Abstract
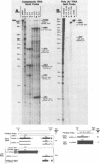
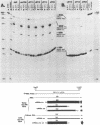
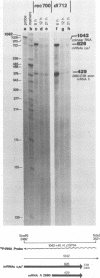
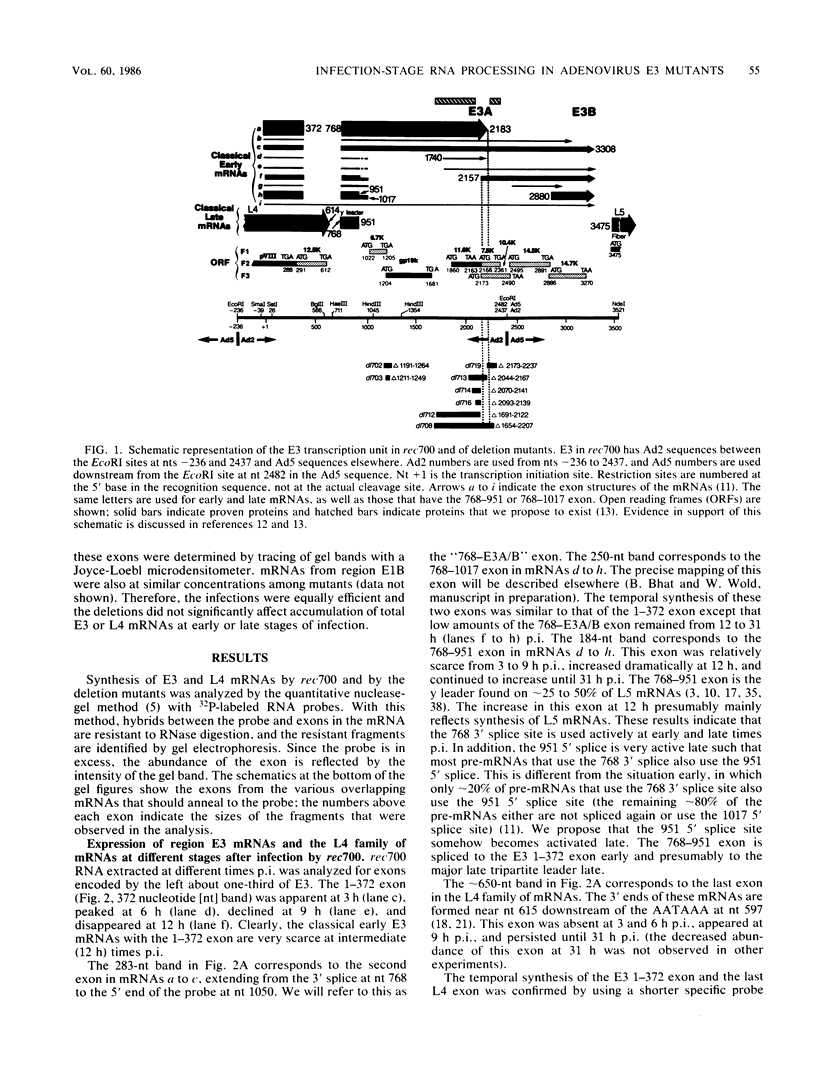
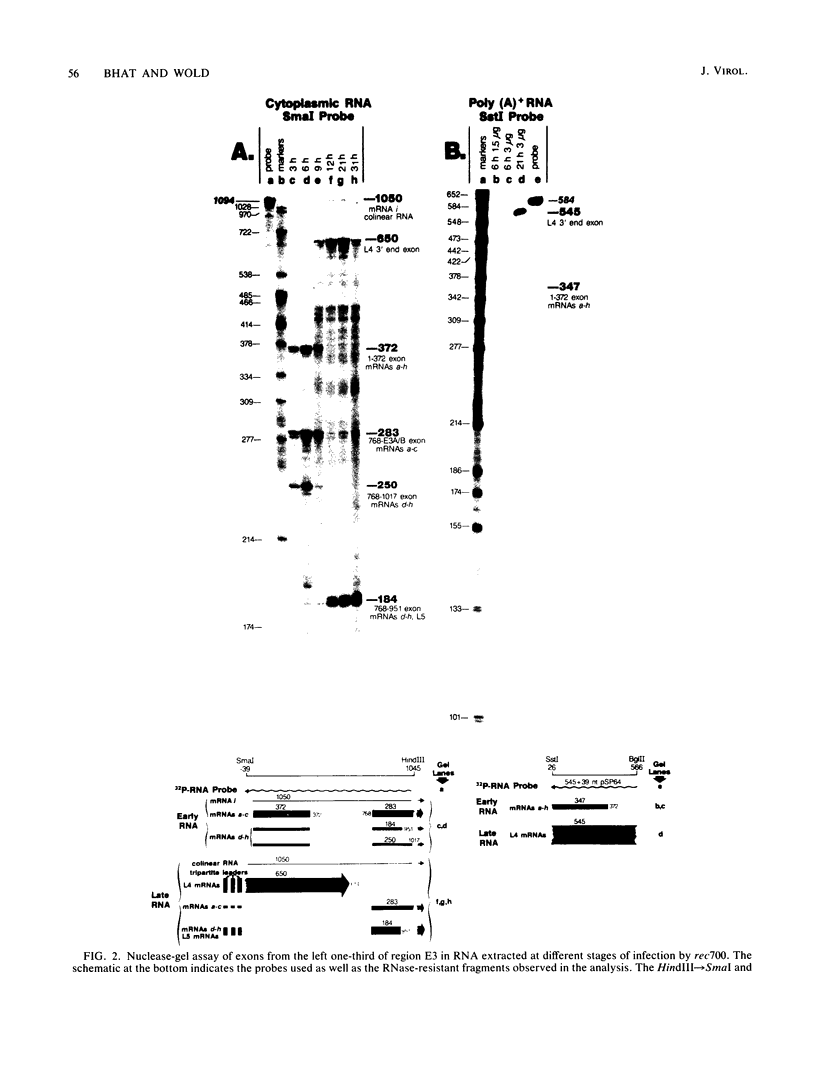
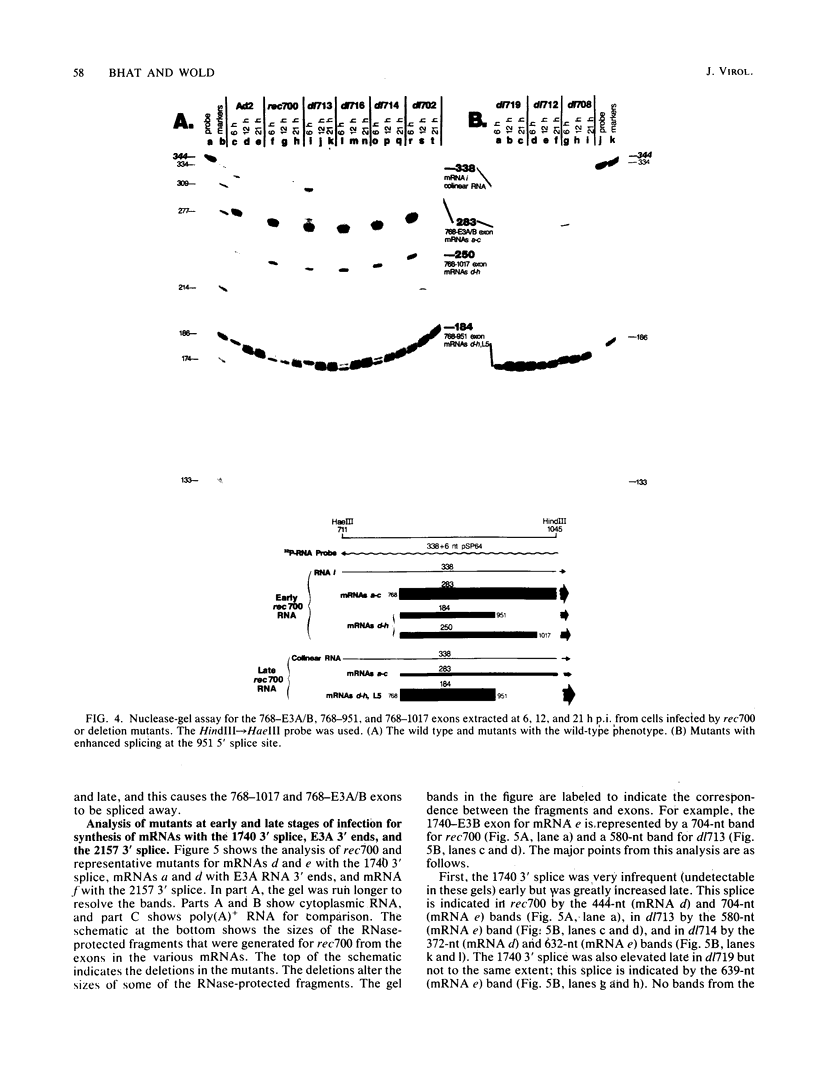
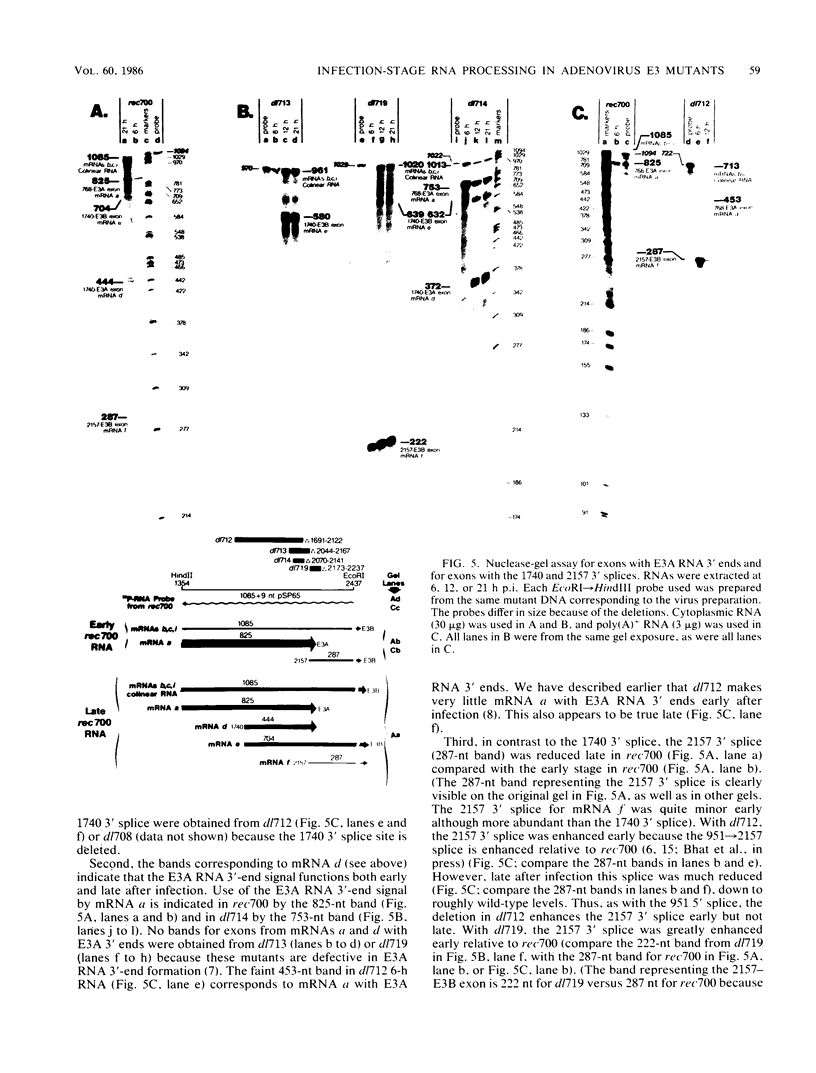
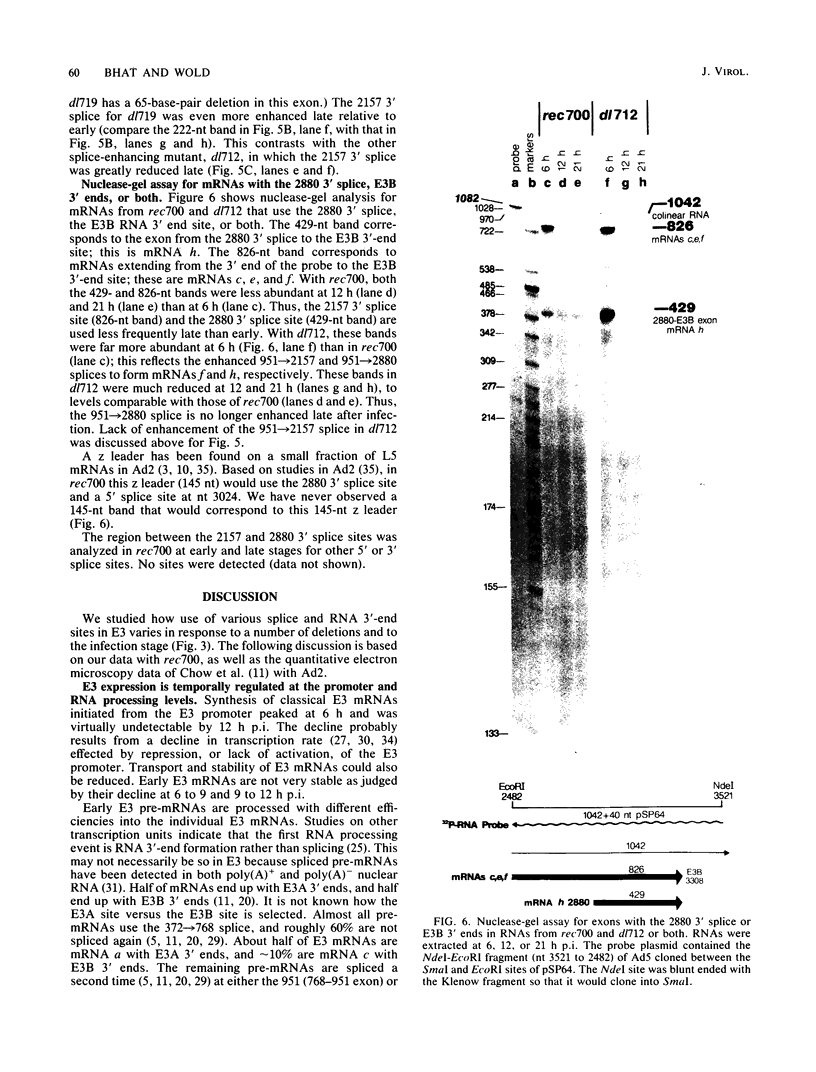
Region E3 of adenovirus encodes about nine overlapping mRNAs (a to i) with different spliced structures and with two major RNA 3' end sites termed E3A and E3B. Synthesis of E3 mRNAs was examined by the nuclease-gel procedure at early and late stages of infection by wild-type virus (rec700) and by several E3 deletion mutants. Our results, together with those obtained by electron microscopy (L. T. Chow, T. R. Broker, and J. B. Lewis, J. Mol. Biol. 134:265-303, 1979), suggest that E3 may be differentially regulated at early and late stages at both the promoter and RNA-processing levels. Early after infection, the E3 promoter is used to make mainly mRNAs a and h. Late after infection, the E3 promoter appears to be shut off and the major late promoter is used to make mainly mRNAs d and e. The late L4 mRNA 3' end site is not used early even though early E3 pre-mRNAs transcribe through the L4 RNA 3' end site. The nucleotide 768-951 exon, which is the y leader on many L5 mRNAs, is very abundant late. (Nucleotide +1 is the major E3 transcription initiation site.) Early after infection, the 951 5' splice site is enhanced 5- to 10-fold in the dl712 (delta 1691 to 2122) group of mutants; late after infection, these mutants resemble the wild type. We speculate that the activity of the 951 5' splice site is regulated at early and late stages; it is suppressed early to permit synthesis of mRNA a, and it is activated late to permit synthesis of mRNAs d, e, and L5. With dl719 (delta 2173 to 2237), the 951----2157 splice is enhanced both early and late; we suggest that this deletion enhances the 2157 3' splice site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G. Anatomy of region L1 from adenovirus type 2. J Virol. 1985 Dec;56(3):879–886. doi: 10.1128/jvi.56.3.879-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bhat B. M., Brady H. A., Wold W. S. Virus deletion mutants that affect a 3' splice site in the E3 transcription unit of adenovirus 2. Mol Cell Biol. 1985 Sep;5(9):2405–2413. doi: 10.1128/mcb.5.9.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B. M., Wold W. S. ATTAAA as well as downstream sequences are required for RNA 3'-end formation in the E3 complex transcription unit of adenovirus. Mol Cell Biol. 1985 Nov;5(11):3183–3193. doi: 10.1128/mcb.5.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B. M., Wold W. S. Adenovirus mutants with splice-enhancing mutations in the E3 complex transcription unit are also defective in E3A RNA 3'-end formation. J Virol. 1986 Mar;57(3):1155–1158. doi: 10.1128/jvi.57.3.1155-1158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binger M. H., Flint S. J. Accumulation of early and intermediate mRNA species during subgroup C adenovirus productive infections. Virology. 1984 Jul 30;136(2):387–403. doi: 10.1016/0042-6822(84)90175-2. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Bhat B., Wold W. S. Mapping the 5' ends, 3' ends, and splice sites of mRNAs from the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):44–54. doi: 10.1016/0042-6822(85)90444-1. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Sayavedra M., Raskas H. J. Strand assignment of polyadenylated nuclear RNAs synthesized early in infection with adenovirus 2. Virology. 1977 Apr;77(2):545–555. doi: 10.1016/0042-6822(77)90480-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Bhat B. M., Pursley M. H., Cladaras C., Wold W. S. Novel deletion mutants that enhance a distant upstream 5' splice in the E3 transcription unit of adenovirus 2. Nucleic Acids Res. 1985 Aug 26;13(16):5771–5788. doi: 10.1093/nar/13.16.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Baker C. C., Moore M. A., Ziff E. B. Poly(A) sites of adenovirus serotype 2 transcription units. J Mol Biol. 1982 Mar 5;155(3):207–233. doi: 10.1016/0022-2836(82)90002-x. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Westphal H. The structure of adenovirus 2 early nuclear and cytoplasmic RNAs. J Mol Biol. 1980 Feb 15;137(1):23–48. doi: 10.1016/0022-2836(80)90155-2. [DOI] [PubMed] [Google Scholar]
- Le Moullec J. M., Akusjärvi G., Stålhandske P., Pettersson U., Chambraud B., Gilardi P., Nasri M., Perricaudet M. Polyadenylic acid addition sites in the adenovirus type 2 major late transcription unit. J Virol. 1983 Oct;48(1):127–134. doi: 10.1128/jvi.48.1.127-134.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Control of adenovirus E1B mRNA synthesis by a shift in the activities of RNA splice sites. Mol Cell Biol. 1984 May;4(5):966–972. doi: 10.1128/mcb.4.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. R., Ziff E. B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3' coterminal mRNAs and five late families. Cell. 1980 Dec;22(3):905–916. doi: 10.1016/0092-8674(80)90568-1. [DOI] [PubMed] [Google Scholar]
- Sittler A., Gallinaro H., Jacob M. In vivo splicing of the premRNAs from early region 3 of adenovirus-2: the products of cleavage at the 5' splice site of the common intron. Nucleic Acids Res. 1986 Feb 11;14(3):1187–1207. doi: 10.1093/nar/14.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålhandske P., Persson H., Perricaudet M., Philipson L., Pettersson U. Structure of three spliced mRNAs from region E3 of adenovirus type 2. Gene. 1983 May-Jun;22(2-3):157–165. doi: 10.1016/0378-1119(83)90099-9. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Svensson C., Josephson S., Aleström P., Chattapadhyaya J. B., Pettersson U., Philipson L. Leader arrangement in the adenovirus fiber mRNA. EMBO J. 1982;1(2):249–254. doi: 10.1002/j.1460-2075.1982.tb01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Magie S. C., Yacoub N. Mapping a new gene that encodes an 11,600-molecular-weight protein in the E3 transcription unit of adenovirus 2. J Virol. 1984 Nov;52(2):307–313. doi: 10.1128/jvi.52.2.307-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Nuclear RNA is spliced in the absence of poly(A) addition. Cell. 1981 Oct;26(1 Pt 1):39–46. doi: 10.1016/0092-8674(81)90031-3. [DOI] [PubMed] [Google Scholar]