Abstract
Objectives
The underlying premise of these investigations was that the lipophilic hormone progesterone, which partitions into and (at relatively high concentrations) impedes the fluid mechanics of the plasmalemma, would perturb integral associations between membrane lipids and exporter pumps that otherwise confer drug resistance. That progesterone can affect susceptibility of ovarian adenocarcinoma cells and xenografts to cisplatin was tested.
Methods
The cisplatin-resistant human cell lines SKOV-3 and OVCAR-3 were treated for 24 hours with cisplatin (0.1 μg/ml) ± progesterone (0.01, 0.1 μg/ml). Cytotoxicity and platinum were measured by MTT assay and inductively coupled plasma mass spectrometry, respectively. Athymic mice were inoculated intraperitoneal (ip) with SKOV-3 cells. Cisplatin (2 mg/kg/week) ± progesterone (25 mg sustained-release pellet) regimens were initiated ip at one week (when micrometastases were present) and continued to six weeks post-xenograft. Tumor burdens, histopathology, and platinum concentrations were assessed upon necropsy at 24 hours after the final injection of cisplatin.
Results
There were no significant in vitro/vivo anticancer effects of cisplatin alone. High-dose progesterone enhanced platinum accretion and induced drug toxicity in both cell lines. Tumorigenesis was suppressed by cisplatin + progesterone. The treatment synergy was related to elevated tumor platinum and morphological evidence of apoptosis.
Conclusion
It appears that the addition of progesterone to ovarian cancer therapeutic modalities represents a step in improving responses.
Keywords: Progesterone, Cisplatin resistance, Ovarian cancer
Introduction
Ovarian cancer of surface epithelial origin is a deadly insidious disease. Symptoms do not typically present until after intraperitoneal (ip) dissemination when tumor implants on the bowel and liver are established. Debulking surgery and platinum-based chemotherapy is the standard course of treatment. Notwithstanding, relapses from residual cells that become drug-resistant usually ensue [1].
Cisplatin has been a first-line drug for ovarian cancer. It enters cells by diffusion and reacts with genomic DNA producing cross-links that result in cell cycle arrest and apoptosis [2]. A sustained intracellular platinum content is the determinant of cytotoxicity and a positive prognosis [3].
Plasma membranes of cells which become refractory to cisplatin overexpress drug efflux pumps [4]. A putative strategy to potentiate drug retention and consequently overcome resistance is to disrupt exporter dynamics by causing membrane rigidification [5]. Progesterone attenuated fluidity of human epithelial ovarian cancer cells [6]. Objectives were to determine if vulnerabilities of cells and xenografts to cisplatin are altered by progesterone.
Materials and methods
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless indicated otherwise. Experiments involving mice were approved by the University of Wyoming Animal Care and Use Committee.
Epithelial ovarian cancer cells
SKOV-3 and OVCAR-3 cells obtained from ascites of patients that had become resistant to cisplatin (American Type Culture Collection/ATCC, Rockville, MD) were propagated in T-75 flasks at 37 C under an atmosphere of 5% CO2 in 15 ml RPMI-1640 medium supplemented with 10% charcoal-stripped/heat-inactivated fetal calf serum, 10 μg/ml insulin, and 1% antibiotic/antimycotic solution (A 9909). Exponentially-growing cells were harvested with 0.25% trypsin/0.03% EDTA.
In vitro effects of cisplatin and(or) progesterone on cellular growth/viability (Experiment 1)
Membrane fluidity was decreased by doses of progesterone ≥0.1 μg/ml [6]. Microplates were seeded with SKOV-3 or OVCAR-3 cells (12,000/well) and incubated for 24 hours with medium only, cisplatin (0.1 μg/ml), progesterone (0.01, 0.1 μg/ml), or cisplatin + progesterone. Each control/treatment was replicated five times. Population responses were assessed using the MTT spectrophotometric method according to the instructions of the manufacturer (ATCC). Yellow MTT salt (3–4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is reduced to purple formazan crystals in the mitochondria of metabolically-active cells. Cells were exposed to MTT reagent for six hours and detergent-solubilized for 16 hours. Samples were read at a wavelength of 570 nm.
In vitro effects of progesterone on cellular platinum accumulation (Experiment 2)
SKOV-3 or OVCAR-3 cells (1 × 106) were incubated for 24 hours with cisplatin or cisplatin + progesterone at the doses indicated for Experiment 1 (n = 6), washed with three changes of PBS, mineralized until complete drying (65% HNO3, 120 C), dissolved (2 ml 2% HNO3with indium standard), and analyzed for platinum by inductively coupled plasma mass spectrometry (ELAN 600; sensitivity = 0.1 ppb) [7].
Chronology of ip SKOV-3 tumor development in athymic mice (Experiment 3)
BALB/c strain nu/nu mice were maintained in a pathogen-free environment under controlled temperature (24 C) and lighting (12L:12D) conditions. Sterilized rodent chow and water were supplied ad libitum.
Cells (5 × 106) suspended in 0.1 ml RPMI medium were injected into the abdominal cavity of 16 mature animals killed by cervical dislocation at 1, 2, 4, or 6 weeks (n = 4). Numbers and diameters of tumors on the surfaces of the liver and mesentery/intestines (three 1 cm segments per mouse) were determined with the aid of a stereo dissecting microscope (Olympus SZX12) and image analysis software (Image J, NIH).
Antitumor effects of progesterone and(or) cisplatin (Experiment 4)
Twenty-four nude mice were inoculated ip with SKOV-3 cells. Equal numbers of animals were assigned to each of four treatment groups (n = 6): vehicle control; cisplatin; progesterone; cisplatin + progesterone. Treatments were started at one week of tumor development. Cisplatin was administered ip in sterile PBS diluent at a rate of 2 mg/kg/week. Progesterone was delivered ip on a continuous basis via a 25 mg 60-day release pellet (Innovative Research of America, Sarasota, FL). Pellets were placed using a stainless steel trochar as per instructions of the manufacturer. Control and cisplatin-only mice were implanted with a blank pellet. Animals were weighed every week. The experiment was terminated 24 hours following vehicle/cisplatin injections at six weeks post-xenograft. Serum (tail vein) and ip fluid samples were collected for progesterone radioimmunoassay [6]. Tumor parameters were assessed as described for Experiment 3.
Tumor-containing specimens were obtained from the mesentery-intestine interface and liver for light microscopic (Olympus BH-2) examination. Tissues were fixed by overnight immersion in Histochoice (Amresco, Solon, OH), washed in PBS, dehydrated in a graded series of ethanol, cleared in xylene, infiltrated with and embedded in paraffin, and serially-sectioned (6 μm thickness). Sections were transferred from a water bath (50 C) onto subbed microscope slides, air-dried, deparaffinized in xylene, rehydrated, stained in hematoxylin and eosin, dehydrated, placed in xylene, and coverslipped with mounting medium. Tumors were categorized (three per organ) as invasive (i.e., had penetrated the parenchyma) or superficial. Total and condensed/pyknotic cells were counted within three fields from each tumor at 1000X magnification.
Kidney, heart, brain, and pooled mesenteric/intestinal tumors from cisplatin-treated mice were excised. Extracts (~ 100 mg tissue) were assayed for platinum [7].
Statistics
Assignments to treatment groups and selections of tissues/areas for morphometric and biochemical analyses were made at random. Subsamples were averaged. Treatment contrasts were made by analyses of variance and protected least significant difference or Student’s t-test. Differences were considered significant at P < 0.05. Data are plotted (figures) as means + standard errors.
Results
Experiments 1 and 2
Metabolic activities in vitro of SKOV-3 and OVCAR-3 cells were not affected by treatments with cisplatin only, low- or high-dose progesterone only, or cisplatin + low-dose progesterone. Cisplatin + high-dose progesterone caused a marked inhibition in both cell lines (Fig. 1). The cytostatic/toxic effect of cisplatin + high-dose progesterone was associated with increased intracellular platinum (Fig. 2).
Fig. 1.
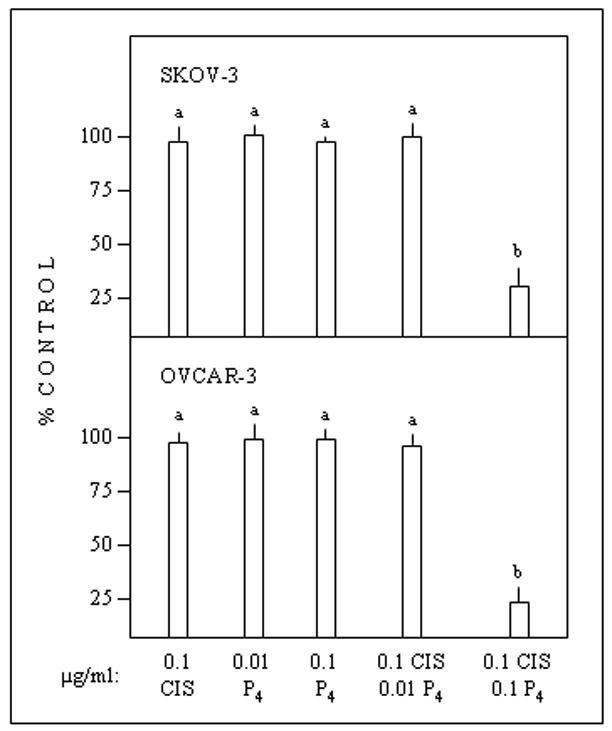
In vitro effects of cisplatin (CIS) and(or) progesterone (P4) on metabolic activity of epithelial ovarian cancer cells (24-hour exposure to treatments). Data are expressed as percentages of control incubates not supplemented with cisplatin and(or) progesterone. Different superscripts (above error bars) denote differences (P < 0.05).
Fig. 2.
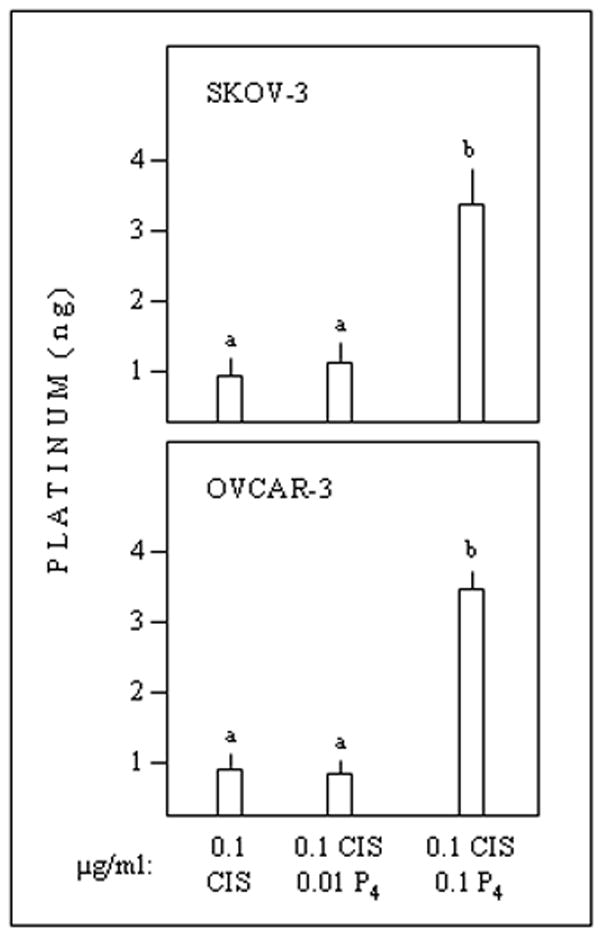
In vitro effects of cisplatin or cisplatin + progesterone on platinum contents of epithelial ovarian cancer cells. Different superscripts denote differences (P < 0.05).
Experiment 3
SKOV-3 tumors were detected in every animal. Discrete colonies of cells were observed on the mesentery and liver with the aid of the dissecting microscope at one and two weeks following inoculation. Macroscopic tumors were evident at four and six (Fig. 3A, B) weeks. Numbers of tumors increased from one to two weeks and then plateaued (Fig. 3C, D). Tumor diameters increased progressively after two weeks (Fig 3E, F). Mesenteric tumors had encroached upon the bowel by six weeks (Fig. 3A).
Fig. 3.
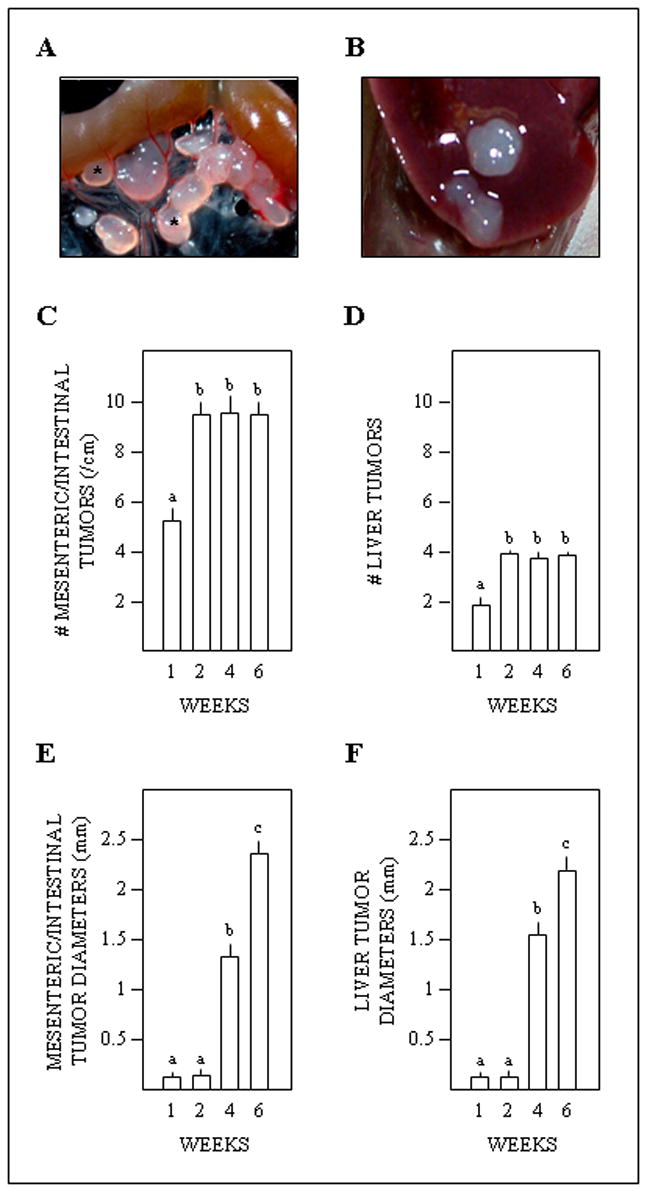
Ovarian (SKOV-3) tumorigenesis in athymic mice. A, representative photograph of tumors (examples are marked by asterisks) along a loop of intestine at six weeks post-inoculation. B, representative photograph of tumors on the liver at six weeks. C, D, E, F, respective effects of weeks post-inoculation on numbers of mesenteric/intestinal tumors, numbers of hepatic tumors, diameters of mesenteric/intestinal tumors, and diameters of hepatic tumors. Different superscripts indicate differences (P < 0.05).
Experiment 4
There were no differences in animal weights due to time or treatments (Fig. 4A).
Fig. 4.

Effects of ip cisplatin (weekly injection) and(or) progesterone (implant) on SKOV-3 tumors in nude mice. The experiment was terminated six weeks post-inoculation at 24 hours after the final vehicle/cisplatin treatment. Different superscripts (B-D, H, J, L) indicate differences (P < 0.05). A, lack of treatment influences on body weights. B, intravenous (iv) and ip progesterone concentrations in hormone-treated mice. C, treatment effects on numbers of ovarian tumors along the mesentery/intestine. D, diameters of mesenteric/intestinal tumors. E, numbers of tumors on the livers of control and cisplatin-treated mice were not significantly different (no tumors existed in progesterone-treated animals). F, diameters of hepatic tumors. G, representative light photomicrographs of superficial (left panel; note no penetration of SKOV-3 cells through the serosa/muscularis) and invasive (right panel; SKOV-3 cells have breached the serosa/muscularis and advanced into the mucosal [epithelial] lining) intestinal tumors. H, percentages of tumors that had permeated the intestines were decreased by progesterone. I, representative sections of mesenteric/intestinal tumors from a control (upper panel; the arrow denotes a condensed/pyknotic cell) and cisplatin + progesterone (lower panel) mouse. J, percentages of mesenteric/intestinal tumor cells that were pyknotic. K, percentages of cells exhibiting pyknosis within liver-resident tumors were not significantly affected by cisplatin. L, platinum concentrations in mesenteric/intestinal tumors were elevated by the combination treatment.
Concentrations of progesterone within ip fluids of mice implanted ip with a progesterone-releasing pellet were approximately 10-fold higher than within the peripheral circulation (Fig. 1B) -mimicking the conditions employed in vitro (0.1, 0.01 μg/ml incubation medium). Intraperitoneal and serum progesterone in control and cisplatin groups were < 0.8 ng/ml.
Numbers and diameters of mesenteric/intestinal (Fig. 4C, D) and hepatic (Fig. 4E, F) tumors were not affected by ip injections with cisplatin alone. Progesterone (continuous delivery) suppressed the ontogeny of mesenteric/intestinal tumors. Livers of animals exposed to progesterone were void of tumors. There was a pronounced interactive inhibitory effect of cisplatin and progesterone on mesenteric/intestinal tumors.
Tumors on the surface of the liver (control and cisplatin groups) had not pervaded the organ capsule by six weeks. Infiltrations of tumor cells through the serosa and muscularis toward the mucosa of the intestine was evident (Fig. 4G). Cisplatin had no bearing on percentages of invasive tumors. Incursions into the intestinal wall were diminished by progesterone (Fig. 4H).
Incidences of pyknosis, a morphologic indicator of cells in apoptotic crisis [8] (Fig. 4I), were elevated within mesenteric/intestinal tumors of mice treated with cisplatin and progesterone. There were no significant differences in apoptotic cells among the control, cisplatin, and progesterone groups (Fig 4J). Likewise, cisplatin (alone) did not elicit a significant cell-killing effect within tumors on the liver (Fig. 4K).
Platinum concentrations of mesenteric/intestinal tumors of animals treat with cisplatin + progesterone were greater than following treatment with cisplatin alone (Fig. 4L). Platinum was undetectable in kidney, heart, and brain.
Discussion
Very little progress has been made in reducing numbers of mortalities due to epithelial ovarian cancer despite aggressive surgery and chemotherapy. Thus, new treatment options are desperately needed [9]. Ancillary roles of hormonal therapies have not been adequately evaluated [10].
This is the first report indicating that progesterone induces cisplatin toxicity in human ovarian adenocarcinoma cell lines (SKOV-3, OVCAR-3) and a preclinical murine xenograft (SKOV-3) model. Previously we found that progesterone suppressed tumorigenesis in athymic mice when administration was initiated prior to an ip inoculation of SKOV-3 cells [11]. Now it is evident that progesterone is of utility when applied after the formation of tumor colonies typical of metastatic-and recurrent-onset conditions. Deposits on the mesentery/intestines are more opposing than their hepatic cohorts which were negated by progesterone alone. Progesterone was ineffective once tumors were well established [11]; this is consistent with the general consensus that progestins are of limited benefit in the treatment of bulky disease [10].
The classical mode of progesterone action involves receptor binding and genomic expression [12]. Progesterone receptors were detected in OVCAR-3 [13], but not SKOV-3 [14] cells. We suspect that the therapeutic gains on cells/tumors invoked by progesterone (both in the absence and presence of cisplatin) were related to a fluidity disorder of the plasmalemma. Indeed, physical state of biological membranes, the equilibrium between molten liquid crystal and solid gel, is a critical determinant of cellular behaviors [15]. The hydrophobic planar ring of progesterone immobilizes the core acyl side-chains of membrane phospholipids hindering rotational mobility [16]. Migration of exocytotic vesicles which liberate proteases that mediate tissue invasion/colonization was thereby inhibited [17]. It seems conceivable that progesterone also antagonized the efficacy of cisplatin excretion. Perhaps cancer cells, which tend to be more fluid than normal cells [18], are prone to functional disturbances that arise from a biophysical effect on the plasma membrane.
We suggest that progesterone be considered in interceptive strategies of ovarian cancer management - upon early diagnosis or cytoreductive surgery. Relatively high doses would likely be required to achieve favorable outcomes. An apparent advantage of ip drug/hormone delivery would be that systemic toxicities (platinum accretion) could be subverted.
Acknowledgments
Supported by NIH CA-97796 and RR-016474.
References
- 1.Hamilton TC. Ovarian cancer, biology. Curr Probl Cancer. 1992;16:5–57. doi: 10.1016/0147-0272(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 2.Fuertesa MA, Castillab J, Alonsoa C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–66. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 5.Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr Drug Targets. 2003;4:23–30. doi: 10.2174/1389450033347172. [DOI] [PubMed] [Google Scholar]
- 6.McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ. Inhibitory effects of progesterone on plasma membrane fluidity and tumorigenic potential of ovarian epithelial cancer cells. Exp Biol Med. 2003;228:308–14. doi: 10.1177/153537020322800310. [DOI] [PubMed] [Google Scholar]
- 7.Ghezzi AR, Aceto M, Cassino C, Gabano E, Osella D. Uptake of antitumor platinum(II)-complexes by cancer cells, assayed by inductively coupled plasma mass spectroscopy (ICP-MS) J Inorg Biochem. 2004;98:73–8. doi: 10.1016/j.jinorgbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Allen RT, Hunter WJ, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–28. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 9.Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer Suppl. 2005;1:3–11. doi: 10.1111/j.1525-1438.2005.15351.x. [DOI] [PubMed] [Google Scholar]
- 10.Rao GG, Miller DS. Hormonal therapy in epithelial ovarian cancer. Expert Rev Anticancer Ther. 2006;6:43–47. doi: 10.1586/14737140.6.1.43. [DOI] [PubMed] [Google Scholar]
- 11.McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ. Effects of progesterone on ovarian tumorigenesis in xenografted mice. Cancer Lett. 2005;221:49–53. doi: 10.1016/j.canlet.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Beato M, Sanchez-Pacheo A. Interaction of steroid hormone receptors with the transcriptional complex. Endo Rev. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- 13.Akahira J, Sukuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H. Differential expression of progesterone isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res. 2002;93:807–15. doi: 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua W, Christianson T, Rougeot H, Rochefort H, Clinton GM. SKOV-3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J Steroid Biochem Molec Biol. 1995;55:279–89. doi: 10.1016/0960-0760(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 15.Marquet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J. 2006;25:3446–57. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000;67:743–57. doi: 10.1016/s0024-3205(00)00669-x. [DOI] [PubMed] [Google Scholar]
- 17.McDonnel AC, Murdoch WJ. High-dose progesterone inhibition of urokinase secretion and invasive activity by SKOV-3 ovarian carcinoma cells: evidence for a receptor-independent nongenomic effect on the plasma membrane. J Steroid Biochem Molec Biol. 2001;78:185–91. doi: 10.1016/s0960-0760(01)00081-4. [DOI] [PubMed] [Google Scholar]
- 18.Sherbet GV. Membrane fluidity and cancer metastasis. Exp Cell Biol. 1989;57:198–205. doi: 10.1159/000163526. [DOI] [PubMed] [Google Scholar]


