Abstract
Early sensory processing can play a critical role in sensing environmental cues. We have investigated the physiological and behavioral function of gain control at the first synapse of olfactory processing in Drosophila. We report that olfactory receptor neurons (ORNs) express the GABAB receptor (GABABR) and its expression expands the dynamic range of ORN synaptic transmission that is preserved in projection neuron responses. Strikingly, we find that different ORN channels have unique baseline levels of GABABR expression. ORNs that sense the aversive odorant CO2 do not express GABABRs nor exhibit any presynaptic inhibition. In contrast, pheromone-sensing ORNs express a high level of GABABRs and exhibit strong presynaptic inhibition. Furthermore, a behavioral significance of presynaptic inhibition was revealed by a courtship behavior in which pheromone-dependent mate localization is impaired in flies that lack GABABRs in specific ORNs. Together, these findings indicate that different olfactory receptor channels may employ heterogeneous presynaptic gain control as a mechanism to allow an animal’s innate behavioral responses to match its ecological needs.
Keywords: Drosophila, olfaction, GABAB, presynaptic inhibition, gain control, dynamic range, two-photon imaging
The stereotypic organization of the Drosophila olfactory system and the identification of the family of odorant receptor genes make the fly an attractive system to study olfactory mechanisms. An adult fly expresses about 50 odorant receptor genes and each ORN typically expresses just one or a few receptor genes (Clyne et al., 1999; Vosshall et al., 1999; Gao et al., 2000; Vosshall et al., 2000; Couto et al., 2005; Fishilevich et al., 2005). ORNs detect odors in the periphery and send axons to glomeruli in the antennal lobe. Each glomerulus receives axons from about 20 ORNs expressing the same receptor genes and dendrites of a few uniglomerular projection neurons (PNs), which propagate olfactory information to higher brain centers (Stocker et al., 1994). This numerically simple olfactory system coupled with genetic markers to label most of the input channels provides an opportunity to dissect synaptic function and information processing.
The Drosophila antennal lobe is populated with GABAergic local interneurons (LNs) that release GABA in many if not all glomeruli (Ng et al., 2002). GABA exerts its modulatory role via two distinct receptor systems, the fast ionotropic GABAA receptor, which is sensitive to the antagonist picrotoxin, and the slow metabotropic GABAB receptor, which is sensitive to the antagonist CGP54626. Pharmacological blockade of the GABA receptors demonstrate that GABA-mediated hyperpolarization suppresses PN response to odor stimulation in a non-uniform fashion (Ng et al., 2002; Wilson and Laurent 2005; Shang et al., 2007). Electron microscopy studies of the insect antennal lobe show that GABAergic LNs synapse with PNs (Distler & Boeckh, 1993), which support the well established olfactory mechanism of lateral inhibition (Mori et al., 1999). GABAergic LNs also synapse onto ORNs and imaging studies in mouse suggest that activation of GABABRs in ORN terminals suppress neurotransmitter release in ORNs (McGann et al., 2005).
We hypothesize that setting the appropriate olfactory gain for environmental cues is important for adjusting an organism’s sensitivity to its environment. A recent study shows that GABABR mediated presynaptic inhibition provides a mechanism to modulate olfactory gain (Olsen & Wilson, 2008). Electrical recordings show that interglomerular presynaptic inhibition suppresses the olfactory gain of PNs to potentially increase the dynamic range of the olfactory response. Likewise, gain modulation may not be uniform among different glomeruli, which could reflect a tradeoff between sensitivity and dynamic range in different olfactory channels. For example, high sensitivity may be crucial for some environmental cues, such as those that require an immediate behavioral response, whereas a larger dynamic range may be more advantageous for other odors where precise spatial and temporal information may be critical for optimal performance.
Here we investigated the physiological and behavioral function of gain control in early olfactory processing. We show that interneuron-derived GABA activates GABABRs on ORN terminals, reducing the gain of ORN-to-PN synaptic transmission. Different types of ORNs exhibit different levels of presynaptic inhibition and this heterogeneity in presynaptic inhibition is preserved in antennal lobe output projection neurons. Interestingly, pheromone-sensing ORNs exhibit high levels of GABABR expression and behavioral experiments indicate that GABABR expression in a population of pheromone ORNs is important for mate localization, suggesting that presynaptic gain control is important for the olfactory channel-specific fine-tuning of behavior.
RESULTS
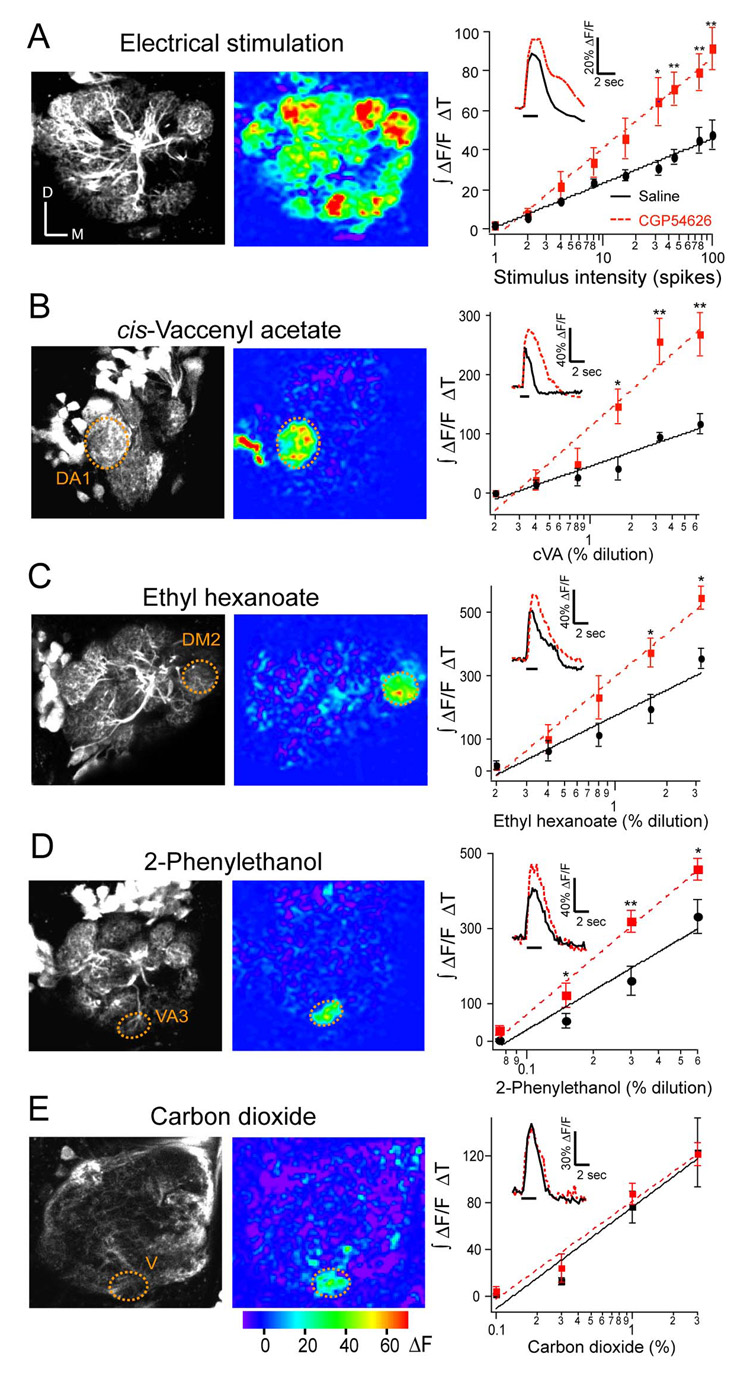
We investigated the role of GABABR in modulating olfactory dynamic range by imaging PN dendritic responses to a range of stimulus intensities in the presence or absence of a GABABR antagonist. Flies bearing GH146-Gal4 and UAS-GCaMP transgenes express the calcium sensor GCaMP (Nakai, et al., 2001) in many PNs (Stocker et al., 1997), allowing the select measurement of calcium response in PN dendrites. Insect dendritic calcium increases are mostly due to influx through nicotinic acetylcholine receptors (Goldberg et al., 1999; Oertner et al., 2001), therefore calcium influx provides an indirect measurement of synaptic potential. We plotted the input-output function of PNs to a range of olfactory nerve stimuli in the presence or absence of the GABABR antagonist (Figure 1A). Addition of CGP54626 significantly increases PN response at high stimulus intensities but not at low intensities, and significantly increased the slope of the input-output function by 105% with no effect on the offset, thus revealing a multiplicative gain modulation. This result suggests that GABABR activation suppresses PN response to expand olfactory dynamic range.
Figure 1. GABAB receptors alter the gain of projection neurons.
Two-photon imaging of PN activity in flies expressing GCaMP in GH146 neurons. (A) Response to electrical stimulation of the olfactory nerve. (B–E) Response to odor stimulation. Gray scale images show antennal lobe structure. Psuedocolored images reveal the response to electrical stimulation or a 2-second odor stimulation. Graphs show the input-output function of PNs in saline (black circles) and in the presence of 25 µM CGP54626 (red squares). Mean integrated fluorescence change over time across preparations is plotted as function of the number of stimuli or odor concentrations. Inset traces are representative of fluorescence change over time. ΔF/F was measured from all glomeruli in the optical section (A), or the outlined regions (B–E). Electrical stimulation was given at 100 Hz, 1 ms in duration and 10 V in amplitude. Concentrations of ethyl hexanoate and 2-phenylethanol were percent dilutions of 300 ppb and 80 ppm, respectively. n, 4–8. Error bars show SEM. * p ≤ 0.05, ** p ≤ 0.01.
Electrical stimulation allows precise control of activity but is an unnatural condition. We therefore investigated antennal lobe gain control with behaviorally relevant odors. The male pheromone odor cis-vaccenyl acetate activates the DA1 glomerulus (Ha and Smith, 2006). The fruit odors ethyl hexanoate and 2-phenylethanol (Stensmyr et al., 2003) activate the DM2 and VA3 glomeruli, respectively. Additionally, the Drosophila stress odorant CO2 activates the V glomerulus to provoke avoidance behavior (Suh et al., 2004). We monitored PN dendritic calcium in response to odor stimulation in the presence or absence of the GABABR antagonist. Blocking GABABRs has different effects on PNs of different glomeruli (Figures 1B–E). The slope of PN response to cis-vaccenyl acetate, ethyl hexanoate and 2-phenylethanol is increased by 153%, 67%, and 43%, respectively, in the presence of CGP54626. In contrast, the slope of PN response to CO2 is not altered by CGP54626. Thus, GABABR activation has different degrees of modulation on the gain of projection neurons in different glomeruli.
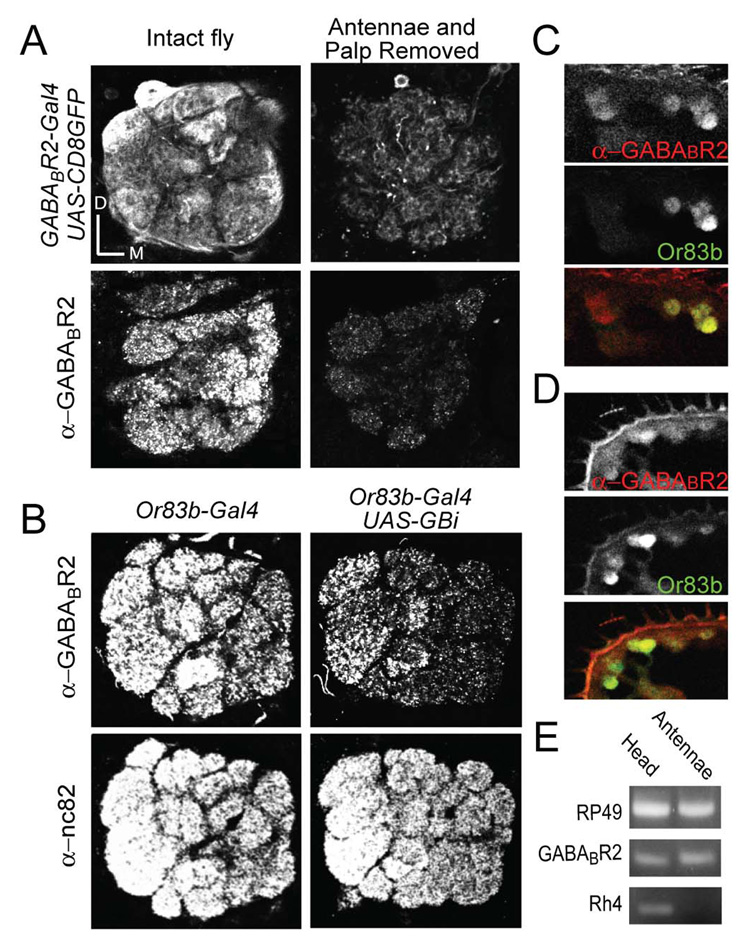
We next investigated the antennal lobe circuitry underlying this GABABR-mediated gain change. We first determined which populations of neurons in the antennal lobe express GABABR. Mammalian GABABR is an obligatory heterodimeric G-protein coupled receptor (Bettler et al., 2004). Similarly, co-expression of Drosophila GABAB-R1 and -R2 is necessary to produce a functional GABABR in heterologous systems (Mezler et al., 2001). To visualize neurons that express GABABR, we therefore generated a transgenic fly line that contains the fusion DNA of the yeast transcription factor Gal4 with 1.5 kb of genomic DNA immediately upstream to the open reading frame of the GABABR2 gene. The expression of GFP in flies bearing GABABR2-Gal4 and UAS-CD8-GFP within the antennal lobes appears to be in axon terminals of ORNs derived from the antennae and maxillary palps (Figure 2A, Supplementary Movie 1). Surgical removal of the olfactory appendages causes complete degeneration of ORN axons within three days (Vosshall et al., 2000), and we observed a dramatic reduction of GFP detection in the antennal lobes (Figure 2A, Supplementary Movie 3). Expression of GABABR2 in the antennae was further confirmed by RT-PCR of isolated antennal tissue. Primers for Drosophila GABABR2 as well as RP49, a ubiquitous ribosomal protein, detected transcripts in both the head and the antennae, while primers for Drosophila Rhodopsin-4 (Rh4), which encodes one of the ultraviolet rhodopsins, do not detect transcript in the antennae (Figure 2E). Thus, ORNs express mRNA for GABABR2. We next mapped the distribution of GABABR with antiserum specific for Drosophila GABABR2 (Hamasaka et al., 2005; Enell et al., 2007). Neurons in the antennae and maxillary palps positive for GABABR2 immunoreactivity largely overlap with the Or83b ORNs (Figure 2C,D). We used RNA interference to further confirm the expression of GABABR2 in ORNs. Expression of the GABABR2-RNAi in Or83b ORNs causes a 40% reduction in GABABR2 immunoreactivity in the antennal lobe, but no change in immunoreactivity of nc82—a neuropil specific antibody (Figure 2B). Immunoreactivity to GABABR2 in the antennal lobe is distributed mainly on ORN axonal terminals, as surgical removal of the olfactory appendages three days prior eliminates most of the staining (Figure 2A). Residual GABABR expression in the absence of ORN axons could be in LNs or PNs, as previously suggested (Wilson & Laurent 2005); our data are not conclusive but suggest that the residual staining is from a population of local interneurons because neurites and cell bodies are confined to the antennal lobe (Supplementary Movie 3). Nevertheless, GABABR within the antennal lobe is mostly distributed on ORN axonal terminals.
Figure 2. Molecular expression of GABAB receptors in olfactory receptor neurons.
(A) Images of the antennal lobe in flies bearing GABABR2-Gal4 and UAS-CD8-GFP (top) and cryosections stained with a GABABR2 antiserum (bottom). The Antennal lobe of normal flies is shown (left) in comparison to the antennal lobe in flies three days after the antennae and maxillary palps were surgically removed (right). (B) Whole mount immunostaining for GABABR2 and the neuropil marker nc82 in the antennal lobe of control flies expressing Or83b-Gal4 (left) and flies expressing Or83b-Gal4 and UAS-GABABR2-RNAi (UAS-GBi) (right). Immunostaining for GABABR2 and GFP in cryosections of the antenna (C) and the maxillary palp (D) in flies expressing Or83b-Gal4 and UAS-CD8-GFP. (E) Products of RT-PCR reactions from entire fly heads or isolated antennae, with primers for GABABR2, RP49 (gene for a ubiquitous ribosomal protein), and Rh4 (a rhodopsin gene).
To investigate the function of GABABR in ORN terminals, we next tested whether GABABR agonists applied exogenously can induce presynaptic inhibition in ORNs. Flies bearing the Or83b-LexA (Lai & Lee, 2006) and LexAop-GCaMP transgenes express the calcium sensor GCaMP in most ORNs, allowing the select measurement of calcium activity in ORN axonal terminals. Electrical stimulation of the olfactory nerve elicits a calcium transient in ORN terminals that is significantly reduced in the presence of 20 µM GABA or 20 µM SKF97541, a selective GABABR agonist (Hamasaka et al., 2005) (Figure 3A). If the GABA effect on presynaptic calcium is caused purely by activation of GABABR, it should be blocked by CGP54626 but not picrotoxin, the selective Drosophila GABABR and GABAAR antagonists respectively (Hamasaka et al., 2005; Wilson & Laurent, 2005). Indeed, the reduction caused by GABA is prevented by 25 µM CGP54626, but not by 125 µM picrotoxin (Figure 3A–B). Given that presynaptic calcium triggers neurotransmitter release, activation of GABABRs should serve to attenuate synaptic transmission. To test this possibility, we performed two-photon imaging of synaptopHluorin (spH), a fluorescent indicator of vesicle exocytosis (Miesenbock et al., 1998), in flies bearing Or83b-Gal4 and UAS-spH transgenes. Electrical stimulation of the olfactory nerve elicits an increase in spH fluorescence that is significantly reduced by the agonist SKF97541 and enhanced by the antagonist CGP54626 (Figure 3C–D). Thus, GABABR activation suppresses ORN presynaptic calcium and neurotransmitter release in Drosophila.
Figure 3. GABAB receptors mediate presynaptic inhibition in olfactory receptor neurons.
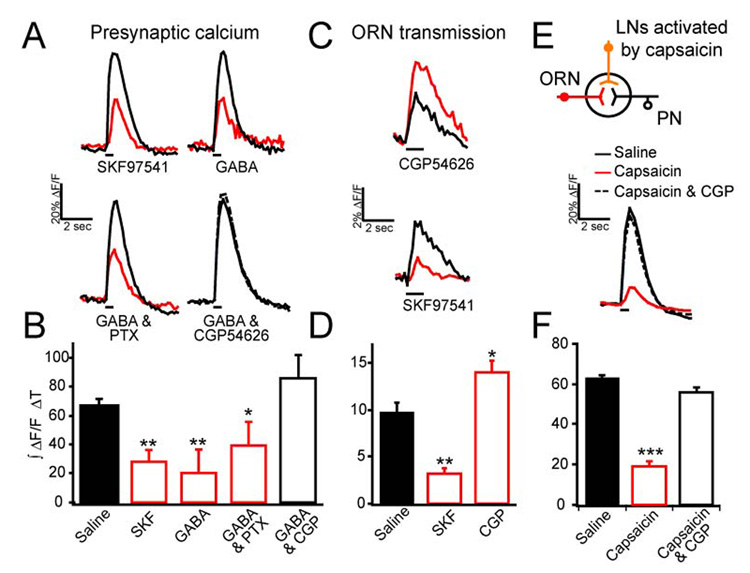
Two-photon imaging of ORNs expressing GCaMP or spH in Or83b neurons was used to monitor response to electrical stimulation of the olfactory nerve. Pharmacological effects on fluorescence change over time: black and red traces show representative responses before and after drug application respectively. (A) Representative traces of GCaMP fluorescence change over time. (B) Bar graph summary of pharmacological effect shows the integrated fluorescence change over time. (C) Representative traces of spH fluorescence change over time; traces are the average of three trials. (D) Bar graph summary of (C). (E) Two-photon calcium imaging of ORN terminals in flies expressing VR1 in LNs. Electrical stimulation of the olfactory nerve elicits a response that is suppressed with 100 µM capsaicin, but is rescued with the addition of 25 µM CGP54626. (F) Bar graph summary of pharmacological effect shows the integrated fluorescence change over time. For all, electrical stimulation was 1 ms in duration and 10 V in amplitude, and 45 pulses (A–B, E–F) or 80 pulses (C–D) at 100 Hz. n, 3–18. Error bars show SEM. * p ≤ 0.05, ** p ≤ 0.01. *** p ≤ 0.001.
We next asked whether a physiologic concentration of GABA released by interneurons causes presynaptic inhibition in ORNs. The GH298-Gal4 line has been shown to label many GABAergic LNs in the antennal lobe (Stocker et al., 1997; Wilson & Laurent, 2005). We therefore examined whether activating these LNs induces presynaptic suppression in ORNs. In these experiments, we used a dual genetic expression system (Lai & Lee, 2006) to express GCaMP in ORNs for calcium imaging and to express the capsaicin sensitive VR1 channel in LNs for exogenous activation of these LNs. Expression of the VR1 channel endows Drosophila neurons with responsivity to capsaicin (Marella et al., 2006). Flies bearing Or83b-LexA, LexAop-GCaMP, GH298-Gal4, UAS-VR1 allow calcium imaging in ORN terminals and activation of many GABAergic LNs by capsaicin. In these flies, ORNs exhibit a significantly reduced calcium response in the presence of 100 µM capsaicin; this reduction is blocked by CGP54626 (Figure 3E–F). Control flies that do not express the VR1 channel (bearing Or83b-LexA; LexAop-GCaMP; UAS-VR1 transgenes) do not exhibit any presynaptic suppression by 100 µM capsaicin (Supplementary Figure 2). Thus activation of the GABAergic LNs is capable of inducing GABABR mediated presynaptic inhibition.
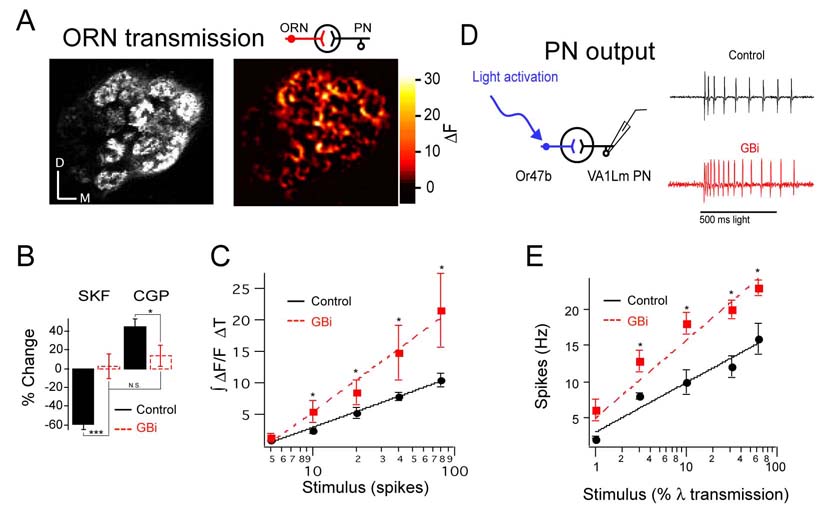
In a simple model, GABABR-mediated gain control in the antennal lobe revealed by pharmacological experiments is entirely attributed to the activation of GABABRs in ORNs. We addressed this issue first by measuring vesicle release in ORNs that lack GABABR2 expression. We performed two-photon imaging of spH in flies bearing Or83b-Gal4, UAS-spH and UAS-GABABR2-RNAi transgenes. Electrical stimulation of the olfactory nerve elicits an increase in spH fluorescence that is not affected by the addition of the agonist SKF97541 or the antagonist CGP54626 (Figure 4B). We plotted the input-output function in control flies and those expressing the RNAi and found that the responses to high intensity stimuli are significantly greater in RNAi expressing flies than that of control flies (Figure 4C). The data are fit with a logarithmic curve and exhibit a 110% increase in the slope, thus indicating an increase in the gain of ORN transmission in flies expressing GABABR2-RNAi, suggesting that GABABR provides a scalable inhibition to ORN presynaptic terminals.
Figure 4. Knockdown of GABAB receptors increases the gain of receptor neuron transmission and projection neuron firing.
(A) Two-photon imaging of ORNs expressing spH in Or83b neurons was used to monitor synaptic transmission in response to electrical stimulation of the olfactory nerve. Gray scale image show antennal lobe structure at one optical plane. Psuedocolored images reveal the response to electrical stimulation. (B) The percent decrease or increase in integrated ΔF/F over time upon addition of SKF97541 or CGP54626, in control flies and those expressing two copies GABABR2-RNAi (GBi) in Or83b neurons. (C) The input-output function of ORN transmission in control and flies expressing GBi in Or83b neurons. Mean integrated fluorescence change over time across preparations is plotted as a function of the number of stimuli. ΔF/F was measured from all glomeruli in this optical section. (D) Loose patch recordings from PNs with dendrites in VA1lm in response to light activation of the Or47b neurons that express two copies of channelrhodopsin-2 in control flies and flies that also express GBi in Or47b neurons. (E) Input-output function of PNs plotted as a function of light intensity. n, 4–10 (B–C) and n, 8 (E). Error bars show SEM. * p ≤ 0.05, *** p ≤ 0.001.
To ask whether knock-down of GABABR in ORNs has a concomitant impact on PN output, we next performed loose patch electrical recordings to monitor PN action potential firing in response to ORN stimulation in the presence or absence of GABABR2-RNAi. To do this, we investigated the ORN to PN transformation in VA1lm glomerulus which detects female odors (van der Goes et al., 2007). Since the ligand for these ORNs has not been identified, we expressed the light sensitive cation channel, channelrhodopsin 2 (ChR2) (Boyden et al., 2005) in Or47b neurons and activated them with light illumination. We plotted the input-output function of VA1lm PNs in control flies bearing the Or47b-Gal4 and UAS-ChR2 transgenes and in flies also expressing GABABR2-RNAi. Indeed expression of RNAi in Or47b neurons increases the gain of the post-synaptic PN firing frequency (Figure 4E). Addition of the GABABR antagonist did not further increase PN firing in RNAi expressing flies, suggesting that GABABR expression in other cell types (see Figure 2A) does not contribute to this gain change (n, 2; data not shown). Thus, GABABR expression in ORNs decreases the gain of PN output.
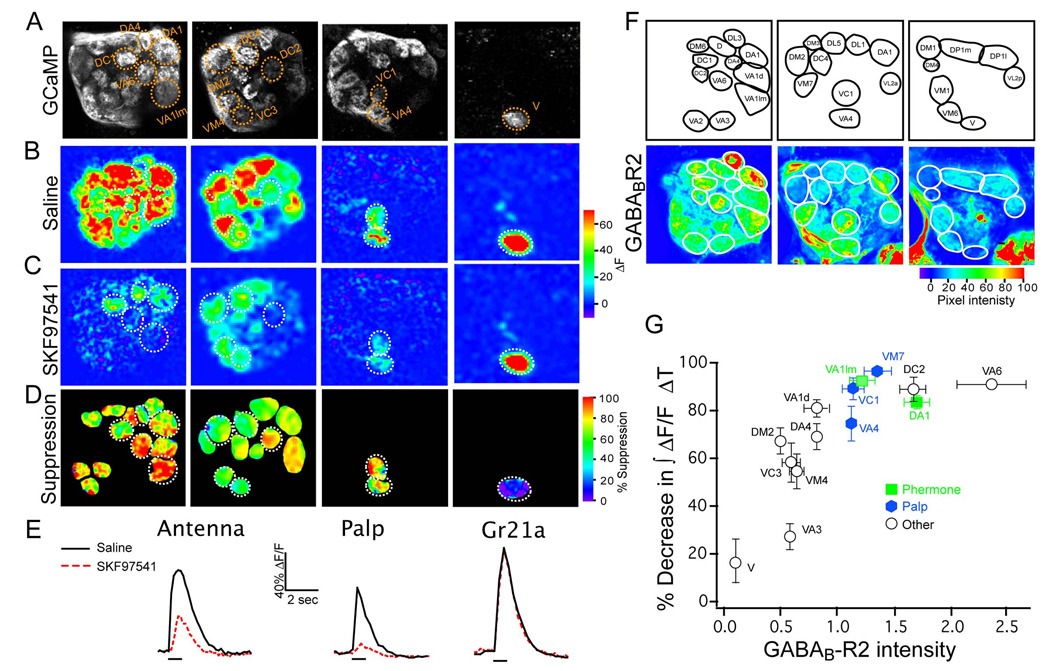
The above experiments indicate that GABABR expression in ORNs is necessary for presynaptic inhibition and for suppression of PN output gain. We reasoned that heterogeneity in the PN olfactory gain (See Figure 1) reflects heterogeneity in the level of presynaptic inhibition. To investigate this, we first asked whether specific ORNs exhibit different amounts of presynaptic inhibition. Presynaptic inhibition was quantified by the percent reduction in calcium activity in the presence of the GABABR agonist. Stimulation of the labial nerve in flies bearing the Or83b-LexA and LexAop-GCaMP transgenes exhibit calcium transients in the palpal axonal terminals that is dramatically reduced by 20 µM SKF97541 (Figure 5A–D, column 3). Similarly, stimulation of the antennal nerve causes a calcium transient that is dramatically reduced in glomeruli receiving axons from pheromone detecting ORNs, while other glomeruli exhibit significantly less reduction in presynaptic calcium (Figure 5A–D, column 1). At the middle layer of the antennal lobe, presynaptic calcium suppression in these ORNs is less than that of the palpal ORNs on the same level (Figure 5A–D, columns 2&3). In the extreme case, the Gr21a CO2 sensing ORNs exhibit no significant suppression of presynaptic calcium by the agonist (Figure 5A–D, column 4). Thus, there is heterogeneity between glomeruli in the magnitude of GABABR-mediated presynaptic suppression; the Gr21a CO2 sensing neurons do not exhibit any significant presynaptic inhibition, while the palpal and pheromone ORNs exhibit significantly stronger levels of inhibition.
Figure 5. Heterogeneity of presynaptic inhibition in olfactory receptor neurons.
(A) Two- photon images of GCaMP expression in Or83b neurons (columns 1–3) and Gr21a neurons (column 4). (B) Electrical stimulation of the olfactory nerve generates calcium influx in axon terminals of antennal ORNs (columns 1,2,4). Stimulating the labial nerve causes calcium influx into axon terminals of the palpal ORNs (column 3). (C) Addition of SKF97541 decreases the calcium response of Or83b neurons to different degrees (columns 1–3), but does not affect Gr21a calcium influx (column 4). (D) The percent suppression is represented by color intensity for particular glomeruli. Images were generated by subtracting images in C from those in B and then dividing the resulting images by those in B. Images were smoothed with a Gaussian filter and a black mask was overlaid on the non-glomerular background. (E) GCaMP fluorescence change is plotted over time for Gr21a and Or83b antennal and palpal neurons; traces for Or83b neurons represent responses from multiple glomeruli in one optical plane. For response to antennal nerve stimulation, ΔF/F was measured from all mid layer glomeruli. (F) GFP intensity from GABABR2-Gal4 reporter line at three different optical planes through the antennal lobe. Images are psuedocolored to emphasize the differences between glomeruli. (G) The suppression of calcium influx by the GABABR agonist is plotted against the reporter intensity for individual glomeruli. n, 3–5. Error bars show SEM, but are not plotted when the SEM is smaller than the symbol. * p ≤ 0.05, ** p ≤ 0.01.
We next asked whether the heterogeneity in presynaptic inhibition matches the heterogeneity observed in the putative GABABR2 expression level. We first addressed this question by correlating the level of presynaptic inhibition with the reporter expression level in flies bearing the GABABR2-Gal4 and UAS-CD8GFP transgenes. GFP fluorescence intensity shows that different glomeruli exhibit different levels of expression (Figure 5F, see Supplementary Table 1 for tests of significance). In the extreme case, the Gr21a ORNs projecting to the V glomerulus have little or no fluorescence intensity (Figure 5F, Supplementary Figure 1). ORNs innervating the VC3 and DM2 glomeruli have relatively weak intensity. In contrast, the VC1, VA4 and VM7 glomeruli that have ORN axons from the maxillary palp via the labial nerve (Couto et al., 2005; Fishilevich et al., 2005), exhibit very high intensity. Additionally, the pheromone sensing glomeruli VA1lm and DA1 (Ha & Smith, 2006; Kurtovic et al., 2007; van der Goes & Carlson, 2007) also exhibit high intensity. Thus, there is a tight correlation between the GABABR2 promoter driven reporter level and the extent of presynaptic inhibition (Figure 5G, see Supplementary table 1 for tests of significance).
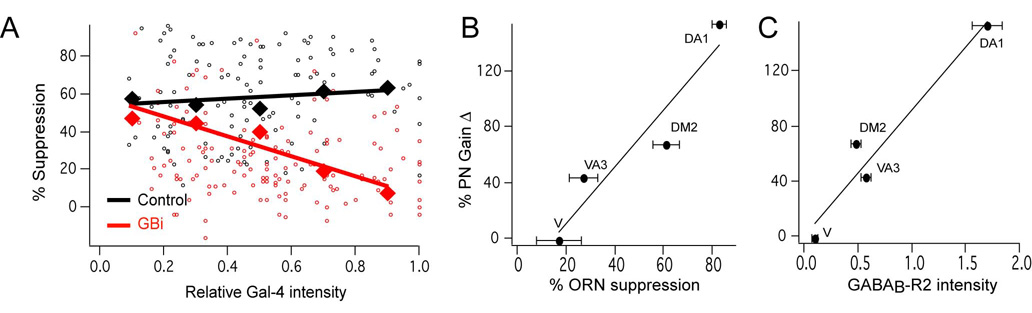
The above experiments suggest that the GABABR level dictates the magnitude of presynaptic inhibition, if so we expect that varying reduction in receptor expression should lead to matching changes in presynaptic inhibition. Using a promoter such as Or83b to express varying levels of GABABR2-RNAi in different ORN types should create a condition to assess the link between receptor and presynaptic inhibition. When two copies of the RNAi transgenes are expressed in ORNs using the Or83b-Gal4 driver, presynaptic inhibition is completely abolished (Figure 4B). However, the expression of one copy of the RNAi transgene in the same condition only partially reduces presynaptic inhibition (Figure 6A). The level of presynaptic inhibition was measured by calcium imaging as percent suppression by SKF97541, in which electrical stimulation of the olfactory nerve was used to elicit calcium transients in ORN terminals in flies bearing Or83b-Gal4 and UAS-GCaMP. The promoter strength of Or83b was measured by the baseline fluorescence. We plotted the percent suppression for individual glomeruli against the Or83b-Gal4 expression level. In control flies without expression of the RNAi, we find that there is no correlation between Gal4 level and presynaptic inhibition; however in flies bearing one copy of the UAS-GABABR2-RNAi transgene, we find an inverse correlation between the Gal4 level and presynaptic inhibition (Figure 6A). Thus differential reduction in GABABR2 expression level with RNAi suppresses presynaptic inhibition accordingly, suggesting that the level of GABABR expression determines ORN sensitivity to presynaptic inhibition.
Figure 6. The level of GABAB receptors in ORNs sets the level of gain modulation for PN response of select glomeruli.
(A) GABABR2-RNAi (GBi) was differentially expressed in different ORN types by the Or83b-Gal4 promoter. Flies expressed GCaMP and one copy of GBi in Or83b neurons. Two-photon imaging of ORN presynaptic calcium was used to measure the calcium response to olfactory nerve stimulation in the presence and absence of SKF97541. The percent suppression in integrated ΔF/F over time is plotted as a function of the Gal4-intensity measured by the baseline GCaMP fluorescence intensity. The Gal4-intensity for each glomerulus is normalized to the brightest glomerulus within each preparation. Each glomerulus is plotted as a small circle and the average within bins (0.2 intensity units in size) are plotted as large diamonds. n, 118 from 5 preparations (control) and 145 glomeruli from 7 preparations (GBi). (B) The percent change in PN gain (from Figure 1) is plotted as a function of the level ORN presynaptic inhibition of the same glomeruli. (C) The percent PN gain change as a function of the GABABR2-Gal4 reporter intensity. n, 4–8 (B–C).
We further examined whether the heterogeneity in presynaptic inhibition is preserved in the antennal lobe output PNs. We compared the percent change in slope, or gain modulation, in PNs (see Figure 1) with the degree of presynaptic inhibition (Figure 6B) and also with the GABABR2-Gal4 intensity (Figure 6C) for four different glomeruli. In the extreme, the V glomerulus ORNs have little or no presynaptic inhibition or GABABR-Gal4 labeling, and blocking GABABR does not alter the PN gain. Conversely, the ORNs projecting to DA1 have a high level of presynaptic inhibition and GABABR-Gal4 intensity, and blocking GABABR causes an increase of 153% in the gain of PN response. Thus, there is a tight correlation between the level of presynaptic inhibition in ORNs and the degree of gain modulation in PNs, suggesting that glomerulus-specific gain modulation may convey important information for olfactory behavior.
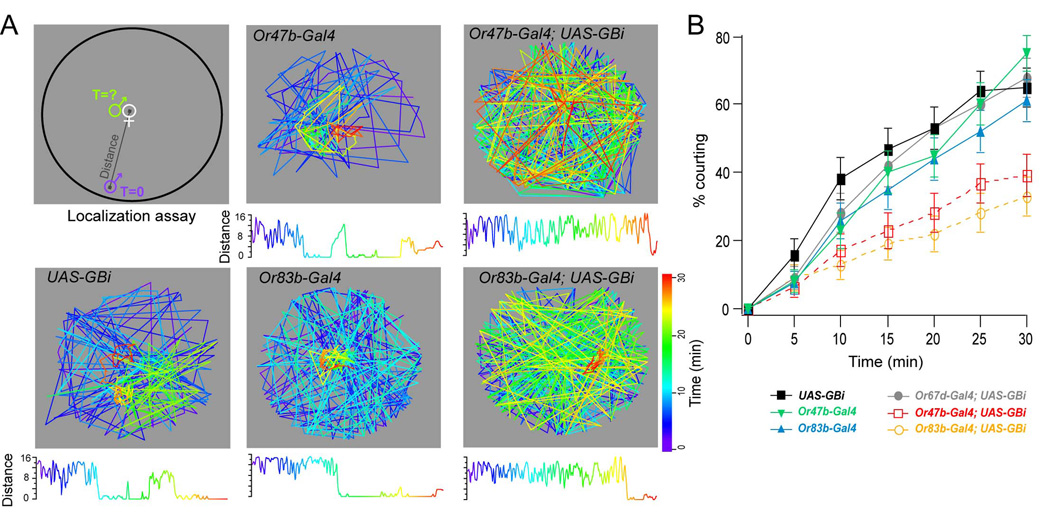
Pheromone sensing ORNs in the DA1 and VA1lm glomeruli exhibit high levels of presynaptic inhibition. We thus hypothesized that gain control in pheromone detection is important for mating behavior. We have adopted an established mating assay in which we measure the latency of courtship exhibited by a single male fly toward a virgin female in the environment with no light. The virgin female was decapitated to prevent movement and any communication from female to male. We used a chamber (40 mm in diameter, 5 mm in height) with mesh screen bottom to create a pheromone gradient for this assay (Figure 7A). We monitored the male with time lapse imaging at a rate of 0.2 Hz for thirty minutes. Upon finding the female, the male exhibits robust courting behavior hallmarked by wing vibration and attempted copulations. In computer analysis, male flies are also found to spend long durations of time in close proximity to the female fly (Figure 7A). Within 30 minutes, approximately 60% of control flies have located the female fly (Figure 7B). In contrast, only about 30% of the male flies expressing GABABR2-RNAi in Or83b ORNs. We next asked whether gain modulation in VA1lm, a glomerulus that responds specifically to female pheromones (van der Goes van & Carlson, 2007), affects male localization of the female fly. We measured courtship latency in male flies expressing GABABR2-RNAi in only Or47b ORNs. Remarkably, these flies exhibit the same courtship latency as those flies expressing GABABR2-RNAi in many ORNs (Figure 7B). We performed further analysis to ask whether impaired localization is due to a reduction in locomotor activity. During the first five minutes in this assay when most of the male flies have not found the female, there is no significant difference in travel distance among experimental groups (Supplementary Figure 3). These results suggest that reduction of olfactory gain in pheromone sensing ORNs facilitates the process of mate localization.
Figure 7. Knockdown of GABAB receptors impairs odor object localization.
(A) A modified courtship assay was used to measure latency in odor object localization. Measurements record the location of the male fly as well as the distance between the male and immobilized female at each time point. The coordinates and distances (mm) as a function of time (color scale) are depicted for representative control flies (columns 1,2) and those expressing GABABRNAi (GBi) in ORNs (column 3). Distance trances were smoothed with a Gaussian filter. (B) Percent of males that initiate courtship behavior as a function of time. n, 53–71. Error bars show SEM. At the 30-minute time point all controls are significantly different for those flies expressing GBi in Or47b and Or83b ORNs. p ≤ 0.01, Z-test.
DISCUSSION
Capturing and amplifying behaviorally relevant stimuli in early sensory processing are critical steps for signal recognition and discrimination. Appropriate implementation of gain control in the first synapse plays an important role in sensory processing (Laughlin, 1994). In this study, we investigated gain control in the first synapse of the Drosophila olfactory system. We used two-photon imaging to monitor activity in selective neural populations in the antennal lobe. Specific blockade of GABABRs reveals a scalable presynaptic inhibition to suppress olfactory response at high odor concentrations. Pharmacological and molecular experiments suggest that GABABRs are expressed in primary olfactory receptor neurons. Furthermore, the level of presynaptic inhibition is different in individual glomerular modules, which is tightly linked to the level of GABABR expression. We have begun to investigate the importance of presynaptic GABABRs in olfactory localization, and found that reduction of GABABR expression in the presynaptic terminal of ORNs impairs the ability of male flies in locating potential mates.
Our study revealed that expression of GABABR2 in ORNs is necessary for presynaptic inhibition. Unexpectedly, we discovered heterogeneity in the levels of presynaptic inhibition among different glomeruli. Varying GABABR2 expression level in ORNs with molecular manipulations is sufficient to produce predictable alterations in presynaptic inhibition in specific glomeruli. Together these experiments argue that presynaptic GABABR expression level is a determinant of glomerulus-specific olfactory gains in the antennal lobe. A recent report revealed that there is a non-linear transformation between ORNs and PNs that is heterogeneous between glomeruli (Bhandawat et al., 2007). In other words, PNs innervating a given glomerulus have a unique response range for its ORN input. Given that ORNs are the main drivers of PN response (Olsen et al., 2007; Root et al., 2007), it is plausible that the heterogeneity in presynaptic inhibition contributes to the heterogeneity in ORN to PN transformations observed by Bhandawat and colleagues. Additionally, heterogeneity in GABA release by LNs (Ng et al., 2002) could also contribute to heterogeneity in presynaptic inhibition. It is interesting to note that when presynaptic inhibition is abolished, heterogeneity remains in the input-output curves of PN response to the four different odors in our experiments (Figure 1), suggesting that other mechanisms such as probability of vesicle release contribute to the heterogeneity as well.
Theoretical analysis of antennal lobe coding has recently suggested that the non-linear synaptic amplification in PNs observed by Bhandawat and colleagues provides an efficient coding mechanism for the olfactory system (Abbott & Luo, 2007). According to this model, the optimal distribution of firing rates across a range of odorants should be flat without clusters. Firing rates of a given ORN responses cluster in an uneven distribution. Conversely, PNs exhibit a more equalized firing rate distribution than ORNs (Bhandawat et al., 2007). According to the optimum coding theory, the high amplification of ORN to PN transformation generates a more even distribution of PN firing rates that should facilitate odor discrimination. However, this model of olfactory coding poses a potential problem. The high gain in this synaptic amplification reduces the dynamic range of PNs, causing a loss of information about concentration variation that could be important for an animal to localize odor objects. Presynaptic inhibition may provide a mechanism to expand the dynamic range of the olfactory system. For some glomerular modules that mediate innate behaviors such as avoidance of the stress odorant CO2 (Suh et al., 2004), there is a potential trade off for odor sensitivity and dynamic range. The lack of GABABR in the CO2 sensing ORNs could be important to maintain high sensitivity.
Pheromones play an important role in Drosophila mating behaviors (Hall, 1994; Greenspan & Ferveur, 2000) and our results indicate that pheromone sensing ORNs have high levels of GABABR, which is correlated with a high level of presynaptic inhibition in these ORNs. We have found that mate localization is impaired in the absence of presynaptic inhibition in one pheromone sensing glomerulus. It is interesting to note that in addition to the pheromone sensing ORNs, the palpal ORNs also exhibit high GABABR expression level. Although the behavioral role of the palpal ORNs has not been determined, it is possible that they are also important for odor object localization. There are two potential mechanisms for the role of GABABR in olfactory localization. GABABR-mediated activity-dependent suppression of presynaptic transmission on a short time scale provides a mechanism for dynamic range expansion. On a longer time scale, activity-dependent suppression provides a mechanism for adaptation, hence a high pass filter to allow the detection of phasic information (McGann et al., 2005). Further experiments will be necessary to determine which property is important for olfactory localization.
Intraglomerular and interglomerular presynaptic inhibition mediated by GABABRs have been described in the mammalian olfactory system. Intraglomerular presynaptic inhibition was suggested as a mechanism to control input sensitivity while maintaining the spatial maps of glomerular activity (McGann et al., 2005). Interglomerular presynaptic inhibition was proposed as a mechanism to increase the contrast of sensory input (Vucinić et al., 2005). A recent report revealed a similar gain control mechanism by interglomerular presynaptic inhibition in the Drosophila olfactory system (Olsen & Wilson, 2008) where GABABR expression in ORNs was shown to scale the gain of PN responses. Interestingly, most if not all of the presynaptic inhibition was suggested to be lateral. In contrast, our study does not seek to distinguish between intra- and interglomerular presynaptic inhibition, however we do find evidence that the VA1lm glomerulus receives significant intraglomerular presynaptic inhibition (Figure 4D–E). Thus, despite significant differences between the insect and mammalian olfactory systems (Hildebrand & Shepherd, 1997), the inhibitory circuit in the first olfactory processing center appears remarkably similar.
Based on whole cell recordings of PNs in response to ORN stimulation, Olsen and Wilson (2008) suggest that both GABAAR and GABABR are expressed in ORNs to mediate presynaptic inhibition and that GABAAR signaling is a large component of lateral presynaptic inhibition. In contrast, our study, which employed direct optical measurements of presynaptic calcium and synaptic vesicle release, suggests that GABABRs but not GABAARs are involved in presynaptic inhibition. To resolve these discrepancies further molecular experiments will be important to determine conclusively whether ORNs express GABAAR and whether the receptor contributes to gain control. Furthermore, the antennal lobe is a heterosynaptic system comprised of at least three populations of neurons that include ORNs, LNs and PNs. Therefore, how these different populations of neurons respond to GABA signaling and what contribution they make to olfactory processing in the antennal lobe is a critical question for future investigation.
We have demonstrated differential presynaptic gain control in individual olfactory input channels and its contribution to the fine–tuning of physiological and behavioral responses. Synaptic modulation by the intensity of receptor signaling is reminiscent of the mammalian nervous system where expression levels of AMPA glutamate receptors play an important role in regulating synaptic efficacy (Shi et al., 1999). Furthermore, presynaptic regulation of GABABR signaling provides a mechanism to modulate the neural activity of individual input channels without much interference with overall detection sensitivity because this mechanism of presynaptic inhibition would only alter responses to high intensity stimuli. In parallel, it is tempting to speculate that global modulation of interneuron excitability should alter the amount of GABA release across channels, thus providing a multi-channel dial of olfactory gain control that may reflect the internal state of the animal.
EXPERIMENTAL PROCEDURES
Experimental Preparations
The following transgenic lines were used: 1) UAS-GCaMP56 (Wang et al, 2003), 2) GH146-Gal4 (Stocker et al., 1997), 3) Or83b-LexAVP16 (Lai & Lee, 2006), 4) LexAop-GCaMP-IRES-GCaMP, 5) Or83b-Gal4 (Wang et al., 2003), 5) UAS-spH (Ng et al., 2001) with the transgene mobilized onto the third chromosome, 6) Gr21a-Gal4 (Scott et al., 2001), 7) UAS-VR1E600K (Marella et al., 2006), 8) GH298-Gal4 (Stocker et al., 1997).
Flies were raised on standard medium at 22–25°C. Female flies age 3–9 days after eclosion were used for experiments. Isolated brain preparations (Wang et al., 2003) were obtained by micro dissection of decapitated flies to remove head cuticle and connective tissues. Neural activity of the fly brain was reduced by dissecting in chilled calcium free AHL saline. The antenna and brain preparation was pinned in a sylgard dish and the olfactory nerves were carefully severed near the base of the antenna with fine forceps. In preparations for labial nerve stimulations, the nerves were similarly preserved and carefully severed far from the brain. After dissection the preparations were rinsed and kept in AHL saline (108 mM NaCl, 5 mM KCL, 2 mM CaCl2, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4, 5 mM trehalose, 10 mM sucrose, 5 mM HEPES [pH 7.5, 265 mOsm]). In experiments using ChR2 stimulation, the saline also contained 50 µM retinal. Loose patch recordings were performed as previously described (Root et al., 2007).
Picrotoxin and CGP54626 (Tocris) were dissolved as 2000x stocks in DMSO. GABA (Sigma) was prepared fresh daily and dissolved as a 1000x stock in AHL saline. SKF97541 (Tocris) was dissolved as a 500x stock in 100 mM NaCl. Capsaicin (Sigma) was dissolved as a 100x stock in ethanol. The appropriate volume (1–4 µL) was first diluted with 100 µL of AHL saline and then added to the preparation to achieve the final concentration.
Two-Photon Imaging and Olfactory Stimulation
Calcium and synaptopHluorin imaging was performed with a custom-built two-photon microscope as described (Wang et al., 2003). Images were captured at 4 frames per second with a resolution of 128×128 pixels. At the end of the experiment, a high resolution Z-stack of images (512×512 pixels) was collected for glomerular identification. Electrical stimulation of the olfactory or labial nerve was delivered with a glass suction electrode that was made by pulling capillary glass to a fine tip, broken with forceps and then fire polished to achieve a diameter that is about 1.5x the diameter of the nerve. The nerve was sucked as a loop into the electrode. A Grass stimulator was used to stimulate the nerve with pulses at 100 Hz, 1 ms in duration and 10 V in amplitude. In odor experiments, a constant carrier airflow of 1 L/min was applied to the antennae in a pipe of 12 mm in diameter. Odor onset was controlled by solenoid valves that mixed a defined percentage of the carrier air with air re-directed through 100 mL bottles containing 20 µL of odorant on a piece of filter paper. Ethyl hexanoate was dissolved 1:10,000 (V/V) in mineral oil. The concentration of odorant inside the bottle was measured to be approximately 80 ppm for 2-phenylethanol and 300 ppb for ethyl hexanoate, using a PID calibrated to isobutylene standard reference. cis-Vaccenyl acetate was applied by redirecting a percentage of the airflow over a piece of filter paper with 1µL of cis-vaccenyl acetate inside a glass pipette located about 5–10 mm from the antennae. CO2 was applied by addition of a small amount of gas to the carrier airflow to achieve the final percentage concentration.
Analysis of imaging data was performed using Igor Pro (Wavemetrics) and a custom macro. Statistical analysis was done using Student’s T test in Excel and results were considered significant if the p value was less than 0.05. Input-output functions were fit with y=m*log(x) + b.
GABABR2 Expression
For GABABR2 immunohistochemistry on brain cryosections and whole mounts was performed as previously described (Enell et al., 2007). Antennal sections were obtained by mounting live fly heads in OCT, Freezing at −20°C on the stage of a cryostat and 12 µM thick section were cut. Slides were immediately fixed with ice-cold 4% paraformaldehyde in 0.1 M NaHPO4 for 10 min. Staining was performed using standard techniques with chick-α-GFP (Abcam), rabbit-α-GABABR2, and nc82 (DSHB) primary antibodies at 1:1000, 1:10,000 and 1:100 respectively. In quantification of differences between control and GABABR2-RNAi flies (Figure 2B), staining intensity was normalized to nc82 staining. The background intensity was first subtracted from Z-projections of the antennal lobe. Then the GABABR2 intensity was normalized to the nc82 to generate a ratio of GABABR2 to nc82 intensity.
RNA was prepared with the RNeasy kit (Qiagen) and RT-PCR was performed using SuperScript One-Step RT-PCR with Platinum Taq (invitrogen) according to manufacture's instructions. Sequence of Primers for RT-PCR: GABABR2-F GCCTGGGAAACTCGACATGTTTCTA; GABABR2-R TTGCTCCAGTTCGCACACCGAGGA; RP49-F ATGACCATCCGCCCAGCATACA; RP49-R TGTGTATTCCGACCAGGTTAC; Rh4F TGTACTGCACACCGTGGGTTGTCCTG; Rh4R AGCTGAAAAAGAAGATGGTGCCCACAAAC.
1.5 kb of genomic DNA upstream of the GABABR2 gene was obtained using the following primers: R2upXbaI AAATCTAGAATAATGTCAGCCATAAGGAT; R2downBamHI AAAGGATCCGTTGACCGCGTGGGCTGTAAA. The genomic DNA was inserted into pTGal4 vector via the xbaI and BamHI restriction sites. The GABABR2-RNAi fly was made by cloning a150 bp inverted repeat of the 3’ end of GABABR2 coding region with a 50 bp linker into the pUAST vector between the EcoRI and Xba1 restriction sites. PCR using the following primers was used to obtain the PCR fragments: GBR2downXbaI AAATCTAGAGGGACTCTTCTCGGTGAGGA; GBR2upEcoRI AAAGAATTCGTAAGGTCAGCCGGAGCTCT; GBR2upXbaI AAATCTAGAGTAAGGTCAGCCGGAGCTCT; GBR2insideDownXbaI AAATCTAGACGCCCTCGAGCAGTTCCGTC. Transgenic flies were generated by standard method.
Behavioral assay
Flies were collected within a few hours of eclosion. Males and females were housed separately in groups of 10–30 flies per vial and aged 3–5 days before experiments. The mating chamber was a small plastic dish (40 mm diameter, 5 mm height) placed upside down on a stainless steel mesh screen (0.178mm openings, Small Parts, Inc.) The mesh screen was suspended approximately two centimeter above water in a clear container. Flies were gently aspirated into the chamber through a small hole that was covered with clear plastic. Experiments were done in a light proof box with illumination from a 660 nm LED panel. Images were captured using a Logitech Quickcam and an acquisition script written in Labview (National Instruments). Significance was tested using a hypothesis test for proportions, the z-test. Movies were analyzed by eye in ImageJ and by use of the object tracker pluggin to track the position of the male over time.
Supplementary Material
Supplementary Figure 1 The V glomerulus is devoid of GABABR2 immunoreactivity. Images show whole mount staining with anti-serum for GABABR2 (left), the neuropil marker nc82 (middle), and merge (right). The V glomerulus is circled.
Supplementary Figure 2 Capsaicin does not affect ORN presynaptic calcium in control flies. Two-photon calcium imaging of ORN terminals in flies bearing Or83b-LexA and LexAOp-GCaMP. (A) Flies expressing VR1 in GABAergic LNs via GH298-Gal4 and UAS-VR1. (B) control flies lacking the GH298-Gal4. Electrical stimulation of the olfactory nerve elicits a response that is suppressed with capsaicin in flies expressing VR1 in GH298 neurons (A) but not in control flies (B). Electrical stimulation was 1 ms in duration and 10 V in amplitude, and 45 pulses at 100 Hz. n, 4. Error bars show SEM. *** p ≤ 0.001.
Supplementary Figure 3 Locomotor activity of flies during the object localization assay. During the object localization assay, the position of the flies was tracked and the distance traveled per minute was calculated. The graph shows distance traveled per minute in the first five minutes of the experiment when less than 15% of flies have located the female. There are no significant differences in locomotor activity between controls and flies expressing GABABR2-RNAi in ORNs.
Supplementary Table 1 P-values for pair-wise comparisons of glomerular presynaptic inhibition and GABABR2 intensity. Pair-wise comparisons between glomeruli were performed using Student’s T Test. P-values for percent physiological suppression (Figure 4F) are in the shaded area of the table, and that for the GABABR2 intensity are in the un-shaded region. Values less than 0.05 are colored red to mark significant differences.
Z-stack through the antennal lobe of flies expressing GFP with the GABABR2-Gal4 driver. Dorsal is up and Medial is right. Images were acquired from a live preparation using two-photon microscopy.
Z-stack through the antennal lobe showing whole mount staining with anti-serum for GABABR2 (Green) and the neuropil marker nc82 (red). Dorsal is up. Images were acquired using confocal microscopy.
Z-stack through the antennal lobe of flies expressing GFP with the GABABR2-Gal4 driver. Dorsal is up and Medial is right. Images were acquired from a live preparation using two-photon microscopy.
Acknowledgments
We would like to thank Richard Axel, Aurel Lazar, Massimo Scanziani, William Kristan and Terry Sejnowski for comments on the manuscript. We kindly thank Phuong Chung for the LexAop-GCaMP flies, Allan Wong for flies with UAS-synaptopHluorin on the third chromosome, and Cynthia Hughes for the UAS-channelrhodopsin2 flies. J.W.W. thanks Massimo Scanziani for helpful discussions. This work was partially supported by a research grant from the Whitehall Foundation to J.W.W., and a grant from the National Institute of Deafness and other Communication Disorders to J.W.W. (R01DC009597). J.W.W. is a Beckman investigator, a Hellman Faculty scholar, and a Searle scholar. K.M. is supported by a fellowship from the Japan Society for the Promotion of Science.
Competing Interest Statement: The authors declare they have no competing interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott LF, Luo SX. A step toward optimal coding in olfaction. Nature Neurosci. 2007;10:1342–1343. doi: 10.1038/nn1107-1342. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Distler PG, Boeckh J. Synaptic connections between identified neuron types in the antennal lobe glomeruli of the cockroach, Periplaneta americana: II. Local multiglomerular interneurons. J Comp Neurol. 1997;383:529–540. doi: 10.1002/(sici)1096-9861(19970714)383:4<529::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Enell L, Hamasaka Y, Kolodziejczyk A, Nassel DR. gamma-Aminobutyric acid (GABA) signaling components in Drosophila: immunocytochemical localization of GABA(B) receptors in relation to the GABA(A) receptor subunit RDL and a vesicular GABA transporter. J Comp Neurol. 2007;505:18–31. doi: 10.1002/cne.21472. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- Goldberg F, Grunewald B, Rosenboom H, Menzel R. Nicotinic acetylcholine currents of cultured Kenyon cells from the mushroom bodies of the honey bee Aapis mellifera. J Physiol. 1999;514(Pt 3):759–768. doi: 10.1111/j.1469-7793.1999.759ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ, Ferveur JF. Courtship behavior in Drosophila. Annual Review of Genetics. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Wegener C, Nassel DR. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol. 2005;65:225–240. doi: 10.1002/neu.20184. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Laughlin SB. Matching coding, circuits, cells, and molecules to signals: general principles of retinal design in the fly’s eye. Progress in Retinal Eye Research. 1994;13:165–195. [Google Scholar]
- Marella S, Fishchler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Mezler M, Muller T, Raming K. Cloning and functional expression of GABA(B) receptors from Drosophila. Eur J Neurosci. 2001;13:477–486. doi: 10.1046/j.1460-9568.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Brotz TM, Borst A. Mechanisms of dendritic calcium signaling in fly neurons. J Neurophysiol. 2001;85:439–447. doi: 10.1152/jn.2001.85.1.439. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci U S A. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Stensmyr MC, Giodano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–724. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila-melangaster—A review. Cell and Tissue Research. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Vucinić D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol. 2006;3:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner G, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 The V glomerulus is devoid of GABABR2 immunoreactivity. Images show whole mount staining with anti-serum for GABABR2 (left), the neuropil marker nc82 (middle), and merge (right). The V glomerulus is circled.
Supplementary Figure 2 Capsaicin does not affect ORN presynaptic calcium in control flies. Two-photon calcium imaging of ORN terminals in flies bearing Or83b-LexA and LexAOp-GCaMP. (A) Flies expressing VR1 in GABAergic LNs via GH298-Gal4 and UAS-VR1. (B) control flies lacking the GH298-Gal4. Electrical stimulation of the olfactory nerve elicits a response that is suppressed with capsaicin in flies expressing VR1 in GH298 neurons (A) but not in control flies (B). Electrical stimulation was 1 ms in duration and 10 V in amplitude, and 45 pulses at 100 Hz. n, 4. Error bars show SEM. *** p ≤ 0.001.
Supplementary Figure 3 Locomotor activity of flies during the object localization assay. During the object localization assay, the position of the flies was tracked and the distance traveled per minute was calculated. The graph shows distance traveled per minute in the first five minutes of the experiment when less than 15% of flies have located the female. There are no significant differences in locomotor activity between controls and flies expressing GABABR2-RNAi in ORNs.
Supplementary Table 1 P-values for pair-wise comparisons of glomerular presynaptic inhibition and GABABR2 intensity. Pair-wise comparisons between glomeruli were performed using Student’s T Test. P-values for percent physiological suppression (Figure 4F) are in the shaded area of the table, and that for the GABABR2 intensity are in the un-shaded region. Values less than 0.05 are colored red to mark significant differences.
Z-stack through the antennal lobe of flies expressing GFP with the GABABR2-Gal4 driver. Dorsal is up and Medial is right. Images were acquired from a live preparation using two-photon microscopy.
Z-stack through the antennal lobe showing whole mount staining with anti-serum for GABABR2 (Green) and the neuropil marker nc82 (red). Dorsal is up. Images were acquired using confocal microscopy.
Z-stack through the antennal lobe of flies expressing GFP with the GABABR2-Gal4 driver. Dorsal is up and Medial is right. Images were acquired from a live preparation using two-photon microscopy.