Abstract
BACKGROUND
Some evidence-based therapies are underused in patients with a poor prognosis despite the fact that the survival gains would be highest among such patient subgroups. The extent to which this applies for acute, life-saving therapies is unknown. The impact of prognostic characteristics and pre-existing conditions on the use of reperfusion therapy among eligible patients with acute ST segment elevation myocardial infarction is examined.
METHODS
Of 2829 acute myocardial infarction patients prospectively identified in 53 acute care hospitals across Ontario, 987 presented with ST segment elevation within 12 h of symptom onset and without any absolute contraindications to reperfusion therapy. The baseline prognosis for each patient was derived from a validated risk-adjustment model of 30-day mortality. Multiple logistical regression was used to examine the relationships among reperfusion therapy, prognosis and the number of pre-existing chronic conditions after adjusting for factors such as age, sex, time since symptom onset and socioeconomic status.
RESULTS
Of the 987 appropriate candidates, 725 (73.5%) received reperfusion therapy (70.8% fibrinolysis, 2.6% primary angioplasty). The adjusted odds ratio of reperfusion therapy fell 4% with each 1% increase in baseline risk of death (adjusted OR 0.96, 95% CI 0.92 to 1.00, P=0.04) and fell 18% with each additional pre-existing condition (adjusted OR 0.82, 95% CI 0.76 to 0.90, P<0.001). The number rather than the type of pre-existing conditions inversely correlated with the use of reperfusion therapy. While the impact of baseline risk and pre-existing conditions was additive, pre-existing conditions exerted a greater impact on the nonuse of reperfusion therapy than did baseline risk.
CONCLUSIONS
A treatment-risk paradox is demonstrable even within a cohort of lower risk patients with ST segment elevation myocardial infarction. These findings are consistent with the view that these clinical decisions are more likely to be attributable to concerns about patient frailty or side effects than to a misunderstanding of treatment benefits.
Keywords: Acute myocardial infarction, Cohort studies, Reperfusion therapy, Utilization
Abstract
HISTORIQUE
Certaines thérapies probantes sont sousutilisées chez les patients présentant un pronostic défavorable, même si grâce à elles, le taux de survie de certains sous-groupes de patients pourrait augmenter. On ne sait pas dans quelle mesure ce phénomène s’applique dans le cadre de thérapies aiguës salutaires. Les répercussions des caractéristiques pronostiques et des pathologies préexistantes sur le recours à la thérapie de reperfusion chez les patients admissibles atteints d’un infarctus aigu du myocarde avec surélévation du segment ST sont analysées.
MÉTHODOLOGIE
Des 2 829 patients atteints d’un infarctus aigu du myocarde repérés prospectivement dans 53 hôpitaux de soins aigus de l’Ontario, 987 présentaient une surélévation du segment ST dans les 12 heures suivant l’apparition des symptômes, sans contre-indication absolue à la thérapie de reperfusion. Le pronostic de départ de chaque patient était dérivé d’un modèle validé de rajustement du risque de décès dans un délai de 30 jours. La régression logistique multiple a été utilisée pour examiner le lien entre la thérapie de reperfusion, le pronostic et le nombre de pathologies chroniques préexistantes après rajustement compte tenu de facteurs comme l’âge, le sexe, la période écoulée depuis l’apparition des symptômes et le statut socioéconomique.
RÉSULTATS
Des 987 candidats pertinents, 725 (73,5 %) ont reçu une thérapie de reperfusion (70,8 % de fibrinolyse et 2,6 % d’angioplastie primaire). Le risque relatif corrigé de la thérapie de reperfusion fléchissait de 4 % par point de pourcentage ajouté au risque de décès de départ (RR corrigé 0,96, 95 % IC 0,92 à 1,00, P<0,04) et chutait de 18 % par pathologie préexistante (RR corrigé 0,82, 95 % IC 0,76 à 0,90, P<0,001). Le nombre plutôt que le type de pathologies préexistantes étaient inversement proportionnel au recours à la thérapie de reperfusion. Bien que les répercussions du risque de départ et des pathologies préexistantes s’additionnent, les pathologies préexistantes avaient plus de répercussions que le risque de départ sur la non-utilisation de la thérapie de reperfusion.
CONCLUSIONS
Un paradoxe entre le risque et le traitement peut être démontré, même au sein d’une cohorte de patients à faible risque atteints d’un infarctus aigu du myocarde avec surélévation du segment ST. Ces observations confirment le point de vue selon lequel ces décisions cliniques sont plus susceptibles d’être attribuables à des inquiétudes quant à la fragilité des patients ou aux effets secondaires qu’à une mauvaise compréhension des bienfaits du traitement.
Available evidence suggests that the absolute impact of therapy in a population of persons with disease will strongly correlate with a patient’s baseline prognosis or risk of adverse events without treatment (1,2). Accordingly, physicians should be most enthusiastic and assiduous about the use of evidence-based therapies in high-risk patients who have the most to gain. This has not been the case for many therapies, where an inverse relationship between baseline risk and application of treatment has been shown (3–13). For example, in the cardiovascular field, elderly patients, patients with lower socioeconomic status and those with greater comorbidity undergo fewer cardiac interventions and may be less likely to receive beta-blockers, acetylsalicylic acid and statins following acute myocardial infarction (AMI), even though these patients have the most to gain (3,4,6–10,14).
This paradoxical pattern of medical decision-making, termed ‘the treatment-risk paradox’ (14), is poorly understood. One possible explanation is that physicians are unaware of existing evidence or lack prognostic sophistication and therefore fail to use indicated treatments in high-risk patients with the most to gain. An equally plausible interpretation is that physicians are generally averse to giving additional treatments to patients with multiple comorbid conditions. This explanation implies that physicians perceive disease-specific interventions as either risky or futile because of the presence of other clinical conditions, the use of multiple medications with potential for drug interactions, and the overall fragility of the patient’s health status.
If the first explanation is correct, then the paradoxical tendency to undertreat in high-risk patients should correlate only with disease-specific prognostic factors, and unrelated comorbid conditions should not affect decision-making. If the second explanation is correct, then the number of comorbid conditions should itself explain undertreatment. Furthermore, the explanatory power of these comorbid conditions should be independent of whether they are immediately relevant to the prognosis of the condition for which treatment is being considered.
The objective of the present study was therefore to explore whether the treatment-risk paradox is explained by the prevalence of pre-existing conditions, disease-specific prognostic factors, both or neither. Our test case involved acute reperfusion therapy among appropriate candidates with ST segment elevation myocardial infarction (STEMI). The analysis was focused by virtue of the nature of the cohort and our eligibility criteria on a group of STEMI patients, whose disease severity fell toward the lower end of the baseline risk spectrum. We believe this test case is helpful on two scores. First, lower risk patients with STEMI represent the end of the prognostic spectrum where treatment gaps are expected to be less obvious than among patients who are at high risk of adverse events due to cardiac-specific factors or general comorbidity (14,15). Thus, this cohort enabled us to test whether the treatment-risk paradox is demonstrable within a lower risk group, thereby showing whether this is a continuous and graded relationship even among relatively lower risk patients. Second, we inferred that the magnitude of the treatment-risk paradox among lower risk patients, if present at all, would be less pronounced than among an unselected cohort of patients from the general population (16).
METHODS
Data source
The Socio-Economic Status and Acute Myocardial Infarction (SESAMI) study is an ongoing prospective study that enrolled 3504 patients (76.2% recruitment rate) admitted to 53 major hospitals with AMI throughout Ontario between December 1999 and February 2003. The inclusion and exclusion criteria used for the present study have been previously described (17,18). Briefly, patients who died prematurely (ie, in the emergency room or the critical care unit within 24 h of admission), those deemed too ill (ie, on ventilators) to participate in longitudinal follow-up, and patients who did not speak English were excluded. Baseline sociodemographic and risk factor profiles were obtained through surveys administered to patients at inception. Clinical data for each patient were linked to the relevant data hospital discharge abstracts (Canadian Institute for Health Information) using encrypted health card numbers for 3407 of the initial 3504 recruits. Of the 3407 patients, 2829 underwent detailed medical chart abstraction related to the index AMI as part of a substudy of SESAMI. Details regarding the training, abstraction and data accuracy of the chart abstraction exercise are reported elsewhere (19). The SESAMI chart abstraction substudy was undertaken on the first 3224 patients enrolled into the study and represented all but three of the original participating institutions. Medical charts could not be located for 317 patients, presumably because of delays in the processing of hospital records among those recently discharged or deceased. An additional 78 patients had information collected on hospitalizations that did not pertain to the index AMI admission; these patients were therefore excluded. While there were no significant differences in the baseline characteristics between participants and nonparticipants of the SESAMI chart abstraction substudy, patients who participated in the SESAMI chart abstraction substudy had a marginally lower 30-day mortality than did chart abstraction nonparticipants (2.4% versus 4.0%, respectively; P=0.04). Clinical data from chart abstraction were linked backwards to hospital discharge administrative databases for the preceding 12 or more years (ie, year 1988). The use of administrative data in conjunction with hospital charts for missing variables has been validated elsewhere (20). Out-of-hospital mortality was ascertained through linkage to Ontario’s Registered Persons Database. The present study received research ethics approval at all participating hospitals.
Appropriate candidates for reperfusion therapy
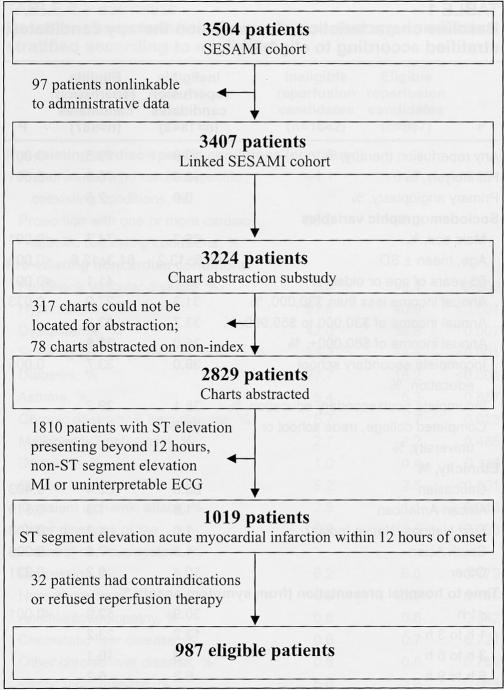
Patients had to meet three criteria to qualify as an appropriate candidate for reperfusion therapy: ST segment elevation on the admission or diagnostic electrocardiogram; less than 12 h from symptom onset to hospitalization; and, no absolute contraindications to reperfusion therapy (active bleeding, suspected aortic dissection, severe hypertension on presentation, recent trauma or pregnancy). The time interval from symptom onset to hospital presentation was obtained from two independent data sources: through self-report as obtained from a baseline survey and as recorded in the medical chart. Patients with discordant information on symptom arrival time intervals (ie, 0 h to 12 h versus 12 h or longer) were excluded from the analysis. Among the 2829 patients included in the analysis, 1019 patients presented to hospital with ST segment elevation within 12 h of symptom onset. An additional 32 patients were excluded because of absolute contraindications or patient refusal for reperfusion therapy, leaving an eligible sample of 987 patients available for analysis (Figure 1).
Figure 1).
Flow chart of the Socio-Economic and Acute Myocardial Infarction (SESAMI) study sample. ECG Electrocardiogram; MI Myocardial infarction
The number of pre-existing conditions in the study population
A pre-existing condition was defined as any cardiac or noncardiac illness or any cardiovascular risk factor that predated the index AMI admission. The number of conditions was tallied and analyzed separately for cardiac-specific and noncardiac diagnoses. Pre-existing cardiac-specific conditions were defined as previous myocardial infarction, angina, congestive heart failure, previous coronary artery bypass graft surgery and previous percutaneous coronary intervention. While the remaining conditions were classified as ‘noncardiac’, altering the categorization of cardiovascular risk factors from noncardiac to cardiac-specific diseases did not significantly alter the results (see below).
The derivation of baseline risk
Baseline risk was defined as the predicted probability of death within 30 days after myocardial infarction. Two established risk-adjustment indexes were tested and performed well in the patient sample (21,22). The algorithm proposed by Krumholz et al (21) showed areas under the receiver operating curve of 0.84 and 0.87 for 30-day and one-year mortality, respectively, with excellent goodness of fit. The second model, derived by Morrow et al (22), was specific to STEMI and consisted of age (in years) divided by 10, then squared, multiplied by the patient’s admission heart rate (beats/min), and then divided by the patient’s systolic blood pressure (mmHg). With respect to the present sample, the second risk index also displayed excellent accuracy (areas under the receiver operating curves of 0.81 and 0.84 for 30-day and one-year mortality, respectively) and precision. The model of Morrow et al (22) had the virtue of excluding any of the pre-existing conditions whose effects on treatment behaviour served as one of the study’s objectives and was therefore used to project baseline risk for each patient (a reanalysis with the index of Krumholz et al [21] did not meaningfully alter the results).
Outcome
Use of reperfusion therapy was defined as either fibrinolytic therapy (ie, with tissue plasminogen activator, tenecteplase or streptokinase) or primary angioplasty.
To examine whether baseline risk and/or pre-existing conditions were associated with higher rates of complications following reperfusion therapy among those receiving fibrinolytic or primary angioplasty, intracerebral hemorrhage rates were compared. Intracerebral events (hemorrhagic or thrombotic) following reperfusion therapy were confirmed through the use of computed tomography imaging.
Analytical techniques
First, the rates of reperfusion therapy use were determined according to each baseline factor in univariate fashion using χ2 analyses (or Fisher’s exact test where appropriate). Multiple logistical regression techniques were used to examine the likelihood of reperfusion therapy in relation to baseline risk (ie, expected 30-day mortality as derived using the index of Morrow et al [22]) and the number of pre-existing conditions. Age, sex, socioeconomic status, time from symptom onset to hospital presentation, and hospital type (on-site catheterization and angioplasty facilities) were adjusted for in each model. The significance of all two-way interactions was also tested.
Next, the relationships between reperfusion therapy, baseline risk and the number of pre-existing conditions after adjustment for all remaining baseline factors were examined.
All multiple logistical regression models were constructed in a similar manner using stepwise backward regression techniques and comparison of the −2 log likelihood ratios between sequential nested models. The number of pre-existing conditions was examined as continuous variables and categorically (when collapsed into quartiles or around their medians where appropriate).
To assess whether the influence on decision-making from coexisting illness varied with the specific comorbidities examined, two sensitivity analyses were undertaken. The first analysis ascertained the comorbidities independently associated with reperfusion therapy, as derived from a multivariate analysis controlling for all other factors. The impact of the number of pre-existing illnesses on treatment propensity was then examined, distinguishing those conditions associated with reperfusion therapy from those not directly related to the therapy in question. The second analysis distinguished those conditions whose variables were each associated with 30-day mortality with those whose variables were not directly related to 30-day mortality outcomes. Finally, given that the efficacy of reperfusion therapy is time-dependent and diminishes markedly beyond 6 h, a sensitivity analysis was conducted in which patients presenting to hospital beyond 6 h from symptom onset were excluded.
Diagnostic tests for collinearity demonstrated no variance inflation factor greater than 5 across any covariate. Statistical significance was defined as P<0.05. SAS statistical software (Version 8.2, SAS Institute, USA) was used for all analyses.
RESULTS
Among 987 patients presenting with acute STEMI within 12 h of presentation who had neither refused treatment nor had absolute contraindications for treatment, 725 patients (73.5%) received reperfusion therapy, 699 patients (70.8%) received thrombolysis and 26 patients (2.6%) received primary angioplasty. The average 30-day crude mortality was 2.6%. The baseline characteristics are shown in Table 1. The correlation between a patient’s baseline risk and the number of pre-existing comorbid conditions was weak (r=0.17, P<0.001). While the average baseline risk among patients with zero or one pre-existing condition (expected 30-day mortality of 2.40%; 95% CI 0.92% to 5.6%) was significantly lower than for patients with two or more pre-existing conditions (expected 30-day mortality of 2.79%; 95% CI 0.95% to 7.67%), the distribution of patients overlapped considerably between the two subgroups.
TABLE 1.
Baseline characteristics of reperfusion therapy candidates, stratified according to eligibility*
| Ineligible reperfusion candidates (n=1842) | Eligible reperfusion candidates (n=987) | P | |
|---|---|---|---|
| Any reperfusion therapy, % | 10.9 | 73.5 | <0.001 |
| Fibrinolysis, % | 10.0 | 70.9 | <0.001 |
| Primary angioplasty, % | 0.9 | 2.6 | |
| Sociodemographic variables | |||
| Male sex, % | 66.3 | 74.7 | <0.001 |
| Age, mean ± SD | 65.6±13.2 | 61.3±12.6 | <0.001 |
| 65 years of age or older, % | 55.7 | 41.1 | <0.001 |
| Annual income less than $30,000, % | 31.3 | 27.9 | 0.033 |
| Annual income of $30,000 to $59,999, % | 33.7 | 33.3 | |
| Annual income of $60,000+, % | 35.0 | 38.8 | |
| Incomplete secondary school education, % | 39.0 | 33.7 | 0.005 |
| Incomplete postsecondary education, % | 28.1 | 29.2 | |
| Completed college, trade school or university, % | 32.9 | 37.1 | |
| Ethnicity, % | |||
| Caucasian | 83.6 | 82.4 | 0.403 |
| African American | 1.6 | 1.3 | 0.519 |
| First Nations (Native Indian) | 1.0 | 1.3 | 0.408 |
| South Asian | 4.8 | 7.3 | 0.006 |
| Other | 10.4 | 9.2 | 0.331 |
| Time to hospital presentation (from symptom onset), % | |||
| <1 h | 30.9 | 52.0 | <0.001 |
| 1 h to 3 h | 17.2 | 23.2 | |
| 3 h to 6 h | 9.6 | 16.1 | |
| 6 h to 9 h | 6.2 | 6.2 | |
| 9 h to 12 h | 3.7 | 2.5 | |
| >12 h | 32.4 | 0 | |
| Clinical characteristics on admission | |||
| Chest pain, % | 88.4 | 96.3 | <0.001 |
| Dyspnea, % | 27.8 | 22.4 | 0.002 |
| <12 h from onset of symptoms, % | 55.5 | 100.0 | <0.001 |
| Diastolic blood pressure, mean ± SD | 82.9±19.1 | 85.0±19.1 | 0.004 |
| Systolic blood pressure, mean ± SD | 148.7±32.0 | 146.5±30.0 | 0.079 |
| Heart rate, mean ± SD | 84.6±24.4 | 78.3±21.1 | <0.001 |
| Respiration rate, mean ± SD | 20.1±5.0 | 19.8±4.5 | 0.112 |
| Acute pulmonary edema, % | 3.7 | 2.0 | 0.013 |
| Rales or crackles, % | 18.3 | 13.6 | 0.001 |
| Wheeze/rhonchi, % | 5.3 | 3.4 | 0.024 |
| Cardiogenic shock, % | 0.6 | 0.9 | 0.341 |
| Cardiac arrest, % | 1.0 | 1.9 | 0.034 |
| ST segment elevation myocardial infarction, % | 20.3 | 100 | <0.001 |
| Non-ST segment elevation myocardial infarction, % | 79.7 | 0 | |
| Anterior wall infarction location, % | 21.8 | 40.9 | <0.001 |
| Inferior wall infarction location, % | 23.9 | 49.7 | |
| Other wall infarction location, % | 52.3 | 9.3 | |
| Baseline risk, median (25th to 75th percentile)† | 2.0 (1.9–2.1) | 1.7 (1.2–2.7) | <0.001 |
| Observed 30-day mortality, % | 2.3 | 2.6 | 0.622 |
| Pre-existing cardiac-specific conditions | |||
| Previous myocardial infarction, % | 28.6 | 18.4 | <0.001 |
| Angina, % | 42.6 | 26.7 | <0.001 |
| Congestive heart failure, % | 4.3 | 1.1 | <0.001 |
| Previous coronary artery bypass graft, % | 8.1 | 3.6 | <0.001 |
| Previous percutaneous coronary intervention, % | 6.7 | 3.2 | <0.001 |
| Number of cardiac-specific pre-existing conditions, mean ± SD | 0.9±1.0 | 0.5±0.8 | <0.001 |
| Proportion with no cardiac-specific coexisting conditions, % | 44.1 | 61.7 | <0.001 |
| Proportion with one or more cardiac-specific coexisting conditions, % | 55.9 | 38.3 | |
| Pre-existing noncardiac conditions | |||
| Peripheral vascular disease, % | 8.0 | 5.1 | 0.003 |
| Hypertension, % | 51.5 | 40.9 | <0.001 |
| Dyslipidemia, % | 42.0 | 36.8 | 0.007 |
| Smoking history (current or former), % | 35.3 | 48.8 | <0.001 |
| Diabetes, % | 27.7 | 18.2 | <0.001 |
| Asthma, % | 5.4 | 5.3 | 0.857 |
| Chronic obstructive lung disease, % | 5.7 | 3.7 | 0.023 |
| Malignancy (metastatic), % | 2.7 | 2.2 | 0.485 |
| Dementia, % | 1.0 | 0.4 | 0.118 |
| Stroke, % | 5.2 | 2.5 | <0.001 |
| Transient ischemic attack, % | 2.5 | 1.1 | 0.013 |
| Other diseases of the central nervous system, % | 0.9 | 0.3 | 0.093 |
| Depression, % | 6.2 | 6.0 | 0.779 |
| Hyperthyroidism, % | 0.5 | 0.1 | 0.180 |
| Anemia/coagulopathy, % | 0.8 | 0.6 | 0.542 |
| Cholestatic liver disease, % | 0.6 | 0.7 | 0.721 |
| Other chronic liver disease, % | 0.6 | 0.5 | 0.759 |
| Peptic ulcer disease, % | 5.0 | 3.6 | 0.089 |
| Bilateral renal artery stenosis, % | 0.4 | 0.3 | 1.000 |
| End stage renal disease (dialysis-dependent), % | 0.8 | 0.3 | 0.201 |
| Any renal insufficiency (serum creatinine ≥140 μmol/L), % | 10.7 | 3.3 | <0.001 |
| Number of noncardiac-specific pre-existing conditions, mean ± SD | 2.1±1.4 | 1.8±1.3 | <0.001 |
| Proportion with no noncardiac-specific coexisting conditions, % | 10.7 | 12.8 | <0.001 |
| Proportion with one noncardiac-specific coexisting condition, % | 26.1 | 32.7 | |
| Proportion with two noncardiac-specific coexisting conditions, % | 27.1 | 28.3 | |
| Proportion with three or more noncardiac-specific coexisting conditions, % | 36.1 | 26.2 | |
| Cumulative number of any pre-existing conditions | |||
| Average number ± SD | 3.0±1.9 | 2.3±1.6 | <0.001 |
| Proportion with no cumulative coexisting conditions, % | 5.9 | 9.2 | <0.001 |
| Proportion with one cumulative coexisting condition, % | 17.5 | 24.7 | |
| Proportion with two cumulative coexisting conditions, % | 21.1 | 27.0 | |
| Proportion with three or more cumulative coexisting conditions, % | 55.4 | 39.1 | |
| Geographical/admitting hospital characteristics | |||
| On-site revascularization at the admitting hospital, % | 14.1 | 16.5 | 0.088 |
| Rural residents, % | 4.7 | 5.7 | 0.271 |
All numbers reflect percentages unless otherwise specified. Percentages were derived from patients without missing data for specified variables;
Baseline risk on admission as derived from a risk adjustment index developed by Morrow et al (22)
Multivariate predictors: Role of baseline risk and pre-existing conditions
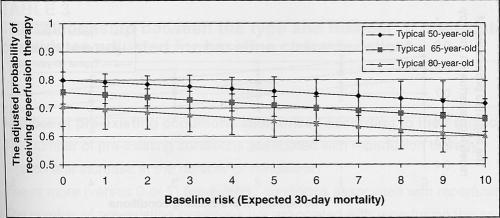
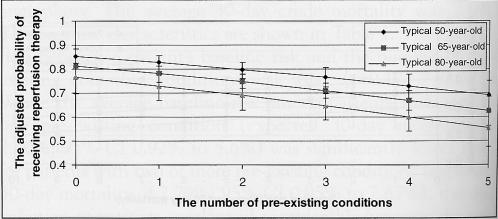
Figure 2 illustrates the probability of receiving reperfusion therapy across baseline risk strata for a typical 50-, 65- and 80-year-old male patient of average income, admitted to an urban community hospital in Ontario within 3 h of symptom onset. Specifically, each 1% increase in the baseline risk of 30-day mortality was associated with a 4% reduction in the odds of receiving reperfusion therapy (adjusted OR 0.96, 95% CI 0.92 to 1.00, P=0.04). However, pre-existing conditions had a much stronger effect on the use of reperfusion therapy than did baseline risk. For example, among typical male 50-, 65- and 80-year-old patients, the odds of receiving reperfusion therapy fell 18% for each additional pre-existing condition (adjusted OR 0.82, 95% CI 0.76 to 0.90, P<0.001) (Figure 3), even after adjusting for baseline sociodemographic or ethnic factors, time to presentation and admitting hospital characteristics.
Figure 2).
The adjusted probability of reperfusion therapy according to age and baseline risk. Baseline risk (expected 30-day mortality) was derived from the risk index by Morrow et al (22), and is stratified according to the typical 50-, 65- and 80-year-old patient. The probability of reperfusion therapy (±95% CI) is adjusted for age, sex, socioeconomic status, ethnicity, hospital type, rural-urban status and hospital arrival times. P=0.04 for the effects of baseline risk; P=0.01 for the effects of age
Figure 3).
The adjusted probability of reperfusion therapy according to age and number of pre-existing conditions. The number of pre-existing conditions represents the cumulative sum of all cardiac and noncardiac comorbid conditions and is stratified according to the typical 50-, 65- and 80-year-old patient. The probability of reperfusion therapy (±95% CI) is adjusted for age, sex, socioeconomic status, ethnicity, hospital type, rural-urban status and hospital arrival times. P=0.001 for the effects of age; P<0.001 for the effect of the number of pre-existing conditions
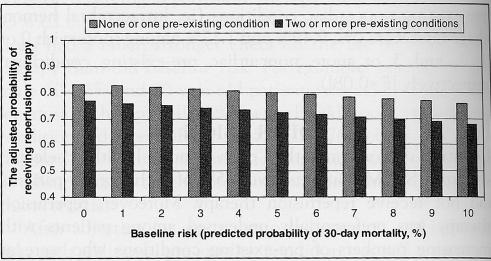
After adjusting for age, time to presentation and baseline risk, each additional pre-existing condition was still associated with a significantly lower likelihood of receiving reperfusion therapy (adjusted OR 0.83, 95% CI 0.76 to 0.91, P<0.001), was equally strong whether derived using noncardiac pre-existing illnesses or cardiac pre-existing conditions, and remained similar in magnitude with or without adjustment for baseline risk. Likewise, baseline risk still remained inversely correlated with reperfusion therapy after adjustments for the number of pre-existing illnesses (adjusted OR 0.96, 95% CI 0.92 to 1.00; P=0.04) (Table 2). In short, the impact of baseline risk and pre-existing conditions was additive, although the latter exerted a much greater impact on reperfusion therapy than did the former (Figure 4).
TABLE 2.
Multivariate analysis illustrating the independent associations of baseline risk, the number of coexisting conditions, age and hospital arrival times with the likelihood of receiving acute reperfusion therapy among ideal candidates*
| Adjusted OR | 95% CI | P | |
|---|---|---|---|
| Age (per year increase) | 0.99 | 0.97–1.00 | <0.001 |
| Hospital arrival times | 0.06 | ||
| <60 min | 1.00 | Reference | |
| 1 h to 2 h | 0.94 | 0.63–1.31 | |
| 3 h to 5 h | 0.70 | 0.46–1.04 | |
| 6 h to 9 h | 0.63 | 0.35–1.14 | |
| 10 h to 12 h | 0.33 | 0.14–0.75 | |
| Number of pre-existing cardiac-specific conditions† | <0.001 | ||
| No conditions | 1.00 | Reference | |
| One condition or more | 0.60 | 0.44–0.81 | |
| Number of pre-existing noncardiac conditions‡ | <0.001 | ||
| None or one condition | 1.00 | Reference | |
| Two conditions | 0.77 | 0.53–1.08 | |
| Three conditions or more | 0.60 | 0.42–0.85 | |
| Number of cumulative pre-existing conditions§ | <0.001 | ||
| None or one condition | 1.00 | Reference | |
| Two conditions | 0.95 | 0.63–1.41 | |
| Three conditions or more | 0.53 | 0.37–0.74 | |
| Baseline risk¶ | 0.96 | 0.92–1.00 | 0.04 |
Patients presenting with ST segment elevation acute myocardial infarction within 12 h of symptom onset with no absolute contraindications or refusal for therapy. Analyses adjusted for age, sex, income, ethnicity, hospital arrival times, baseline risk, number of pre-existing conditions, hospital characteristics (ie, on-site revascularization facilities) and geographical residents (rural-urban). The effects of sex, income, ethnicity, hospital characteristics and rural-urban status are P>0.1 for each, and are therefore not presented in the above model;
Pre-existing cardiac-specific conditions include pre-existing angina, previous myocardial infarction, previous congestive heart failure, previous percutaneous transluminal coronary angioplasty and previous coronary artery bypass graft;
Pre-existing noncardiac conditions include diabetes, hyperlipidemia, hypertension, smoking history, chronic obstructive pulmonary disease, asthma, depression, dementia, stroke, transient ischemic attack, other central nervous system disorders, cancer (metastatic), cholestasis, chronic liver disease, dialysis, renal insufficiency (140 mmol/L or greater), bilateral renal artery stenosis, coagulopathy/anemia, peptic ulcer disease, peripheral artery disease and thyroid disorders;
Cumulative number of pre-existing conditions includes pre-existing cardiac-specific and noncardiac conditions;
Baseline risk was derived using a risk-adjustment index from Morrow et al (22)
Figure 4).
The adjusted probability of reperfusion therapy according to baseline risk and the number of pre-existing conditions. The number of pre-existing conditions represents the cumulative sum of all cardiac and noncardiac comorbid conditions, and is stratified into two subgroups: those with no or one pre-existing condition and those with two or more pre-existing conditions. Baseline risk (expected 30-day mortality) was derived from the risk adjustment index by Morrow et al (22). The probability of reperfusion therapy is also adjusted for age, sex, socioeconomic status, ethnicity, hospital type, rural-urban status and hospital arrival times. P=0.03 for the effects of baseline risk; P<0.01 for the effect of the number of pre-existing conditions
The effect of the number of pre-existing conditions on the use of reperfusion therapy was independent of the type of comorbid diseases tallied, and was as marked when the numbers of conditions comprised individual comorbid factors most closely related to reperfusion therapy or 30-day mortality as it was when comprising factors not associated with therapeutic use or outcomes (Table 3). Finally, the importance of pre-existing conditions on reperfusion therapy underutilization persisted even after excluding patients who presented to hospital between 6 h and 12 h from the onset of symptoms.
TABLE 3.
The relationship between the type and number of pre-existing conditions and acute reperfusion therapy among ideal candidates adjusted for baseline characteristics*
| Adjusted OR | 95% CI | P | |
|---|---|---|---|
| Number of pre-existing conditions categorized according to their relationship with reperfusion therapy† | |||
| The number of pre-existing conditions associated with reperfusion therapy (each unit increase in the number of conditions) | 0.73 | 0.63–0.84 | <0.001 |
| Two or more (versus 0 or 1) pre-existing conditions associated with reperfusion therapy | 0.56 | 0.41–0.75 | <0.001 |
| The number of pre-existing conditions not associated with reperfusion therapy (each unit increase in the number of conditions) | 0.84 | 0.73–0.96 | 0.009 |
| Two or more (versus 0 or 1) pre-existing conditions not associated with reperfusion therapy | 0.68 | 0.59–0.91 | 0.01 |
| Number of pre-existing conditions classified according to their relationship with death at 30 days‡ | |||
| The number of pre-existing conditions associated with death at 30 days (each unit increase in the number of conditions) | 0.54 | 0.29–1.00 | 0.05 |
| Two or more (versus 0 or 1) pre-existing conditions associated with death at 30 days | 0.54 | 0.29–1.00 | 0.05 |
| The number of pre-existing conditions not associated with death at 30 days (each unit increase in the number of conditions) | 0.83 | 0.76–0.90 | <0.001 |
| Two or more (versus 0 or 1) pre-existing conditions not associated with death at 30 days | 0.65 | 0.48–0.89 | 0.008 |
Patients presenting with ST segment elevation acute myocardial infarction within 12 h of symptom onset with no absolute contraindications or refusal for therapy. Analyses adjusted for age, sex, income, ethnicity, hospital arrival times, hospital characteristics (ie, on-site revascularization facilities) and geographical residents (rural-urban). The effects of sex, income, ethnicity, hospital characteristics and rural-urban status are P>0.1 for each, and are therefore not presented in the above model;
Pre-existing conditions that independently predict reperfusion therapy in multivariate analyses include angina (adjusted OR 0.64, 95% CI 0.43 to 0.93), previous myocardial infarction (adjusted OR 0.63, 95% CI 0.42 to 0.97), previous coronary artery bypass graft surgery (adjusted OR 0.40, 95% CI 0.18 to 0.90), cancer (adjusted OR 0.28, 95% CI 0.09 to 0.86), diabetes (adjusted OR 0.56, 95% CI 0.37 to 0.85), stroke (adjusted OR 0.36, 95% CI 0.13 to 0.99) and smoking (adjusted OR 1.57, 95% CI 1.11 to 2.22);
Pre-existing conditions that independently predict death at 30 days in multivariate analyses include anemia/coagulopathy (adjusted OR 24.2, 95% CI 2.30 to 255.5), central nervous system disorders (other than stroke, transient ischemic attacks or dementia) (adjusted OR 150.7, 95% CI 8.86 to 999.9) and chronic obstructive pulmonary disease (adjusted OR 9.74, 95% CI 2.74 to 34.6)
Complications following reperfusion therapy
Only four strokes were recorded following reperfusion therapy (all confirmed by computed tomography), three of which were attributable to intracerebral hemorrhages (0.41% intracerebral hemorrhage rate among those receiving reperfusion therapy). Intracerebral hemorrhage rates did not correlate with either baseline risk (P=0.34) or the number of pre-existing cardiac-specific conditions (P=0.55). However, intracerebral hemorrhage rates did increase with a rise in the number of pre-existing noncardiac conditions (ie, intracerebral hemorrhage rates were 0%, 0.49% and 1.17% among those with 0 or1, 2, and 3 or more noncardiac pre-existing conditions, respectively [P=0.08]).
DISCUSSION
Our study demonstrated that, even among a healthier selected cohort of STEMI patients, over 25% of such eligible patients did not receive reperfusion therapy. Moreover, reperfusion therapy was preferentially underused among patients with increasing numbers of pre-existing conditions who were at highest baseline risk of future cardiovascular events.
Randomized clinical trial evidence has shown significant mortality reduction when reperfusion therapy is given to patients presenting with acute STEMI within 12 h of symptom onset (23–37). However, in our prospective cohort study involving more than 50 hospitals, 26.5% of otherwise appropriate candidates did not receive reperfusion therapy. Similar underuse of reperfusion therapy has been demonstrated among acute STEMI patients presenting within 6 h of symptom onset in the United States (15,38). Likewise, in the Global Registry of Acute Coronary Events (39), only 70% of eligible STEMI patients received reperfusion therapy. While the use of primary angioplasty in our study was low when compared with international standards (40,41), the rates of primary angioplasty were comparable with other jurisdictions within Canada (19,42).
Our results also confirm earlier studies that have shown the use of evidence-based pharmacotherapies to diminish paradoxically with increasing baseline risk of future events (6,8,11,13,14,38,39,43–46). While the magnitude of effect for baseline risk on the use of reperfusion therapy was modest, the relationship was independent of age and hospital arrival times – both of which have also been shown to be independent predictors of reperfusion therapy use.
The treatment-risk paradox is still poorly understood, notwithstanding the fact that the same pattern of decision-making has been demonstrated for many conditions and treatments. To understand the key determinants of these decisions, we asked, at the outset of our analysis, whether a condition-specific prognosis or the number of comorbid conditions was the more powerful predictor of reperfusion therapy nonuse. In this context, the tendency of physicians to withhold potentially life-saving therapies was more strongly dependent on the number of pre-existing comorbid conditions at presentation than on baseline risk, per se. However, each factor was independently associated with reperfusion therapy, and the effects of the two factors combined were additive. For example, the likelihood of reperfusion therapy fell 18% for each additional increase in the number of pre-existing conditions at hospital entry, and fell 4% for each additional per cent increase in the risk of death at 30 days – relationships that were not explained by absolute contraindications to therapy and were independent of the type of comorbid illnesses.
Why would baseline risk and the number of pre-existing conditions lead to undertreatment even in this acute setting?
One possibility is that physicians perceive treatment to be futile among patients with greater illness severity and disease complexity. Indeed, some evidence suggests that the biological responsiveness of thrombolytic therapy diminishes with age (47) – an independent determinant of clinical prognosis and the number of pre-existing conditions at AMI presentation. However, studies have also demonstrated that the potential survival benefit of any therapy in the population is driven more by baseline risk than by therapeutic efficacy (48). Nevertheless, perceptions of futility may be driven, in part, by misinformed decision-making regarding the potential benefits of therapy among high-risk patients.
Another possibility, suggested by Redelmeier et al (49), is that physicians are reluctant to manage multiple, unrelated conditions simultaneously. However, this seems far more germane for chronic pharmacotherapy than for the acute situation of STEMI.
A third, and more persuasive, possibility is that physicians are concerned that a patient with multiple comorbidities may suffer adverse effects from active treatment, and that the hippocratic doctrine of ‘primum non nocere’ overrides considerations of net benefit in persons who actually have much to gain from treatment. Indeed, our study confirmed a modest relationship between therapeutic complication rates and the noncardiac, comorbid disease burden. Harmful side effects stemming from therapy are usually obvious and attributable, unlike their downstream prognostic benefits, which are less tangible and more often probabilistic, particularly for preventive treatments. Thus, when dealing with frail patients or those with multiple comorbid conditions, physicians may opt for a more conservative approach in their decision-making behaviour, fearing errors of commission more than errors of omission.
The present study has several noteworthy limitations. First, our analysis relied on observational data and logistic regression models, which can only draw inference on physician decision-making thought processes. Second, while we did impose rigorous criteria to create our ‘eligible patient group’ and excluded any patient with ‘absolute contraindications’ to therapy, subtle or relative contraindications to reperfusion therapy were not captured and may have partially accounted for the gaps in reperfusion therapy care observed in the study. Finally, by design, our sample was drawn from a prospective study that required individual consent. As a result, the patients under study may have been ‘healthier’ than corresponding patients from the general population. However, as noted, our study confirmed the presence of the treatment-risk paradox even within a selected group of patients whose expected mortality covered mostly the lower end of the baseline risk continuum. We accordingly expect that the effects would be larger among unselected STEMI patients than among those observed here. As well, we adjusted for many other factors, such as time from symptoms to hospitalization, as well as available clinical and sociodemographic characteristics.
We should emphasize that the rate of life-threatening complications required to negate potential survival benefits from reperfusion therapy and similar treatments, while lower in patients with higher degrees of comorbidity, rarely ever approaches the incidence expected in actual practice (48). Thus, in a context such as the one studied, the absolute net benefits from treatment remain clear-cut in those patients who have both an adverse condition-specific prognosis and general comorbidities. This paradoxical relationship between anticipated treatment yield and likelihood of intervention constitutes a continuing limit on the potential population payoff from various evidence-based therapies. One possible solution, albeit counter to conventional wisdom, involves closer attention to teasing out the net benefits for potential high-yield subgroups, ideally using multifactorial analyses that avoid focusing post hoc on one or two determinants of prognosis. By highlighting these high-yield subgroups more clearly in reports of clinical trial results, systematic reviews, undergraduate and postgraduate medical education and continuing education, professional opinion leaders may be able to modify physician behaviour and ensure that the benefits of modern, evidence-based therapies are made available to all.
ACKNOWLEDGEMENTS
This study was supported by an operating grant from the Canadian Institutes of Health Research (CIHR). The Institute for Clinical Evaluative Sciences (ICES) is supported in part by a grant from the Ontario Ministry of Health. The results, conclusions and opinions are those of the authors, and no endorsement by the Ontario Ministry of Health, ICES or CIHR is intended or should be inferred. Dr Alter is a New Investigator of the CIHR, funded by both the CIHR and the Heart and Stroke Foundation of Canada
APPENDIX
| Socio-Economic Status and Acute Myocardial Infarction (SESAMI) study co-investigators | |
|---|---|
| Dr David A Alter (Principal Investigator), Ms Karey Iron, Dr Peter C Austin, Dr Jack I Williams, Dr Jack V Tu, Dr Jane Irvine, Dr Christopher Morgan, Dr Blair Wheaton, Dr C David Naylor, Dr Cameron Mustard | |
| SESAMI collaborators | |
| Hospital | Physician |
| Alexandra Hospital | Dr Marguerite Hanoman |
| Belleville General Hospital | Dr Henry Kafka |
| Cambridge Memorial Hospital | Dr A Shekhar Pandey |
| Chatham-Kent Health Alliance | Dr Linda Sinnaeve |
| Credit Valley Hospital – Mississauga | Dr Maurice Druck |
| Grand River Hospital Corporation – Kitchener | Dr Claus H Rinne |
| Greater Niagara General – Niagara Falls | Dr Yun Kai Chan |
| Grey Bruce – Owen Sound Site | Dr George Kuruvilla |
| Guelph General Hospital | Dr Dan Schwarz |
| Halton Healthcare Services Corp – Oakville | Dr Donald Peat |
| Hamilton – General Division | Dr James Veliano |
| Hamilton – Henderson Site | Dr Akbar Panju |
| Hamilton – McMaster Medical Centre | Dr Koon Teo |
| Hamilton – St Joseph’s | Dr Dominic Raco |
| Hotel Dieu Grace Hospital – Windsor | Dr Raj Chetty |
| Humber River Regional – Church | Dr Robert Bauer |
| Humber River Regional – Finch | Dr Teosar Bhesania |
| Joseph Brant Memorial Hospital – Burlington | Dr Ian Darcel |
| Lake of the Woods | Dr William Cameron |
| Lakeridge Health Corporation – Oshawa | Dr Rakesh Bhargava |
| London Health Sciences Centre – University Site | Dr Gerald Wisenberg |
| London Health Sciences Centre – Victoria Campus | Dr Kumar Sridhar |
| Mount Sinai Hospital – Toronto | Dr Gary Newton |
| North Bay General Hospital | Dr Blair Bowker |
| North York General Hospital | Dr Emory Burke |
| Orillia Soldiers’ Memorial Hospital | Dr John MacFayden |
| Ottawa Heart Institute | Dr Rick Davies |
| Perth and Smith Falls | Dr Robert Del Grande |
| Peterborough Regional Health Centre | Dr William Hughes |
| Rouge Valley Health System (Ajax/Pickering Site) | Dr Rolland Leader |
| Rouge Valley Health System (Centenary Site) | Dr Narendra Singh |
| Royal Victoria Hospital – Barrie | Dr Bruce R Burke |
| St Catharines General Hospital | Dr Sven Pallie |
| St Catharines – Hotel Dieu Hospital | Dr Peter Fernandez and Dr Peter Bolli |
| St Joseph’s Hospital – Toronto | Dr Maria DeVilla |
| St Mary’s General Hospital – Kitchener | Dr Claus H Rinne |
| St Michael’s Hospital – Toronto | Dr David Fitchett |
| Sarnia General Hospital | Dr S Nasir Ali |
| Sault Ste Marie | Dr Hui Lee |
| The Scarborough Hospital – General Division | Dr Ted Davies |
| The Scarborough Hospital – Grace Division | Dr Robert Nitkin |
| South Lake Regional Health Centre – Newmarket | Dr David Fell |
| Sudbury Regional Hospital | Dr Shah Nawaz and Dr Grama Ravi |
| Sunnybrook – Toronto | Dr David Alter |
| Thunder Bay (Port Arthur and McKellar Sites) | Dr Chris Lai |
| Timmins and District Hospital | Dr Malvinder Parmar |
| Toronto East General Hospital | Dr Chuck Lefkowitz |
| Toronto General Hospital and Toronto Western Hospital | Dr Robert Mark Iwanochko |
| Trillium Health Centre – Mississauga Site | Dr Thomas Rebane |
| William Osler Health Centre (Brampton Site/Peel) | Dr Milan Gupta |
| William Osler Health Centre (Etobicoke Hospital Campus) | Dr Keith Kwok |
| York Central Hospital – Richmond Hill | Dr Eric Gangbar |
REFERENCES
- 1.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–33. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 2.Parker AB, Naylor CD. Subgroups, treatment effects, and baseline risks: Some lessons from major cardiovascular trials. Am Heart J. 2000;139:952–61. doi: 10.1067/mhj.2000.106610. [DOI] [PubMed] [Google Scholar]
- 3.Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–67. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 4.Alter DA, Khaykin Y, Austin PC, Tu JV, Hux JE. Processes and outcomes of care for diabetic acute myocardial infarction patients in Ontario: Do physicians undertreat? Diabetes Care. 2003;26:1427–34. doi: 10.2337/diacare.26.5.1427. [DOI] [PubMed] [Google Scholar]
- 5.Amar J, Vaur L, Perret M, Bailleau C, Etienne S, Chamontin B PRATIK study investigators. Hypertension in high-risk patients: Beware of the underuse of effective combination therapy (results of the PRATIK study) J Hypertens. 2002;20:779–84. doi: 10.1097/00004872-200204000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Aronow WS. Underutilization of lipid-lowering drugs in older persons with prior myocardial infarction and a serum low-density lipoprotein cholesterol >125 mg/dl. Am J Cardiol. 1998;82:668–9. A6–A8. doi: 10.1016/s0002-9149(98)00401-9. [DOI] [PubMed] [Google Scholar]
- 7.Berger AK, Radford MJ, Wang Y, Krumholz HM. Thrombolytic therapy in older patients. J Am Coll Cardiol. 2000;36:366–74. doi: 10.1016/s0735-1097(00)00723-3. [DOI] [PubMed] [Google Scholar]
- 8.Califf RM, DeLong ER, Ostbye T, et al. Underuse of aspirin in a referral population with documented coronary artery disease. Am J Cardiol. 2002;89:653–61. doi: 10.1016/s0002-9149(01)02335-9. [DOI] [PubMed] [Google Scholar]
- 9.De Wilde S, Cook DG, Carey IM, Hilton SR, Whincup PH. Underuse of statins among older people. Lancet. 2003;362:746–7. doi: 10.1016/s0140-6736(03)14216-x. [DOI] [PubMed] [Google Scholar]
- 10.Dudley NJ, Bowling A, Bond M, et al. Age- and sex-related bias in the management of heart disease in a district general hospital. Age Ageing. 2002;31:37–42. doi: 10.1093/ageing/31.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest. 1999;116:603–13. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 12.Portnoi VA. The underuse of warfarin treatment in the elderly. Arch Intern Med. 1999;159:1374–5. doi: 10.1001/archinte.159.12.1374. [DOI] [PubMed] [Google Scholar]
- 13.Solomon DH, Finkelstein JS, Katz JN, Mogun H, Avorn J. Underuse of osteoporosis medications in elderly patients with fractures. Am J Med. 2003;115:398–400. doi: 10.1016/s0002-9343(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 14.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: The treatment-risk paradox. JAMA. 2004;291:1864–70. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Murillo JE, Chen J, et al. Thrombolytic therapy for eligible elderly patients with acute myocardial infarction. JAMA. 1997;277:1683–8. [PubMed] [Google Scholar]
- 16.Weiss CO, Varadhan R. Risk-treatment paradox in use of statins. JAMA. 2004;292:169. doi: 10.1001/jama.292.2.169-a. [DOI] [PubMed] [Google Scholar]
- 17.Alter DA, Brandes S, Irvine J, Iron K. Impact of socioeconomic status on cardiovascular outcomes in Canada. Expert Rev Pharmacoeconomics Outcomes Res. 2003;3:691–702. doi: 10.1586/14737167.3.6.691. [DOI] [PubMed] [Google Scholar]
- 18.Alter DA, Iron K, Austin PC, Naylor CD SESAMI Study Group. Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–7. doi: 10.1001/jama.291.9.1100. [DOI] [PubMed] [Google Scholar]
- 19.Tu JV, Donovan LR, Lee DS, et al. Quality of Cardiac Care in Ontario: EFFECT (Enhanced Feedback for Effective Cardiac Treatment) Toronto: Institute for Clinical Evaluative Sciences; 2004. [Google Scholar]
- 20.Norris CM, Ghali WA, Knudtson ML, Naylor CD, Saunders LD. Dealing with missing data in observational health care outcome analyses. J Clin Epidemiol. 2000;53:377–83. doi: 10.1016/s0895-4356(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 21.Krumholz HM, Chen J, Wang Y, Radford MJ, Chen YT, Marciniak TA. Comparing AMI mortality among hospitals in patients 65 years of age and older: Evaluating methods of risk adjustment. Circulation. 1999;99:2986–92. doi: 10.1161/01.cir.99.23.2986. [DOI] [PubMed] [Google Scholar]
- 22.Morrow DA, Antman EM, Giugliano RP, et al. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: An InTIME II substudy. Lancet. 2001;358:1571–5. doi: 10.1016/S0140-6736(01)06649-1. [DOI] [PubMed] [Google Scholar]
- 23.A prospective trial of intravenous streptokinase in acute myocardial infarction (I.S.A.M.). Mortality, morbidity, and infarct size at 21 days. The I.S.A.M. Study Group. N Engl J Med. 1986;314:1465–71. doi: 10.1056/NEJM198606053142301. [DOI] [PubMed] [Google Scholar]
- 24.Long-term effects of intravenous anistreplase in acute myocardial infarction: Final report of the AIMS study. AIMS Trial Study Group. Lancet. 1990;335:427–31. [PubMed] [Google Scholar]
- 25.Randomised trial of late thrombolysis in patients with suspected acute myocardial infarction. EMERAS (Estudio Multicentrico Estreptoquinasa Republicas de America del Sur) Collaborative Group. Lancet. 1993;342:767–72. [PubMed] [Google Scholar]
- 26.Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6–24 hours after onset of acute myocardial infarction. Lancet. 1993;342:759–66. [PubMed] [Google Scholar]
- 27.A comparison of reteplase with alteplase for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. N Engl J Med. 1997;337:1118–23. doi: 10.1056/NEJM199710163371603. [DOI] [PubMed] [Google Scholar]
- 28.Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) Lancet. 1986;1:397–401. [PubMed] [Google Scholar]
- 29.Long-term effects of intravenous thrombolysis in acute myocardial infarction: Final report of the GISSI study. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) Lancet. 1987;2:871–4. [PubMed] [Google Scholar]
- 30.Guerci AD, Gerstenblith G, Brinker JA, et al. A randomized trial of intravenous tissue plasminogen activator for acute myocardial infarction with subsequent randomization to elective coronary angioplasty. N Engl J Med. 1987;317:1613–8. doi: 10.1056/NEJM198712243172601. [DOI] [PubMed] [Google Scholar]
- 31.Randomized factorial trial of high-dose intravenous streptokinase, of oral aspirin and of intravenous heparin in acute myocardial infarction. ISIS (International Studies of Infarct Survival) pilot study. Eur Heart J. 1987;8:634–42. [PubMed] [Google Scholar]
- 32.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber TL, Miller DH, Silvasi DA, Moses JW, Borer JS. Randomized double-blind trial of intravenous streptokinase for acute myocardial infarction. Am J Cardiol. 1986;58:47–52. doi: 10.1016/0002-9149(86)90239-0. [DOI] [PubMed] [Google Scholar]
- 34.Van de Werf F. Thrombolysis for acute myocardial infarction. Haemostasis. 1994;24:65–8. doi: 10.1159/000217086. [DOI] [PubMed] [Google Scholar]
- 35.van de Werf F. More evidence for a beneficial effect of platelet glycoprotein IIb/IIIa-blockade during coronary interventions. Latest results from the EPILOG and CAPTURE trials. Eur Heart J. 1996;17:325–6. doi: 10.1093/oxfordjournals.eurheartj.a014858. [DOI] [PubMed] [Google Scholar]
- 36.White HD, Norris RM, Brown MA, et al. Effect of intravenous streptokinase on left ventricular function and early survival after acute myocardial infarction. N Engl J Med. 1987;317:850–5. doi: 10.1056/NEJM198710013171402. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox RG, von der Lippe G, Olsson CG, Jensen G, Skene AM, Hampton JR. Effects of alteplase in acute myocardial infarction: 6-month results from the ASSET study. Anglo-Scandinavian Study of Early Thrombolysis. Lancet. 1990;335:1175–8. [PubMed] [Google Scholar]
- 38.Barron HV, Bowlby LJ, Breen T, et al. Use of reperfusion therapy for acute myocardial infarction in the United States: Data from the National Registry of Myocardial Infarction 2. Circulation. 1998;97:1150–6. doi: 10.1161/01.cir.97.12.1150. [DOI] [PubMed] [Google Scholar]
- 39.Eagle KA, Goodman SG, Avezum A, Budaj A, Sullivan CM, Lopez-Sendon J GRACE Investigators. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: Findings from the Global Registry of Acute Coronary Events (GRACE) Lancet. 2002;359:373–7. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RH, Sadiq I, Goldberg RJ, et al. GRACE Investigators. Effectiveness of primary percutaneous coronary intervention compared with that of thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2004;147:253–9. doi: 10.1016/j.ahj.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Zahn R, Schiele R, Schneider S, et al. Primary angioplasty versus no reperfusion therapy in patients with acute myocardial infarction and a pre-hospital delay of > 12–24 hours: Results from the pooled data of the maximal individual therapy in acute myocardial infarction (MITRA) registry and the myocardial infarction registry (MIR) J Invasive Cardiol. 2001;13:367–72. [PubMed] [Google Scholar]
- 42.Pilote L, Merrett P, Karp I, et al. Cardiac procedures after an acute myocardial infarction across nine Canadian provinces. Can J Cardiol. 2004;20:491–500. [PubMed] [Google Scholar]
- 43.Hirsch AT, Gotto AM., Jr Undertreatment of dyslipidemia in peripheral arterial disease and other high-risk populations: an opportunity for cardiovascular disease reduction. Vasc Med. 2002;7:323–31. doi: 10.1191/1358863x02vm453ra. [DOI] [PubMed] [Google Scholar]
- 44.Miller M, Byington R, Hunninghake D, Pitt B, Furberg CD. Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) Investigators. Arch Intern Med. 2000;160:343–7. doi: 10.1001/archinte.160.3.343. [DOI] [PubMed] [Google Scholar]
- 45.Rojas-Fernandez CH, Kephart GC, Sketris IS, Kass K. Underuse of acetylsalicylic acid in individuals with myocardial infarction, ischemic heart disease or stroke: Data from the 1995 population-based Nova Scotia Health Survey. Can J Cardiol. 1999;15:291–6. [PubMed] [Google Scholar]
- 46.Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest. 2001;119:720–5. doi: 10.1378/chest.119.3.720. [DOI] [PubMed] [Google Scholar]
- 47.Thiemann DR, Coresh J, Schulman SP, Gerstenblith G, Oetgen WJ, Powe NR. Lack of benefit for intravenous thrombolysis in patients with myocardial infarction who are older than 75 years. Circulation. 2000;101:2239–46. doi: 10.1161/01.cir.101.19.2239. [DOI] [PubMed] [Google Scholar]
- 48.Alter DA, Manuel DG, Gunraj N, Anderson G, Naylor CD, Laupacis A. Age, risk-benefit trade-offs, and the projected effects of evidence-based therapies. Am J Med. 2004;116:540–5. doi: 10.1016/j.amjmed.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 49.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–20. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]