normal for CrCl 90 mL/min or greater; mildly impaired for CrCl 60 mL/min to 89 mL/min; moderately impaired for CrCl 30 mL/min to 59 mL/min; and severely impaired for CrCl less than 30 mL/min. Sixty-eight and 1026 patients were excluded because their baseline creatinine and weight on admission, respectively, were not available; 23 patients with CrCl less than 5 mL/min or more than 180 mL/min were also excluded. As a result, the study population was comprised of 3510 ACS patients, who were stratified into four groups of renal function. Because missing admission weight accounted for the vast majority of patients excluded from the main analyses using CrCl, the analyses were repeated using serum creatinine (available in 98.5% of patients).
The primary outcome was cumulative all-cause mortality at one year. Deaths during index hospitalizations were recorded on case report forms, and vital status at one-year follow-up was determined by standardized telephone interview for hospital survivors. The robust end point of overall mortality was chosen due to the inherent inaccuracy in classifying the cause of death, especially among patients with renal dysfunction who may have chronically elevated cardiac biomarkers.
Statistical analysis
Continuous data are shown as medians with 25th and 75th percentiles, and categorical data as frequencies and percentages. Group comparisons were made using the χ2 test and the Kruskal-Wallis test for discrete and continuous variables, respectively. Kendall’s tau-b test was used to evaluate trends. Renal function was analyzed as a categorical variable using the aforementioned cutoff points of CrCl. To assess the independent prognostic value of renal dysfunction on one-year mortality, a multivariable logistic regression model was developed (with backward elimination for P>0.10) adjusting for age, heart rate, systolic blood pressure, Killip class, cardiac biomarker status and electrocardiographic changes (ST deviation and bundle branch block). Selection of these predictor variables was based on a registry-derived risk model (12), which was previously validated in the Canadian ACS Registry cohort (13). Diabetes was also considered because of its association with renal dysfunction. Model discrimination was evaluated by the c-statistic (area under the receiver-operating-characteristic curve) (14), and calibration by the Hosmer-Lemeshow goodness-of-fit test for which a low P value indicates lack of fit (15). The correlation matrix of beta coefficients was examined for the potential problem of multicollinearity because CrCl was calculated using age, which was also included in the multivariable model. To evaluate treatment heterogeneity among patients with and without renal dysfunction, an interaction term (a product term with renal dysfunction, a dichotomous variable for CrCl less than 60 mL/min versus 60 mL/min or more) was added to the regression model. An interaction term for renal dysfunction and cardiac biomarker status was also tested to determine whether the association with outcome differed between the groups with unstable angina versus myocardial infarction. McNemar test was used to compare medication use at discharge with one-year follow-up. Statistical significance was set at a two-sided P<0.05. Analyses were performed using SPSS version 12.0.0 (SPSS Inc, USA).
RESULTS
Of 3510 ACS patients in the study cohort, 1152 (32.8%) had normal renal function; 1253 (35.7%) had mild renal dysfunction, 944 (26.9%) had moderate renal dysfunction, and 151 (5.1%) had severe renal dysfunction. Their baseline characteristics are shown in Table 1.
TABLE 1.
Baseline demographic and clinical characteristics on presentation
| Normal renal function (n=1152) | Mild renal dysfunction (n=1253) | Moderate renal dysfunction (n=944) | Severe renal dysfunction (n=161) | |
|---|---|---|---|---|
| Age (years)* | 55 (48, 61) | 66 (59, 72) | 75 (70, 80) | 79 (73, 85) |
| Female sex (%) | 18 | 28 | 48 | 47 |
| Current smoker (%) | 44 | 27 | 16 | 8 |
| Hypertension (%) | 42 | 47 | 60 | 68 |
| Diabetes (%) | 23 | 23 | 27 | 32 |
| Dyslipidemia (%) | 47 | 47 | 42 | 40 |
| Prior angina (%) | 40 | 53 | 62 | 68 |
| Prior myocardial infarction (%) | 25 | 31 | 38 | 46 |
| Prior heart failure (%) | 4 | 7 | 17 | 35 |
| Prior percutaneous coronary intervention (%) | 15 | 15 | 13 | 11 |
| Prior coronary artery bypass graft surgery (%) | 9 | 14 | 14 | 16 |
| Heart rate (beats/min)* | 72 (62, 84) | 70 (60, 84) | 74 (63, 91) | 79 (65, 93) |
| Systolic blood pressure (mmHg)* | 147 (129, 164) | 149 (130, 169) | 148 (129, 170) | 142 (127, 170) |
| Killip class (%) | ||||
| I | 90 | 85 | 73 | 59 |
| II | 8 | 12 | 21 | 34 |
| III/IV | 2 | 3 | 6 | 7 |
| ST depression (%) | 19 | 23 | 24 | 35 |
| ST elevation or bundle branch block (%) | 39 | 36 | 37 | 43 |
| Serum creatinine (μmol/L)* | 80 (71, 89) | 91 (79, 103) | 107 (88, 126) | 207 (148, 327) |
| Abnormal cardiac biomarker (%) | 42 | 42 | 44 | 56 |
Median (25th, 75th percentiles)
Across the strata of worsening renal function, advanced age, diabetes, hypertension, previous myocardial infarction and heart failure were more prevalent (all P<0.01). Patients with renal dysfunction more frequently presented with higher heart rate, higher Killip class and ST depression on the admission electrocardiogram (all P<0.01).
The use of fibrinolytic therapy, coronary angiography, percutaneous coronary intervention and coronary artery bypass graft surgery during index hospitalization are summarized in Table 2. Treatment differed substantially among the four groups; patients with renal dysfunction were significantly less likely to undergo coronary angiography and percutaneous coronary intervention (P<0.001 for both). Fibrinolytic therapy was also less often administered to patients with renal dysfunction (P<0.001), even among those considered ‘ideal candidates’, as defined by the presence of ST elevation or left bundle branch block within 12 h of symptom onset, systolic blood pressure of 180 mmHg or less and no history of serious bleeding. The rate of coronary artery bypass graft surgery was slightly higher among patients with normal renal function and mild renal impairment, compared with those with moderate to severe renal dysfunction (4.3% versus 2.9%, respectively; P=0.04). Overall, the most common final diagnosis was unstable angina (39%), followed by non-Q wave myocardial infarction (33%) and Q wave myocardial infarction (29%). In-hospital vital status was available for all patients, with an overall mortality of 2.1%.
TABLE 2.
In-hospital management and final diagnosis
| Normal renal function (n=1152) | Mild renal dysfunction (n=1253) | Moderate renal dysfunction (n=944) | Severe renal dysfunction (n=161) | |
|---|---|---|---|---|
| Fibrinolytic therapy* (%) | 67 | 63 | 41 | 28 |
| Coronary angiography (%) | 44 | 44 | 31 | 19 |
| PCI (%) | 19 | 17 | 12 | 9 |
| CABG (%) | 3.1 | 5.5 | 3.1 | 1.9 |
| Final diagnosis (%) | ||||
| Unstable angina | 37 | 40 | 40 | 39 |
| NQMI | 31 | 32 | 34 | 40 |
| QMI | 32 | 28 | 26 | 21 |
Percentage among eligible patients, including those who presented within 12 h of symptom onset with ST elevation or left bundle branch block, systolic blood pressure of 180 mmHg or less, and no history of serious bleeding. CABG Coronary artery bypass graft surgery; NQMI Non-Q wave myocardial infarction; PCI Percutaneous coronary intervention; QMI Q wave myocardial infarction
Among patients who survived until hospital discharge, those with renal dysfunction were also less often prescribed acetylsalicylic acid, beta-blockers and lipid-lowering agents (all P<0.001) (Table 3). The use of angiotensin-converting enzyme inhibitors was highest among patients with moderate renal dysfunction, and lowest among those with severe renal dysfunction, who were most likely to be treated with calcium channel blockers.
TABLE 3.
Medication use at discharge and at one year among survivors
| Normal renal function | Mild renal dysfunction | Moderate renal dysfunction | Severe renal dysfunction | P | |
|---|---|---|---|---|---|
| At discharge (n) | 1150 | 1236 | 909 | 143 | |
| ASA (%) | 91 | 87 | 86 | 87 | <0.001 |
| Beta-blocker (%) | 83 | 78 | 69 | 71 | <0.001 |
| ACEI (%) | 55 | 58 | 60 | 42 | NS |
| Calcium channel blocker (%) | 21 | 29 | 36 | 42 | <0.001 |
| Lipid-lowering agent (%) | 63 | 59 | 47 | 36 | <0.001 |
| At one year (n) | 1019 | 1088 | 752 | 88 | |
| ASA (%) | 87 | 85 | 77 | 75 | <0.001 |
| Beta-blocker (%) | 68 | 66 | 63 | 63 | 0.02 |
| ACEI (%) | 57 | 57 | 53 | 42 | 0.03 |
| Calcium channel blocker (%) | 20 | 25 | 33 | 36 | <0.001 |
| Lipid-lowering agent (%) | 76 | 72 | 59 | 50 | <0.001 |
ACEI Angiotensin-converting enzyme inhibitor; ASA Acetylsalicylic acid; NS Not significant
At one-year, 218 patients (6.2%) were lost to follow-up, and vital status was not available for these patients. Their baseline characteristics, including CrCl, were similar to those of the remaining cohort. The cumulative one-year mortality is depicted in Figure 1. In bivariate analysis, compared with the group with normal renal function, the unadjusted ORs for one-year mortality were 2.38 (95% CI 1.55 to 3.67; P<0.001), 5.88 (95% CI 3.91 to 8.85; P<0.001) and 24.01 (95% CI 14.76 to 39.19; P<0.001) for the group with mild, moderate and severe renal dysfunction, respectively. After adjusting for other known prognosticators, moderate and severe renal dysfunction remained independent predictors of one-year mortality (Table 4). The c-statistic was 0.81 and the Hosmer-Lemeshow P=0.64, indicating good model discrimination and calibration, respectively. Inclusion of diabetes as a covariate in the multivariable model did not weaken the independent prognostic impact of renal dysfunction. The relationship between renal dysfunction and survival was similar regardless of cardiac biomarker status (P=0.49 for interaction). The in-hospital use of percutaneous coronary intervention, coronary artery bypass graft surgery and the discharge use of acetylsalicylic acid were all independently associated with a substantial survival benefit at one year (Table 5). There was also a trend suggesting that the discharge prescription of a beta-blocker was associated with better outcome. The associations between these treatments and improved survival were similar irrespective of the presence or absence of renal dysfunction.
Figure 1).
Cumulative one-year mortality; P<0.001 for trend
TABLE 4.
Multivariable logistic regression model for one-year mortality
| Independent predictors | Adjusted OR | 95% CI | P |
|---|---|---|---|
| Age* | 1.73 | 1.45 to 2.05 | <0.001 |
| Heart rate† | 1.17 | 1.11 to 1.24 | <0.001 |
| Systolic blood pressure‡ | 0.94 | 0.89 to 0.98 | 0.004 |
| Killip class§ | <0.001 | ||
| II | 1.86 | 1.36 to 2.54 | <0.001 |
| III | 1.70 | 0.96 to 3.01 | 0.07 |
| IV | 3.66 | 1.18 to 11.37 | 0.025 |
| Bundle branch block or ST deviation | 1.37 | 1.03 to 1.82 | 0.03 |
| Abnormal cardiac biomarker | 1.99 | 1.39 to 2.85 | <0.001 |
| Renal dysfunction | <0.001 | ||
| Mild | 1.42 | 0.88 to 2.31 | 0.15 |
| Moderate | 1.82 | 1.08 to 3.08 | 0.025 |
| Severe | 6.29 | 3.37 to 11.77 | <0.001 |
Per decade increase;
Per 10 beats/min increase;
Per 10 mmHg increase;
Compared with Killip class I
TABLE 5.
Association of treatment with one-year mortality
| Treatment | Adjusted OR* | 95% CI | P | P for heterogeneity† |
|---|---|---|---|---|
| In-hospital PCI | 0.60 | 0.37 to 0.96 | 0.03 | 0.25 |
| In-hospital CABG | 0.11 | 0.01 to 0.78 | 0.03 | 0.29 |
| Discharge use of ASA | 0.43 | 0.31 to 0.60 | <0.001 | 0.15 |
| Discharge use of beta-blocker | 0.76 | 0.56 to 1.02 | 0.07 | 0.37 |
Adjusted for the independent prognosticators shown in Table 4.
For patients with creatinine clearance less than 60 mL/min versus at least 60 mL/min. ASA Acetylsalicylic acid; CABG Coronary artery bypass graft surgery; PCI Percutaneous coronary intervention
Medication use at one year was available for 2947 survivors (98.4%) and is presented in Table 3. Overall, when compared with discharge, there was a significant increase in the use of lipid-lowering agents, but a significant reduction in the use of acetylsalicylic acid and beta-blockers (all P<0.001). The less frequent use of acetylsalicylic acid, beta-blockers and lipid-lowering agents among patients with renal dysfunction persisted at one-year follow-up.
DISCUSSION
The present study demonstrates that renal dysfunction afflicts a significant proportion of less selected patients across the broad spectrum of ACS in Canada. While these patients also have more comorbidities at baseline, renal dysfunction remains a strong independent predictor of one-year mortality. Furthermore, patients with renal dysfunction are less likely to undergo coronary angiography and revascularization, and to receive evidence-based medical therapies both at discharge and at one year. Nevertheless, these treatments are independently associated with better survival in both patients with and without renal dysfunction.
Chronic kidney disease has become an enormous threat to public health worldwide. Its prevalence is expected to rise due to the epidemic of diabetes and hypertension in an aging population. The detrimental impact of renal disease on the cardiovascular system has now been recognized (1,16). The early seminal work of Herzog et al (17) documented a dismal outcome among end-stage renal disease patients after acute myocardial infarction, with one- and five-year mortality rates of 59% and 73%, respectively. Subsequently, other investigators have shown that even less severe renal impairment is an adverse prognosticator among elderly patients in the Cooperative Cardiovascular Project (CCP) (2), among patients admitted to coronary care units in large academic centres (5,6,18) and those enrolled in the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial (19). Renal dysfunction was also found to confer incremental prognostic value beyond validated risk models, both in the setting of non-ST elevation ACS (3) and ST elevation myocardial infarction (4). However, these important studies were limited to more selected patient populations, such as the elderly (2), those with myocardial infarction (2,4–6,19–21), those enrolled in clinical trials (3,4,19,20) or those admitted to a single tertiary care centre (5,6,18). For instance, patients with renal impairment were often underrepresented in large clinical trials, and the applicability of these studies to the general population has not been confirmed. Using data from Global Registry of Acute Coronary Events (GRACE), Santopinto et al (22) recently demonstrated that decreased CrCl was an independent predictor of hospital death among less selected patients with ACS. However, the prognostic impact of mild renal dysfunction (CrCl 60 mL/min to 90 mL/min) was not specifically assessed, and long-term outcomes have not yet been reported. In a recent study of 2706 veterans with ACS, moderate and severe renal dysfunction were associated with less aggressive treatment and higher seven-month mortality (23). The generalizability of these findings in this predominantly elderly male population with only 16% having normal renal function remains to be established.
In addition to confirming the work of previous investigators, the present study provides several new insights. The multi-centre recruitment of less selected patient populations, including younger patients and those with unstable angina, enhances the generalizability of the findings. The results indicate that moderate and severe renal dysfunction were independent predictors of all-cause mortality up to one year after ACS. The observation that mild renal dysfunction was no longer associated with higher mortality after adjusting for other confounders is in accord with some studies (6,20), but not others (2–5,19). This finding should be interpreted with caution because of inadequate power to exclude a clinically important excess mortality risk (95% CI 0.88 to 2.31). Moreover, the longer duration of follow-up may account for the discrepancy observed because the increased short-term risk tends to dissipate over time (2).
The reported underutilization of standard medical therapies among patients with renal dysfunction in early 1990s (2,5,7,8) preceded several landmark clinical trials that have since expanded indications of these therapies. For example, beta-blockers were once thought to be contraindicated in heart failure, and the role of angiotensin-converting enzyme inhibitors and statins after myocardial infarction had not been well established (24,25). Indeed, McCullough et al (7) observed a significant increase in the use of acetylsalicylic acid and beta-blockers from 1990 to 1998. In addition, Shlipak et al (2) suggested that the treatment disparities observed in their study might be less pronounced in younger patients and may have changed considerably over time. Therefore, the re-evaluation of medication use among contemporary, less-selected ACS patients appears warranted. We found that there were still significant trends with lesser use of fibrinolytics, acetylsalicylic acid, beta-blockers and lipid-lowering agents across the strata of worsening renal function. While the overall greater use of these evidence-based therapies compared with that reported in previous investigations is encouraging, there remains an important gap in the care of these vulnerable patients.
The present study also provides an opportunity to assess the impact of recent trials that support an early invasive strategy in the management of high-risk ACS (26,27). The rates of coronary angiography and percutaneous coronary intervention were significantly lower among patients with renal dysfunction despite their poor outcome. In contrast, the rate of coronary artery bypass graft surgery was only slightly less, suggesting that severe multivessel disease may be relatively more common in these patients.
Several reasons may explain the apparent undertreatment of this high-risk population. Physicians may be skeptical that patients with renal dysfunction, who were generally excluded from clinical trials, would derive similar therapeutic benefits. The potential excess toxicities of therapies and the high prevalence of comorbidities that are perceived as contraindications may further discourage aggressive treatment. Yet studies suggest that, at least among carefully selected patients, certain treatment benefits appear to outweigh the risks (18,28). While several studies have reported the relationship between more intensive treatment and lower in-hospital or short-term mortality, Wright et al (5) showed that the discharge use of acetyl-salicylic acid and beta-blocker was also associated with more favourable long-term outcome. Our observations confirm their findings, and extend the association with better one-year survival to early revascularization.
To the best of our knowledge, the present study is among the first to examine medication use among patients with renal dysfunction at one year after an ACS. Use of acetylsalicylic acid, beta-blockers and lipid-lowering therapy among these patients was persistently lower than among those with preserved renal function. Of note, the temporal decline in the use of acetylsalicylic acid and beta-blockers was evident across the groups, although there was an overall increase in the use of lipid-lowering agents.
Several study limitations should be considered in interpreting our results. The lack of truly random site selection, the potential for nonconsecutive patient enrollment, and exclusion of early deaths as reflected by the low in-hospital mortality rate, may limit the generalizability of our findings. Because weight was not recorded, CrCl could not be determined in 22% of the patients in the registry. However, when serum creatinine (available in 98.5% of patients) was analyzed, similar results were obtained (data not shown). Because contraindications to medical therapies were not routinely collected on the case report form, we were unable to clearly identify the ‘ideal candidates’ for various therapies. Nevertheless, the association of treatment with better outcome was seen in the entire study cohort, rather than just those ‘ideal candidates’ in whom the benefit to risk ratio should be even more favourable. This observed association, albeit promising, may reflect unmeasured confounding. The question of whether more intensive treatment improves outcome can only be rigorously assessed by randomized, controlled trials. It is also unclear to what extent the treatment patterns observed were in fact related to advanced age and other comorbidities associated with renal dysfunction. Finally, vital status at one year was unavailable for the 6% of patients who were lost to follow-up, although there were no systematic differences in their baseline characteristics compared with those of the remaining cohort (data not shown).
CONCLUSIONS
Renal dysfunction is common and independently portends a higher one-year mortality among patients presenting with a broad spectrum of ACS. Underutilization of current evidence-based therapies, which are associated with similar and substantial survival benefits, may contribute to the worse outcome of patients with renal dysfunction and ACS. Thus, there exists an important opportunity to improve the quality of care. Future initiatives should elucidate the reasons for undertreatment and the optimal management strategy targeted for this high-risk population.
ACKNOWLEDGEMENTS
The authors thank Sue Francis for her expert secretarial assistance, and all the study investigators, coordinators and patients for their invaluable contributions to the Canadian Acute Coronary Syndromes (ACS) Registry
Footnotes
FUNDING: The present research was sponsored by the Canadian Heart Research Centre and Key Pharmaceuticals, Division of Schering Canada Inc. The funding source had no role in data collection, analysis, interpretation or in the decision to submit the manuscript for publication. Dr Andrew Yan is supported by the Canadian Institutes of Health Research Fellowship Award, the Canadian Heart Research Centre Fellowship Award and the Detweiler Travelling Fellowship.
REFERENCES
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–62. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–80. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CM, Pinto DS, Murphy SA, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535–43. doi: 10.1016/j.jacc.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: A high-risk combination. Ann Intern Med. 2002;137:563–70. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Beattie JN, Soman SS, Sandberg KR, et al. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–200. doi: 10.1053/ajkd.2001.24522. (Erratum in 2001;38:701) [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J. 2002;144:226–32. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- 8.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–8. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 9.Yan AT, Tan M, Fitchett D, et al. One-year outcome of patients after acute coronary syndromes (from the Canadian Acute Coronary Syndromes Registry) Am J Cardiol. 2004;94:25–9. doi: 10.1016/j.amjcard.2004.03.024. (Erratum in 2005;95:438) [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- 12.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 13.Yan AT, Jong P, Yan RT, et al. Clinical trial – derived risk model may not generalize to real-world acute coronary syndrome patients. Am Heart J. 2004;148:1020–7. doi: 10.1016/j.ahj.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 15.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 16.McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725–8. doi: 10.1016/s0735-1097(02)02955-8. [DOI] [PubMed] [Google Scholar]
- 17.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003;41:718–24. doi: 10.1016/s0735-1097(02)02956-x. [DOI] [PubMed] [Google Scholar]
- 19.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen CR, Brendorp B, Rask-Madsen C, Kober L, Kjoller E, Torp-Pedersen C. The prognostic importance of creatinine clearance after acute myocardial infarction. Eur Heart J. 2002;23:948–52. doi: 10.1053/euhj.2001.2989. [DOI] [PubMed] [Google Scholar]
- 21.Menon V, Sarnak MJ, Lessard D, Goldberg RJ. Recent trends in hospital management practices and prognosis after acute myocardial infarction in patients with kidney disease. Am J Cardiol. 2004;94:1290–3. doi: 10.1016/j.amjcard.2004.07.116. [DOI] [PubMed] [Google Scholar]
- 22.Santopinto JJ, Fox KA, Goldberg RJ, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: Findings from the global registry of acute coronary events (GRACE) Heart. 2003;89:1003–8. doi: 10.1136/heart.89.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masoudi FA, Plomondon ME, Magid DJ, Sales A, Rumsfeld JR. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J. 2004;147:623–9. doi: 10.1016/j.ahj.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 24.ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: Systematic overview of individual data from 100,000 patients in randomized trials. Circulation. 1998;97:2202–12. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- 25.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 26.FRagmin and Fast Revascularization during InStability in Coronary artery disease Investigators. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354:708–15. [PubMed] [Google Scholar]
- 27.Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–87. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 28.Januzzi JL, Cannon CP, DiBattiste PM, Murphy S, Weintraub W, Braunwald E. Effects of renal insufficiency on early invasive management in patients with acute coronary syndromes (The TACTICS-TIMI 18 Trial) Am J Cardiol. 2002;90:1246–9. doi: 10.1016/s0002-9149(02)02844-8. [DOI] [PubMed] [Google Scholar]