Abstract
Receptor-based radiopharmaceuticals are of great current interest in early molecular imaging and radiotherapy of cancers, and provide a unique tool for target-specific delivery of radionuclides to the diseased tissues. In general, a target-specific radiopharmaceutical can be divided into four parts: targeting biomolecule (BM), pharmacokinetic modifying (PKM) linker, bifunctional coupling or chelating agent (BFC), and radionuclide. The targeting biomolecule serves as a “carrier” for specific delivery of the radionuclide. PKM linkers are used to modify radiotracer excretion kinetics. BFC is needed for radiolabeling of biomolecules with a metallic radionuclide. Different radiometals have significant difference in their coordination chemistry, and require BFCs with different donor atoms and chelator frameworks. Since the radiometal chelate can have a significant impact on physical and biological properties of the target-specific radiopharmaceutical, its excretion kinetics can be altered by modifying the coordination environment with various chelators or coligand, if needed. This review will focus on the design of BFCs and their coordination chemistry with technetium, copper, gallium, indium, yttrium and lanthanide radiometals.
Keywords: radiopharmaceuticals, radionuclides, target-specific delivery, diagnosis, radiotherapy
1. Introduction
Radiopharmaceuticals are drugs containing a radionuclide, and are used routinely in nuclear medicine for diagnosis or therapy of diseases [1–7]. Almost all radiopharmaceuticals are administered via intravenous injection. They are mostly small organic or inorganic compounds with definite composition. Radiopharmaceuticals can also be macromolecules such as monoclonal antibodies and antibody fragments that are not stoichiometrically labeled with a radionuclide. Depending on their medical applications, radiopharmaceuticals can be divided into two primary classes: diagnostics and therapeutics. They can also be classified according to their biodistribution characteristics: those whose biodistribution is determined exclusively by their chemical and physical properties; and those whose ultimate distribution is determined by their receptor binding capability or other biological interactions. The latter class is often called target-specific radiopharmaceuticals.
A diagnostic radiopharmaceutical is the molecule labeled with a gamma-emitting isotope for single photon emission computed tomography (SPECT) or a positron-emitting isotope for positron emission tomography (PET) [1–7]. In general, diagnostic radiopharmaceuticals are used in very low concentrations (10−6 – 10−8 M), and are not intended to have any pharmacological effects. The aim of the diagnostic application is the detailed description of morphologic structure of organs or tissues and above all the testing of their physiological function through accumulation of the radiopharmaceutical. Diagnostic radiopharmaceuticals are predominantly metal complexes with an organic chelator for metal-essential agents or a chelator-biomolecule conjugate for target-specific radiopharmaceuticals. In some cases, they can be organic molecules attached with a non-metallic radionuclide, such as 18F and 125I. Diagnostic radiopharmaceuticals provide a non-invasive method of assessing the disease or disease states by SPECT or PET. They are also useful for monitoring the efficacy of a specific therapeutic treatment [1–7].
Therapeutic radiopharmaceuticals are molecules designed to deliver therapeutic doses of ionizing radiation to the diseased sites. The main obstacles for radiotherapy to assume a wider role in clinical practice are the availability of therapeutic isotopes and techniques for their specific localization in diseased tissues, such as tumors [8]. Therapeutic doses of radiation can be delivered in three different ways: external beam irradiation, implantable “seeds” or systemic administration. Brach therapy involves the use of “seeds”, which are physically placed at the tumor site and will remain there unless they are surgically removed. Brach therapy plays a vital role in the care of prostate cancer patients. It is only useful for treatment of accessible tumors. Systemic administration of radiopharmaceuticals that are designed for target-specific delivery of the therapeutic radionuclide at tumor sites provides opportunities for treatment of the disseminated metastatic tumors [9]. Ideally, therapeutic radiopharmaceuticals should localize in tumor site in sufficient concentration to deliver a cytotoxic radiation dose to tumor cells, and at the same time clear rapidly from blood and non-cancerous organs to minimize radiation damage to normal tissues.
A target-specific radiopharmaceutical is based on the receptor binding of a radiolabeled receptor ligand in the diseased tissue [10–14]. In general, a target-specific radiopharmaceutical can be divided into four parts: targeting biomolecule (BM), pharmacokinetic modifying (PKM) linker, bifunctional coupling or chelating agent (BFC), and radionuclide. The targeting biomolecule serves as a “carrier” for specific delivery of radionuclide to the diseased tissue, which is known to contain a substantial concentration of the targeted receptor. The radiolabeled receptor ligand binds to receptors with high affinity and specificity, which results in selective uptake of the radiopharmaceutical. Many biomolecules, including monoclonal antibodies, small peptides, or non-peptide receptor ligands, have been successfully used for target-specific delivery of radionuclides. Table 1 lists selected commercial target-specific radiopharmaceuticals approved by FDA (Food and Drug Administration) for diagnosis or treatment of diseases, such as thrombosis and cancer. Figure 1 illustrates the structures of two small peptide-based target-specific radiopharmaceuticals. The approval of 90Y-labeled anti-CD20 monoclonal antibody (Zevalin®, IDEC Pharmaceuticals Corp.) represents the most significant milestone in the use of radiolabeled MoAbs for radioimmunotherapy (RAIT) of cancers [15–20]. Many excellent reviews have appeared recently covering a broad range of topics related to target-specific diagnostic and therapeutic radiopharmaceuticals [5–15, 21–43].
Table 1.
Selected target-specific diagnostic and therapeutic radiopharmaceuticals.
| Radiopharmaceutical | Trade Name | Primary Uses |
|---|---|---|
| Indium-111 Capromab pendetide | ProstaScint® | imaging of prostate cancer |
| Indium-111 pentetreotide | Octreoscan® | imaging of neuroendocrine tumors |
| Indium-111 satumomab pendetide | OncoScint® | imaging of metastatic disease associated with colorectal and ovarian cancer |
| Tc-99m Apcitide | AcuTect® | synthetic peptide for imaging deep vein thrombosis |
| Tc-99m Arcitumomab | CEA-Scan® | monoclonal antibody for imaging colorectal cancer |
| Tc-99m Depreotide | Neotect® | for imaging somatostatin receptor-positive tumors |
| Y-90 Ibitumomab Tiuxetan | Zevalin® | for treatment of Non-Hodgkin's Lymphoma |
| I-131 Tositumomab | Bexxar® | for Treatment of Non-Hodgkin's Lymphoma |
Figure 1.
Structures of two selected target-specific radiopharmaceuticals. The name in the bracket indicates the commercial kit preparation of the corresponding radiopharmaceutical.
Radionuclide is the radiation source. Selection of radionuclide is largely dependent on medical application of the radiopharmaceutical. For diagnostic radiopharmaceuticals, 99mTc, 111In, 62/64Cu and 67/68Ga, will be the choice of radionuclides for SPECT or PET imaging. While 64Cu and 68Ga are particularly useful for PET, 99mTc is of particular importance for SPECT imaging due to its optimal nuclear properties (6.02 h half-life with 140 keV gamma photons) and its easy availability at low cost. In contrast, β−emitters (e.g. 90Y, 177Lu and 186/188Re) are useful for development of therapeutic radiopharmaceuticals. 90Y is of particular interest since it is a pure β-emitter with a long penetration range (~12 mm), which may yield a more homogeneous dose distribution even when the radiotracer is heterogeneously distributed in the tumor.
An important aspect during the radiopharmaceutical development is to improve target-to-background (T/B) ratios by modifying excretion kinetics of radiolabeled biomolecules with various PKM linkers. Figure 2 shows several types of PKM linkers (cationic, anionic, neutral or metabolically cleavable). The linker can be a simple hydrocarbon chain to increase lipophilicity, a peptide sequence (such as polyaspartic acid) to improve hydrophilicity and renal clearance, or a poly(ethyleneglycol) linker to slow extraction by hepatocytes. It has been reported that linker groups have significant effect on biodistribution of 111In and 99mTc-labeled antibodies [44–46]. Metabolizable linkers have been used for 111In-labeled somatostatin analogs [47]. A tetrapeptide linker Gly-Gly-Gly-L-(p-NO2)-Phe-CONH2 that is cleaved between Gly and Phe residues has been used to modify pharmacokinetics of 90Y-labeled antibodies [48–51]. A sugar moiety was used to increase tumor/liver ratios of 125I and 18F-labeled cyclic RGD (Arg-Gly-Glu) peptides [52–55]. The di(cysteic acid) linker was used to minimize liver accumulation of the radiolabeled nonpeptide integrin αvβ3 antagonists [56–59]. It has been reported that introduction of the polyethylene glycol (PEG) linker can improve not only tumor uptake but also excretion kinetics of the 125I and 18F-labeled cyclic RGD monomer [60], and 64Cu-labeled cyclic RGD dimer [61]. PEG4 and amino acid linkers have also been used to improve excretion kinetics of 111In and 99mTc-labeled E[c(RGDfK)]2 [62, 63]. The ultimate goal of using PKM linkers is to modify the radiotracer excretion kinetics so that its T/B ratios can be optimized by minimizing its uptake in non-tumor organs while maintaining its high tumor uptake [5, 6, 12].
Figure 2.
PKM linkers useful for modification of pharmacokinetics of radiopharmaceuticals.
BFC is needed for radiolabeling of biomolecules with a metallic radionuclide. BFC is covalently attached to the targeting molecule either directly or through a PKM linker, and strongly coordinates to the radiometal. The choice of BFC is largely determined by the nature and oxidation state of the radiometal. Different radiometals require BFCs with different donor atoms and chelator frameworks. Therefore, it is important to understand the coordination chemistry of BFCs with any given radiometal to be labeled. An ideal BFC is that which is able to form a stable radiometal chelate with high thermodynamic stability and kinetic inertness.
Since many reviews have covered a broad range of topics related to the target-specific radiopharmaceuticals [5, 6, 21–43, 64–68], this critical review will focus on the fundamental coordination chemistry of BFCs with technetium, copper, gallium, indium, yttrium and lanthanide metals. Whenever possible, references from the last 10 years will be used. The author would apologize to those whose work has not been presented in this review, and for the omission of 18F-labeled and iodinated biomolecules as diagnostic and therapeutic radiotracers, details of which can be found in recent review articles [15, 16, 20, 21, 69, 70].
2. Radiometals for Diagnostic and Therapeutic Radiopharmaceuticals
2.1. Why Metallic Radionuclides?
Receptor-based radiopharmaceuticals provide a unique tool for target-specific delivery of radionuclide. The overwhelming majority of diagnostic radiopharmaceuticals currently available in nuclear medicine are either radiometal complexes or target-specific biomolecules labeled with metallic radionuclides (Table 1), such as 99mTc and 111In. It should be noted that the choice of radionuclide depends largely upon its nuclear properties (half-life, type of radiation, energy, and presence or lack of other particulate radiation emissions) and potential for wide clinical applications of the radiotracer. For example, 18F is an excellent PET isotope, and has been widely used for the development of target-specific PET radiotracers for research purposes. However, the wide clinical applications of the 18F-based target-specific radiotracers will be limited due to its short-half life (t1/2 = 110 min), difficulties in radiosynthesis and chromatographic purification under GMP (Good Manufacturing Practice) conditions, and high cost to maintain the radionuclide production infrastructure. In this respect, metallic radionuclides, such as 68Ga and 64Cu, become viable alternatives to 18F in part because of their availability, which makes it much more feasible to develop target-specific radiotracers with wider clinical applications. 68Ga is readily available from the 68Ge-68Ga generator. 64Cu has a much longer half-life (t1/2 = 12.7 h) and can be produce with very high specific activity. In addition, the use of metallic radionuclides offers many opportunities for the design and development of new target-specific radiotracers. Different radiometals have significant difference in their coordination chemistry. Since the radiometal chelate can have a significant impact on biological properties, the biodistribution of a target-specific radiopharmaceutical can be systematically changed by either modifying the coordination environment around the radiometal with a variety of chelators or by the use of various coligands if the radiometal chelate contains two or more ligands.
2.2. Radiometals for SPECT
The diagnostic radionuclide is often a gamma-emitting isotope for SPECT or positron-emitter for PET.Table 2 lists several metallic radionuclides useful for scintigraphy and SPECT imaging. In general, generator-produced radiometals are ideal since the daughter radionuclide that can be easily separated from the parent isotope by ion-exchange chromatography. For SPECT imaging, 99mTc remains the most widely used isotope due to its optimal nuclear property and easy availability at low cost. 111In is also useful for gamma and SPECT imaging, and is often used as the imaging surrogate for 90Y analogs since 90Y is a pure β-emitter.
Table 2.
Metallic radionuclides useful for scintigraphic imaging.
| Radiouclide | Half-life | γ-Energy (keV) | Decay Mode | Source |
|---|---|---|---|---|
| 67Ga | 78.3 h | 93 (10%), 185 (24%) 296 (22%) |
EC | Generator |
| 99mTc | 6.02 h | 141 (89%) | IT | Generator |
| 111In | 2.83 d | 171 (88%), 247 (94%) | EC | Cyclotron |
2.2.1. Technetium-99m
99mTc is produced from 99Mo, a fission product with a half-life of 2.78 days. In a 99Mo-99mTc generator, 99MoO42− is absorbed to an alumina column and 99mTc is formed by decay of 99Mo. 99mTcO4− is eluted from the column with saline. The 99mTc produced by the generator is never carrier-free because thirteen percent of 99Mo decays directly to the long-lived isotope 99Tc (t1/2 = 2.13 × 105 y). The specific activity (the amount of radioactivity per unit mass of the radionuclide) of eluted 99mTc is dependent upon prior-elution time (the time interval between elutions). The total technetium (99mTc and 99Tc) concentration in the generator eluant is in the range of 10−7 – 10−6 M.
2.2.2. Gallium-67
Among several radionuclides of gallium, 67Ga is the most utilized due to its ability to identify inflammation and soft tissue tumors. 67Ga is a cyclotron-produced radionuclide by the 68Zn(p, 2n)-67Ga nuclear reaction, and has a half-life of 78 h. 67Ga decays to 67Zn by electron capture with emission of several gamma photons of 93 keV (40%), 184 keV (20%), 300 keV (17%) and 393 keV (5%). 67Ga is separated from the target (68Zn) by solvent extraction with isopropyl ether, and is then back extracted from ether to HCl, which is then evaporated to dryness. Citric acid is often added to prevent hydrolysis of 67Ga(III). The pH is adjusted to near neutral and finally the 67Ga-citrate solution is sterilized for clinical use [64, 68].
2.2.3. Indium-111
111In is a cyclotron-produced isotope by the 111Cd(p, n)-111In nuclear reaction, and has a half-life of 67.9 h (2.83 days). 111In is separated from the cadmium using solvent extraction, ion exchange, or both even though co-precipitation with ferric hydroxide has also been used. 111In decays by electron capture with two γ-photon emissions at 173 and 247 keV (89% and 95% abundance, respectively), and has been widely used in gamma scintigraphy.
2.3. Radiometals for PET
Table 3 lists metallic radionuclides useful for PET imaging. In general, it is highly desirable that the radionuclide has no radiation decays other than 511-keV gamma photons from positron annihilation. This will minimize impairment of spatial resolution due to high β+ energy and reduce radiation burden to the patient. A generator-based isotope would be ideal to achieve high specific activity for target-specific radiopharmaceuticals so that their target uptake can be maximized. It is also much easier for dose preparation, quality control, transportation and delivery using a generator produced isotope. The half-life of parent isotope should be long while the half-life of daughter isotope should be short. Radiolabeling should be readily completed, preferably over 10 – 30 min. In addition, the cost for parent isotope production and availability of the enriched source should also be considered.
Table 3.
Selected metallic radionuclides useful for PET imaging.
| Isotope | Half-life (h) | Decay Mode | Eβ+ (keV) | Production Method |
|---|---|---|---|---|
| 61Cu | 3.3 | β+ (62%) | 1220, 1150 | cyclotron, 61Ni(p, n)61Cu |
| EC (38%) | 940, 560 | |||
| 62Cu | 0.16 | β+(98%) | 2910 | 62Zn/62Cu generator |
| EC (2%) | ||||
| 64Cu | 12.7 | β+ (19%) | 656 | cyclotron, 64Ni(p, n)/64Cu |
| EC (41%) | ||||
| β−(40%) | ||||
| 68Ga | 1.1 | β+ (90%) | 68Ge/68Ga generator | |
| EC (10%) | 1880, 770 | |||
| 89Zr | 78.5 | β+ (23%) | 897 | cyclotron, 89Y(p, n)/89Zr |
| EC (77%) | ||||
| 94mTc | 0.9 | β+ (72%) | 2.47 | cyclotron, 63Cu(α, nγ)/66Ga |
2.3.1. Copper-62
62Cu is a generator-produced radionuclide from the decay of 62Zn. It has a half-life of 9.7 min, which allows repeated dosing without imposing a significant radiation burden to the patient [71–73]. The 62Zn-62Cu generator is made up of Dowex ion exchange column. Carrier free 62Cu is eluted from the column with 2 N HCl. The 62Zn-62Cu generator only lasts for 1 – 2 days due to the short half-life (9.3 h) of 62Zn. This makes PET imaging with 62Cu-labeled biomolecules very expensive. However, the cost of 62Zn-62Cu generators may be significantly reduced as their usage increases [71]. A commercially available 62Zn-62Cu generator has been successfully used in Phase III clinical trials [74]. There is significant interest in the 62Cu radiopharmaceuticals, such as 62Cu-PTSM, for heart and tumor imaging by PET [71–78].
2.3.2. Copper-61 and Copper-64
61Cu has a relatively high β+ emission rate (61%) with maximum β+ energy of 1.22 MeV and a half-life of 3.32 h. It also has two gamma rays with Eγ = 283 (13%) and 380 keV (3%). Several nuclear reactions can be used for the production of 61Cu. These include nuclear reactions [59Co(α, 2n)61Cu] (40 MeV), [natNi(α, p)61Cu] (21 MeV), and [61Ni(p, n)61Cu]. The latter methods are often free from 64Cu radio-impurity. Although the nuclear properties are very attractive for PET imaging, 61Cu has not been used to the same extent as 64Cu, which has a low β+ emission rate (18%) with maximum β+ energy of 0.66 MeV and a half-life of 12.7 h. The longer half-life of 64Cu is much more feasible for the radiolabeling of small biomolecules for the development of target-specific radiopharmaceuticals. 64Cu can be produced by proton irradiation of natNi or enriched 68Zn. Both methods suffer from low yield and co-production of 61Cu and 67Cu radioimpurities [71, 72]. The enriched 64Ni target has been used for production of 64Cu with very high specific activity (>10,000 Ci/mmol). Using this method, Ci amounts of 64Cu can be produced on demands. 64Cu has been successfully for radiolabeling of small biomolecules for imaging tumors [61, 79–86].
2.3.3. Gallium-68
68Ga is also a generator-produced isotope with a half-life of 68 min. 68Ga decays by positron emission and hence 511-keV annihilation radiation [64, 66]. The photon abundance is 178%. In general, the generator is made up of alumina loaded in a plastic or glass column. Carrier free 68Ge in HCl is neutralized in EDTA solution and absorbed on the column. 68Ga is eluted from the column with 0.05 M EDTA solution. Alternatively, 68Ge is absorbed on a stannous dioxide column and 68Ga is eluted with 1 N HCl. This generator can be eluted quite frequently since maximum yield is obtained in a few hours. Due to the long half-life (271 days) of 68Ge, the 68Ge-68Ga generator can be used for 1 – 2 years, allowing PET imaging at facilities without the on-site cyclotron. The half-life of 68Ga is long enough to permit multiple-step radiotracer syntheses and data requisition over longer periods. Thus, cameras with the highest sensitivity are not prerequisite for obtaining high quality images. With properly designed radiotracers, 68Ga could become the radionuclide as useful for PET imaging as 99mTc for planar and SPECT imaging. However, there is a lack of efficient production methods for 68Ge. As a result, 68Ga is often considered the most cost-prohibitive radionuclide for PET imaging [71]. Right now, 68Ge-68Ga generators are available from several commercial sources in Russia, Europe and the United States.
2.3.4. Technetium-94m
94mTc has a half-life of 52 min and β+ energy of 2.47 MeV (72%). It can be obtained from a number methods, including 94Mo(p, n)/94mTc (13.5 – 11 MeV), natNb(3He, 2n)/94mTc (18 – 10 MeV), 92Mo(α, pn)/94mTc (26 – 18 MeV). To obtain sufficient yield using small cyclotrons, the reaction 94Mo(p, n)/94mTc is preferred. Access to this isotope makes it possible to use PET to estimate the uptake of 99mTc radiotracers. The quantitative superiority of PET permits modeling of radiotracer kinetics and dosimetry measurements. Commercially kits for 99mTc radiotracers (e.g.99mTc-Sestamibi and 99mTc-Tetrofosmin) might be used to prepare 94mTc analogs. The use of dual isotopes 99mTc/94mTc (SPECT/PET) may provide much better imaging quality. The integration of PET and SPECT radiotracer would pave the way for better exploitation of current strengths of both imaging modalities. However, the availability of 94mTc for clinical applications remains a significant challenge.
2.3.5. Zirconium-89
89Zr has a half-life of 78.5 h with a β+ emission (897 keV, 23%) and EC (77%). 89Zr is produced by cyclotron from the nuclear reaction [89Y(p, n)/89Zr], but the separation of 89Zr is requires both solvent extraction and ion-exchange chromatography [64, 66]. A simplified production method using the [89Y(d, 2n)/89Zr] reaction requires only one-step ion-exchange separation. Due to its long half-life, it is an attractive isotope for 89Zr-labeling of biomolecules. Theoretically, all the BFCs, such as DTPA and DOTA derivatives, for 111In and 90Y-labeling can be used for 89Zr-labeling of biomolecules.
2.4. Radiometals for Therapeutic Radiopharmaceuticals
Table 4 lists some selected isotopes useful for radiotherapy. Identifying an appropriate radionuclide for radiotherapy often requires weighing various factors [5, 6,9, 12, 68], including tumor uptake and tumor retention, blood clearance, rate of radiation delivery, half-life and specific activity of the radionuclide, and the feasibility of large-scale production of the radionuclide in an economical fashion. The main objective for the receptor-based target-specific radiotherapy is to deliver a tumorcidal radiation dose to tumor cells without causing unmanageable side-effects. Other practical considerations in selecting a radionuclide for a given targeting biomolecule in target-specific tumor radiotherapy are availability and quality. The radiochemical purity has to be sufficient and reproducible, as trace amounts of impurities (particularly the radionuclide impurities) can affect the radiolabeling and radiochemical purity of the radiopharmaceutical. The receptor sites in tumors are typically limited in number. This requires that the chosen radionuclide have high specific activity, which depends primarily on the production method. Trace metal contaminants must be minimized as they often compete with the radionuclide for the “cold” BFC-BM conjugate and their metal complexes may compete for receptor binding with the radiolabeled BFC-BM conjugate. Among various radionuclides, 90Y and radiolanthanides are of particular interest. There are several lanthanide isotopes to choose: low energy β-emitter 177Lu, medium energy β-emitters, 153Sm, and high-energy β-emitters, 166Ho and 90Y. Depending on the tumor size and location, the choice of the β–;emitter may be different. For example, medium or low energy β–emitters such as 177Lu are better for smaller metastases while high-energy β–emitters such as 90Y are used for larger tumors. Chelation chemistry of 90Y and lanthanide radionuclides is well developed and understood. In addition, β-emitters have relatively long penetration range (2 – 12 mm), which is particularly important for solid tumors with high heterogeneity. The β-particle emitters yield a homogeneous dose distribution due to the “cross-fire effect” even when they are heterogeneously distributed in the tumor.
Table 4.
Selected radionuclides useful for radiotherapy.
| Nuclide | Half-life (days) | Energy (MeV) | Maximum Range (mm) | Gamma (keV) | Source | Specific* Activity |
|---|---|---|---|---|---|---|
| 67Cu | 2.58 | 0.575 | 1.8 | 185 (40%) | accelerator | low |
| 90Y | 2.66 | 2.27 | 12.0 | ---- | generator | high |
| 153Sm | 1.95 | 0.80 | 3.0 | 103 (28%) | reactor | low/medium |
| 166Ho | 1.1 | 1.6 | 8.0 | 81 (6.3%) | reactor or generator | high |
| 177Lu | 6.7 | 0.497 | 1.5 | 208 (28%) | reactor | medium/high |
| 186Re | 3.7 | 1.02 | 5.0 | 137 (9%) | accelerator or reactor | low/medium |
| 188Re | 0.71 | 2.12 | 11.0 | 155 (15%) | reactor generator | high |
The specific activity of a radionuclide depends on the source and method of production, as well as the technique of separation. In general, generator-produced radionuclides, such as 90Y and 188Re, have a higher specific activity than those accelerator- or reactor-produced radioisotopes. High specific activity can also be achieved by chemical purification of the desired radionuclide from the parent element after direct (n,γ)-activation of the target.
2.3.1. Yttrium-90
90Y is a generator-produced radionuclide, resulting from the decay of 90Sr. It decays with the high energy β–particle (Emax = 2.28 MeV, 100% abundance) to form 90Zr. 90Y has a half-life of 2.7 days, which is short enough to achieve a critical dose rate and at the same time is long enough to allow the radiopharmaceutical to be manufactured, transported and delivered for clinic use. The specific activity for 90Y is very high, and is well suited for development of receptor-based therapeutic radiopharmaceuticals. For quantitative imaging, the corresponding 111In-labeled BFC-BM conjugate is often used as a surrogate to determine the biodistribution characteristics and radiation dosimetry of the 90Y-labeled BFC-BM conjugate.
2.3.2. Samarium-153
153Sm has three β-emissions (30% 0.64 MeV, 50% 0.71 MeV, and 20% 0.81 MeV) and a γ-emission (28% 103 keV) with a half-life of 1.95 days. It can be produced in large amount with high specific activity by neutron activation of enriched 152Sm [65]. The short half-life of 153Sm allows for the delivery of fractionated dose regimes while the 103-keV gamma ray is useful for biodistribution determination via gamma imaging.
2.3.3. Holmium-166
166Ho emits a beta particle with maximum energy of 1.85 MeV (maximum penetration range ~9 mm) and a small portion of gamma rays (80.6 keV at 6.6% and 1.38 MeV at 0.9%), which are useful for biodistribution determination of the therapeutic radiopharmaceutical via gamma imaging. It has a half-life of 26.78 h. 166Ho is produced with relatively high specific activity by neutron capture reaction [165Ho(n, γ)166Ho] [65].
2.3.4. Lutetium-177
177Lu is a reactor-produced radionuclide. It has three β-emissions (12% 0.176 MeV, 9% 0.384 MeV, and 79% 0.497 MeV) and two γ-emissions (6.4% 113 keV and 11% 208 keV) with a half-life of 6.75 days. One method for the production of 177Lu involves irradiation of enriched 176Lu in a reactor. By this method, 177Lu can be prepared in high yield and medium high specific activity at low cost. The specific activity of 177Lu from University of Missouri Research Reactor is routinely more than 20 Ci/mg [65].
2.3.5. Rhenium-186
Rhenium has two isotopes (186Re and 188Re). 186Re has a half-life of 3.68 days with a β-emission (Emax = 1.07 MeV, 91% abundance) and a gamma-photon (E = 137 keV, 9% abundance) which should allow imaging during therapy. 186Re is a reactor-produced radionuclide. There is only one possibility to produce 186Re by the irradiation of 185Re with neutrons (185Re(n, γ)186Re). The yield of 186Re depends on the amount of Re in the target, the energy of the neutrons available, and the neutron reflux. The specific activity is from low to medium, but a carrier-free product is not possible.
2.3.6. Rhenium-188
188Re has a half-life of 16.98 h with a high-energy β-emission (Emax = 2.12 MeV, 85% abundance) and 155 keV gamma photons (15% abundance). 188Re can be prepared either from the nuclear reaction (187Re(n, γ)188Re) or from the 188W-188Re generator. The generator-produced 188Re is carrier-free and has very high specific activity. The major advantage of using 188Re is the inexpensive and readily available 188W-188Re generator, which has a long useful shelf-life. The 155-keV gamma photons are useful for biodistribution determination and radiation dosimetry calculation of the 188Re-labeled BFC-BM conjugate.
2.3.7. Copper-67
Among several copper isotopes, 67Cu has the longest half-life (t1/2 = 62 h). 67Cu decays by three β-emissions (45% 0.40 MeV, 3% 0.48 MeV, and 20% 0.58 MeV), and two γ-emissions with energies of 93 (17%), and 185 (48%) keV. The γ-emissions permit imaging of the radionuclide distribution during therapy; but they may become problematic due to extra radiation burden to normal organs if 67Cu is used in therapeutic radiopharmaceuticals. 67Cu is produced by bombarding a natural Zn target with 200 MeV protons (68Zn(p, 2p)) in a accelerator. It is difficult to obtain very high specific activity using this production method. More recently, the production of 67Cu on low and medium-energy cyclotrons has been reported. Kastleiner et al predicted that up to 400 mCi of 67Cu can be obtained at saturation using a small cyclotron (Ep = 17 – 18 MeV; 80 µA beam current [72].
3. BFCs for 99mTc and 186/188Re-Lableing of Biomolecules
3.1. Why 99mTc?
Nearly 80% of radiopharmaceuticals currently available in clinical nuclear medicine are 99mTc compounds due to ideal nuclear properties of 99mTc. The 6 h half-life is long enough to allow a radiopharmacist to carry out radiosynthesis and prepare the dose, and for nuclear medicine practitioners to collect clinically useful images. At the same time, it is short enough to permit administration of millicurie amounts of 99mTc radiopharmaceutical without causing a significant radiation dose to the patient. The monochromatic 140 KeV photons are readily collimated to give images of high spatial resolution. Furthermore, 99mTc is readily available from the 99Mo-99mTc generators at low cost.
3.2. Diverse Redox Chemistry of Technetium
One of the characteristics of technetium is its diverse redox chemistry. Table 5 summarizes various oxidation states of technetium. Since there is no effective chemistry that can be used to attach 99mTcO4− to biomolecules, the Tc(VII) in 99mTcO4− has to be reduced to a lower oxidation state. When 99mTcO4− is reduced, the oxidation state of Tc depends upon the reducing agent, chelator, and reaction conditions. The rich and diverse redox chemistry makes it difficult to control the oxidation state and solution stability of Tc chelates. At the same time, it also provides opportunities to modify structures and properties of technetium complexes by the choice of chelators with high affinity for a specific oxidation state of Tc. Technetium chemistry has been reviewed recently [10, 11, 87].
Table 5.
Various oxidation states and stereochemistry of technetium.
| Oxidation State | Example | Coordination Geometry | Coordination Number | Magnetic Moment (µB) |
|---|---|---|---|---|
| +7 (d0) | [TcH9]2− | Trigonal prism | 9 | diamagnetic |
| TcO4− | Tetrahedron | 4 | diamagnetic | |
| +6 (d1) | TcO42− | Tetrahedron | 4 | 1.60 |
| +5 (d2) | [Tc(NCS)6]− | Octahedron | 6 | diamagnetic |
| [Tc(Diars)2Cl4]2− | dodecahedron | 8 | 0.9 | |
| TcOCl4− | Square pyramid | 5 | diamagnetic | |
| [TcO(l, l-ECD)] | Square pyramid | 5 | diamagnetic | |
| [TcO(d,l-HM-PAO)] | Square pyramid | 5 | diamagnetic | |
| [TcO2(tetrofosmin)]+ | Octahedron | 6 | diamagnetic | |
| +4 (d3) | [TcCl6]2− | Octahedron | 6 | 4.05 |
| +3 (d4) | [Tc(Diars)2Cl2]+ | Octahedron | 6 | diamagnetic |
| +2 (d5) | [TcCl3(PhP(OEt)2)4] | Octahedron | 6 | 1.4 |
| +1 (d6) | [Tc(CNC(CH3)3)6]+ | Octahedron | 6 | diamagnetic |
| [Tc(CO)5([9]aneN3)]+ | Octahedron | 6 | diamagnetic | |
| 0 (d7) | [Tc2(CO)10] | Octahedron | 6 | diamagnetic |
| −1(d8) | [Tc(CO)5]− | Trigonal bipyramid | 5 | diamagnetic |
3.3. Isomerism
Another aspect of technetium chemistry is isomerism, including geometric isomers, epimers, enantiomers, and diastereomers [4, 8, 10, 11, 88]. Figure 3 shows selected examples of isomerism in technetium chelates. Epimers are often found in square pyramidal or octahedral oxotechnetium complexes containing chelating ligands with substituents on the ligand backbone or a tertiary amine-N donor atom. Formation of epimers is due to the relative orientation (anti and syn) of substituents to the [Tc=O]3+ core [10, 88]. Enantiomers are often found in Tc(V)-oxo complexes, such as [TcO(MAG3)]−, due to asymmetrical bonding of chelator to the [Tc=O]3+ core even though the free chelator does not have a chiral center. Enantiomers are also formed when the Tc chelate contains a pro-chiral chelator, such as tricine in ternary ligand Tc complexes (Figure 3). Enantiomers are indistinguishable by NMR methods; but they are separable under chiral chromatographic conditions (chiral solid phase or chiral mobile phase) by HPLC. If a Tc complex contains two or more chiral centers, diastereomers may be formed, and are often separated by reversed phase HPLC methods. Isomers often have different lipophilicity and biodistribution patterns. This is particularly true for small Tc complexes as their biological properties are determined by physical and chemical properties of the Tc chelate. For example, [TcO(map)]− (map = 2,3-bis(mercaptoacetamido)propanoate), has two epimers (anti and syn) due to the disposition of the COOH group on the chelate ring relative to the Tc=O moiety. It was reported that in humans 58% of syn isomer was excreted at 30 min as compared to only 19% of anti isomer [89]. For receptor-based target-specific radiopharmaceuticals, the target uptake is largely dependent on receptor binding affinity of the radiolabeled receptor ligand, receptor population and the blood clearance, which is determined by physical properties of both the targeting biomolecule and Tc chelate. Formation of isomers for the Tc chelate may have significant impact on biological properties of a target-specific radiopharmaceutical. Therefore, the choice of BFCs should be those which form technetium complexes with minimal isomerism.
Figure 3.
Examples of isomerism in technetium complexes.
3.4. Challenges for 99mTc-Labeling of Biomolecules
99mTc radiopharmaceuticals are used in very low concentrations (10−8 to 10−6 M). Therefore, the radiolabeling kinetics must be taken into consideration in the development of 99mTc radiopharmaceuticals. 99mTc is obtained from the 99Mo-99mTc generator as 99mTcO4− in saline. This requires that the radiolabeling be performed in aqueous solution. Due the short half-life (t1/2 = 6.02 h) of 99mTc, radiosynthesis must be completed within 30 min. The radiochemical purity (RCP) of the radiopharmaceutical must be greater than 90% since injection of a mixture of different 99mTc-containing species will decrease organ specificity, and needlessly increases the radiation burden to patient. Since all 99mTc radiopharmaceuticals are administered by intravenous injection, radiosynthesis has to be conducted under sterile and pyrogen free conditions. This requirement virtually eliminates any chromatographic purification of the desired 99mTc radiopharmaceutical. Each of these constrains provides a unique challenge for inorganic chemistry. Fortunately, most of these challenges have been successfully met with the development of coordination chemistry of technetium and new 99mTc-labeling techniques.
3.5. Requirements for Ideal BFCs
An ideal BFC is that which is able to form a stable 99mTc complex in high yield at very low concentration of the BFC-BM conjugate. To achieve this goal, the BFC must selectively stabilize an intermediate or lower oxidation state of Tc so that the 99mTc complex is not subject to redox reactions. Oxidation state changes are often accompanied by transchelation of 99mTc from a 99mTc-BFC-BM complex to the native chelating ligands in biological systems. The BFC should form a 99mTc complex which has thermodynamic stability and kinetic inertness with respect to dissociation or release of 99mTc. The BFC should form the 99mTc complex with a minimum number of isomers since different isomeric forms of the 99mTc-chelate may result in significantly different biological and pharmacokinetic characteristics of the 99mTc-BFC-BM complex. Finally, the conjugation group should be easily attached to the targeting biomolecule.
3.6. Kit Formulation
Due to the 6 h half-life of 99mTc, a kit formulation is required for 99mTc radiopharmaceuticals. The kit formulation is particularly important for consistency and reproducibility in the RCP performance during 99mTc-labeling. In general, the kits are sterile, pyrogen free, and non-radioactive mixtures, which are dried by lyophilization and stored under nitrogen in glass vials. For target-specific 99mTc radiopharmaceuticals, a kit contains a BFC-BM conjugate and a reducing agent, if necessary. Kit components are often dissolved in a buffer system, which is used for pH control during the manufacturing and radiolabeling processes. Sometimes a bulking agent is needed so that kit components can crystallize on the crystals of the bulking agent. Other components (antioxidants, solubilizing agents, and weak transferring ligands) may be needed to improve the yield and solution stability of the 99mTc radiopharmaceutical. In many cases, 99mTc-labeling can be accomplished simply by adding 99mTcO4− to the kit. It must be emphasized that all kit components be non-toxic and suitable for intravenous injection. The amount of BFC-BM conjugate in the kit formulation has to be sufficiently high so that high RCP can be achieved for the intended 99mTc radiopharmaceutical. However, a large amount of the BFC-BM may result in receptor site saturation, blockage of the receptor binding of the 99mTc radiotracer, as well as unwanted side effects. To avoid these problems, the BFC-BM concentration in each kit has to be very low (e.g. 20 µg/mL or ~ 2 × 10−6 M for a BFC-BM conjugate with molecular weight of 1000 Daltons). Otherwise, post-labeling purification is needed to remove excess unlabeled BFC-BM conjugate, which is time consuming and not amenable for the kit formulation. Compared to the total Tc concentration (~5 × 10−7 M) in 100 mCi of 99mTcO4− (eluted with 24 h prior-elution time), 20 µg of the BFC-BM conjugate is not in overwhelming excess. It should be noted that the amount of BFC-BM conjugate also depends largely on receptor population, receptor ligand binding affinity and possible side effect caused from the BFC-BM conjugate. A careful study is recommended to determine the optimum amount of BFC-BM conjugate in the kit formulation.
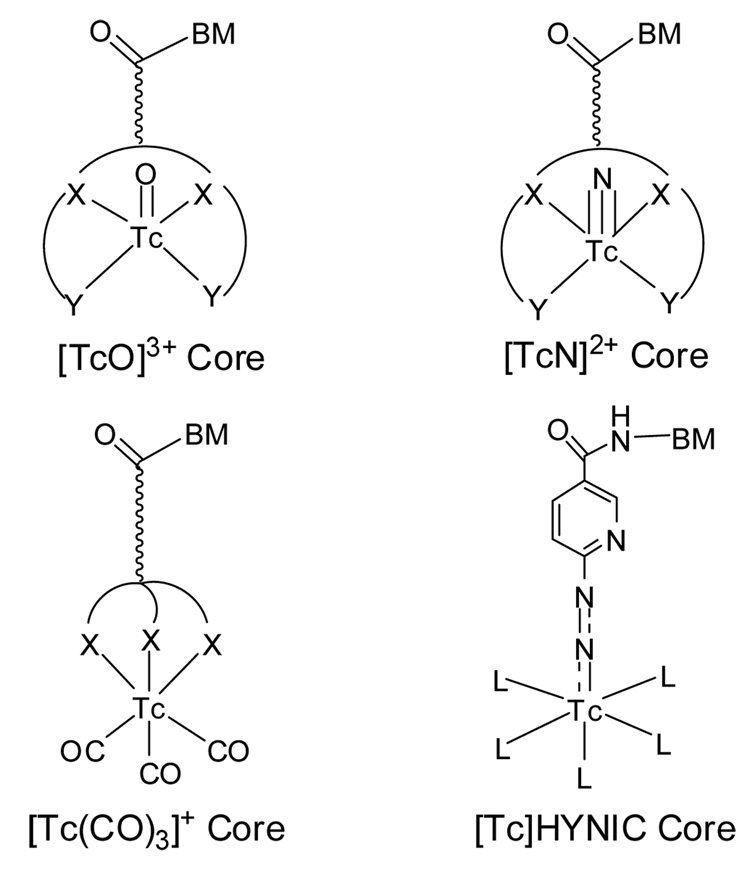
3.7. Technetium Cores
Figure 4 shows some selected Tc cores, which have been used for 99mTc-labeling of biomolecules, including antibodies, antibody fragments, small peptides, and nonpeptide receptor ligands. Since Tc chemistry and Tc cores have been reviewed in detail recently [3, 4, 8, 10, 11, 87], we will focus on the bifunctional coupling systems and their related coordination chemistry with the [Tc=O]3+, [Tc≡N]2+, [Tc(CO)3]+ and [Tc]HYNIC cores.
Figure 4.
Technetium cores useful for the 99mTc-labeling of biomolecules.
3.7.1. [Tc=O]3+ Core
The [Tc=O]3+ core is very stable in the presence of a good chelator in aqueous solution. It is the most frequently used technetium core for 99mTc-labeling of biomolecules (Table 4). The [Tc=O]3+ core forms square pyramidal Tc(V)-oxo complexes with tetradentate chelators, such as N2S2 diamidedithiols (Figure 5: DADS), N3S triamidethiols, N2S2 monoamidemonoaminedithiols (Figure 5: MAMA), and N2S2 diaminedithiols (Figure 6: DADT). N2S2 DADS chelators contain two amide-N and two thiolate-S donors, and form stable anionic oxotechnetium complexes with the [Tc=O]3+ core [89–91]. Like N2S2 DADS, N3S triamidethiols also form very stable anionic Tc(V)-oxo complexes [91–93]. Fritzberg and coworkers first reported the use of 4,5-bis(thioacetamido)pentanoate (mapt) as the BFC in labeling antibodies and their fragments with 99mTc by the preformed chelate approach [94–96]. Other N2S2 DADS and N3S triamidethiols have also been used for 99mTc-labeling of small RGD peptides [97–99]. N2S2 DADT chelators (Figure 5) represent another class of BFCs that bind the [Tc=O]3+ core strongly to form stable Tc(V) complexes. They can be tribasic utilizing two thiolate-S atoms, one deprotonated amine-N and one neutral amine-N to form neutral Tc(V)-oxo complexes, or dibasic using two thiolate-S and two amine-N donor atoms to form cationic Tc(V)-oxo complexes [100–108]. N2S2 MAMA chelators (Figure 5) contain a amine-N, an amide-N and two thiolate-S donors, and bind to the [Tc=O]3+ core to form neutral complexes, [99mTcO(MAMA)] [109]. The N2S2 MAMA-type BFCs have been used for 99mTc-labeling of progesterone receptor ligands [110–112], platelet glycoprotein IIb/IIIa receptor antagonists [97, 98], and dopamine transporters [113]. Small peptides, such as Gly-Ala-Gly-Gly and Gly-Ser-Cys (Figure 6) have also been proposed as BFCs for 99mTc-labeling of biomolecules [114, 115]. The attachment of the tripeptide chelating sequences can be easily incorporated into solid-phase peptide synthesis. These tripeptide sequences form stable technetium complexes with the [Tc=O]3+ core. As a matter of fact, the tripeptide sequence with N3S donor atoms has been used to bind the [Tc=O]3+ core in 99mTc-P280, a FDA-approved thrombosis imaging agent [116–119].
Figure 5.
Thiol-containing BFCs and their technetium complexes.
Figure 6.
Tripeptide sequences as BFCs.
3.7.2. [Tc≡N]2+ Core
The [Tc≡N]2+ core is isoelectronic with [Tc=O]3+. The nitrido ligand is a powerful π-electron donor and shows a high capacity to stabilize the Tc(V) oxidation state. The [Tc≡N]2+ core forms Tc(V)-nitrido complexes with various chelators [120–126]. The [99mTc≡N]2+ core has been used for 99mTc-labeling of small peptides and benzodiazepine receptor ligands [127–129]. The PXP bisphosphine ligands (Figure 7) are used as coligands to stabilize the [99mTc≡N]2+ core, and the BFCs containing thiolate-S, amine-N or carboxylate-O donors are attached to the peptide or benzodiazepine receptor ligands. It has been demonstrated that the [99mTcN(PXP)]2+ fragment reacts with the cysteine to form asymmetrical 99mTc-nitrido complexes in very high specific activity [127].
Figure 7.
Examples of the 99mTc-nitrido core for the labeling of biomolecules.
3.7.3. [99mTc(CO)3]+ Core
Alberto et al first reported synthesis of Tc(I) and Re(I) complexes [M(H2O)3(CO)3]+ (M = 99mTc and 188Re) by direct reduction of [99mTc]pertechnetate or [188Re]perrhenate with sodium borohydride in aqueous solution [130–132]. The yield of the 99mTc or 188Re complex was > 95%. In [99mTc(H2O)3(CO)3]+, all three water molecules are labile with respect to substitution [133–138]. A variety of BFCs can be used for the 99mTc-labeling of biomolecules [139–148]. Figure 8 shows examples of bidentate and tridentate chelators containing imidazoles, pyridines, pyrazoles, amides, amines, carboxylic acids or combination thereof. Since it is a natural amino acid, histidine is of particular interest as the BFC for 99mTc-labeling of monoclonal antibodies and small peptides [144, 146]. The diverse coordination chemistry of the [99mTc(CO)3]+ core offers a tremendous opportunity for development of new BFCs. However, monodentate and bidentate chelators often form 99mTc(I)-tricarbonyl complexes with low solution stability, which results in high protein binding and high background activity in the blood stream. In contrast, tridentate chelators form 99mTc(I)-tricarbonyl complexes with high stability and rapid clearance from blood and other major organs. Alberto and coworkers reviewed organometallic radiopharmaceuticals recently [149, 150].
Figure 8.
Examples of bidentate and tridentate BFCs for 99mTc- and 186/188Re-labeling of biomolecules. The R group may be a biomolecule or a linker attached to the biomolecule.
3.7.4. [Tc]HYNIC Core
Abrams et al first reported the use of 6-hydrazinonicotinamide (Figure 9: HYNIC) for 99mTc-labeling of polyclonal IgG [151, 152]. Since then, HYNIC has been used for 99mTc-labeling of antibody fragments [153], chemotactic peptides [154–157], somatostatin analogs [158–163], liposomes [164], and antisense oligonucleotides [165, 166]. Since HYNIC can only occupy one or two coordination sites, a coligand, such as tricine, is often needed to complete the coordination sphere of technetium. The advantage of using HYNIC as the BFC is its high 99mTc-labeling efficiency and the choice of coligands such as tricine and glucoheptonate, which allows easy modification of hydrophilicity and pharmacokinetics of the 99mTc-labeled biomolecules. However, the use of tricine as coligand suffers two major drawbacks: (1) solution instability of [99mTc(HYNIC-BM)(tricine)2] (Figure 9), and (2) presence of multiple species in solution due to different bonding modalities of HYNIC and coligands [167, 168]. To overcome these problems, Liu et al developed several versatile ternary ligand systems (HYNIC, tricine and water-soluble phosphine or pyridine analogs) that form ternary ligand technetium complexes [99mTc(HYNIC-BM)(tricine)(phosphine)] (Figure 9) in high yield and high specific activity [168–171]. These ternary ligand 99mTc complexes have very high solution stability, and often show two peaks in their radio-HPLC chromatograms if the biomolecule contains one or more chiral centers. The presence of two peaks is due to the resolution of two diasteromers resulting from chiral centers on the peptide backbone and the chiral Tc chelate [168–171]. The 1:1:1:1 composition for Tc:HYNIC:L:tricine was determined through a series of mixed ligand experiments [169, 170], and has been confirmed by FAB-MS and LC-MS at both 99mTc and 99Tc levels [171, 172]. Many coligands (Figure 9) have been used for 99mTc-labeling of small biomolecules, such as chemotactic peptides [168] and LTB4 receptor antagonists [173, 174] for imaging infection/inflammation, cyclic RGD peptides for imaging integrin αvβ3-positive tumors [175–179], and a GPIIb/IIIa receptor antagonist for diagnosis of thrombi [169, 170, 180, 181].
Figure 9.
HYNIC, coligands and their binary and ternary ligand 99mTc complexes.
3.8. Radiolabeling Approaches
The choice of 99mTc-labeling approaches depends on biomolecules (antibody versus small biomolecules) and the purpose of study (proof of concept versus product development). In the last three decades, a large number of techniques have been developed for 99mTc-labeling of biomolecules, including monoclonal antibodies, small peptides and non-peptide receptor ligands. They are often classified into three main categories: direct labeling approach, pre-labeling (or preformed chelate) approach, and post-labeling approach.
3.8.1. Direct Labeling
The direct labeling approach (Chart I) usually uses a reducing agent such as SnCl2 to convert the disulfide linkages into free thiols, which bind strongly to the Tc. This approach is that it is easy to carry out [182–186]; but it applies only to antibodies or antibody fragments because many small biomolecules do not have any disulfide bonds, or in many cases the disulfide bond is too critical for maintaining their biological properties to be reduced. There are several critical questions to be answered for this approach. These include: oxidation state of technetium, number of 99mTc bonded to biomolecule, number of 99mTc-species in the radiolabeled kit, and impact of 99mTc-labeling on biological activity of the targeting biomolecule? In addition, there is little control over solution stability of the 99mTc radiotracer.
Chart I.
Direct Labeling Approach
3.8.2. Pre-Labeling Approach
The pre-labeling or “pre-formed chelate” approach (Chart II) involves formation of the 99mTc-BFC chelate, and conjugation of the 99mTc-BFC chelate to a biomolecule in a separate step. This approach has been successfully used in labeling antibodies and their fragments with 99mTc [94–96]. In this approach, the chemistry is better defined, and the targeting biomolecule is not exposed to sometimes harsh conditions in the chelation step. For research purposes, this approach is very useful to demonstrate the proof of concept in a short period of time before making extensive efforts in preparing the BFC-BM conjugate. However, the multiple-step radiosynthesis is too complex and time consuming for routine clinical use, and makes it very difficult to develop a kit formulation.
Chart II.
The Pre-Labeling Approach
3.8.3. Post-Labeling Approach
In the post-labeling, or indirect labeling, approach (Chart III), a BFC is first attached to the biomolecule to form BFC-BM conjugate. Once the BFC-BM conjugate is prepared, radiolabeling can be accomplished by direct reduction of 99mTcO4− in the presence of a sufficient amount of the BFC-BM conjugate or by ligand exchange with an intermediate 99mTc complex, such as [99mTc]glucoheptonate. This approach combines the ease of direct labeling with well-defined chemistry of the preformed chelate approach. This is the most practical approach for kit formulation and for development of commercial products.
Chart III.
The Post-Labeling Approach
3.9. 99mTc-Labeling Efficiency
99mTc-labeling efficiency is a term used to describe the ability of a BFC to achieve a high radiolabeling yield (> 90%) of its 99mTc complex. High radiolabeling efficiency is required for BFC in target-specific radiopharmaceuticals. However, there is little experimental data to compare the 99mTc-labeling efficiency of various BFCs. In general, there are several factors influencing 99mTc-labeling efficiency of a BFC. These include identity of donor atoms, BFC concentration, reaction temperature and time, and pH value in the mixture [187]. If BFC concentration is fixed, the conditions for 99mTc-labeling depend largely upon the nature of donor atoms. For example, high pH and heating at 100 °C for 30 min is required for successful 99mTc-labeling of N3S triamidethiols and N2S2 DADS at low concentrations (10−5 – 10−6 M) while N2S2 MAMA chelators are well labeled under milder conditions. For the N2S2 DADT chelators, the ligand exchange with [99mTc]glucoheptonate can be completed within 60 min at room temperature [187]. In general, HYNIC and N2S2 DADT chelators are better BFCs for small biomolecules with high receptor binding affinity mainly due to their high 99mTc-labeling efficiency. In some cases, N3S triamidethiol, N2S2 DADS, or N2S2 MAMA can also be used as BFCs if the use of a large amount (> 100 µg/mL) of BFC-BM conjugate does not cause unwanted side effects. For example, there is 100 µg of bibapcitide (P280) in each lyophilized AcuTect™ vial. This allows the use of less than 50 mCi of [99mTc]pertechnetate for radiolabeling [116–119].
3.10. 99mTc-Labeling of BFC-BM Conjugates
Once it is decided to use the indirect labeling approach, the next question will be how to synthesize the 99mTc-BFC-BM radiotracer. In general, there are three different approaches for successful preparation of the 99mTc-BFC-BM. These include direct reduction, ligand exchange, and reduction-exchange. The choice of synthetic route is largely dependent on both BFC and targeting biomolecule.
3.10.1. Direct Reduction
Using the reduction route, 99mTcO4− is reduced in one step in presence of a BFC-BM conjugate [10, 11, 188]. The conditions employed in these preparations are dictated by short half-life of 99mTc, low concentration of 99mTcO4−, and chemical stability of the BFC-BM conjugate. The reaction generally produces a mixture of reduced 99mTc-species, and in many cases the chemical form and oxidation state in these 99mTc-containing species are not known. However, the 99mTc-BFC-BM complex can be prepared as a single predominant product by the choice of reducing agent and BFC under well-controlled conditions. Many reducing agents can be used to reduce 99mTcO4− during radiolabeling. These include stannous chloride, borohydride, dithionate, dithionite, hypophosphoric acid, hydroxamine, formamidine sulfinic acid, and water soluble phosphines. While most of these reductants can be used for synthesis of simple Tc complex radiopharmaceuticals, only a few of them have been used in commercial kits for routine preparation of target specific radiopharmaceuticals.
Sn(II) is the most commonly used reducing agent in commercial kits for the rapid preparation of 99mTc radiopharmaceuticals due to its fast reduction kinetics. However, the use of Sn(II) often leads to several problems. For example, during the synthesis of a 99mTc radiopharmaceutical, initial reduction of 99mTcO4− leads to rapid formation of the Tc(VI) intermediate 99mTcO42−, which is unstable with respect to disproportionation. The reduction of 99mTcO4− can lead to the formation of Tc(IV), which undergoes rapid hydrolysis in aqueous solution to form 99mTcO2. Sn(IV) also undergoes rapid hydrolysis to form insoluble SnO2. The formation of colloids (99mTcO2/SnO2) compromises the radiolabeling yield of the radiotracer. Therefore, a weak chelating agent such as glucoheptonate (GH) is often used to stabilize Sn(II) and Tc in its intermediate oxidation state.
3.10.2. Ligand Exchange
The second route for successful 99mTc-labeling of a BFC-BM conjugate is the two-step ligand exchange synthesis. This route involves reduction of 99mTcO4− by a reducing agent in the presence of a chelating agent such as glucoheptonate to form the [99mTcO(GH)2]n− intermediate, which is then allowed to react with the BFC-BM conjugate under milder conditions to give the 99mTc-BFC-BM complex. This route is often used for the 99mTc-labeling of biomolecules that are sensitive to harsh reaction conditions (e.g high pH and heating at elevated temperatures). [99mTcO(GH)2]n− has been used for 99mTc-labeling of small peptides [97–99, 116–119]. [99mTcO(L)2]n− (L = tricine, mannitol, and glucamine) have also been used for 99mTc-labeling of HYNIC-conjugated biomolecules, such as small peptides [151–172]. Unlike tetradentate thiol-containing chelators, which replace glucoheptonate ligands in [99mTcO(GH)2]n−, HYNIC reacts with the [Tc=O]3+ core [151–157]. The exchange ligand is the oxo-O atom while Tc(V) is reduced to Tc(III) when HYNIC binds to the Tc [10, 11, 32, 33]. Tricine or glucoheptonate serves two purposes: as a ligand to stabilize the reduced 99mTc in its Tc(V) oxidation state, and as a coligand to stabilize the [99mTc]HYNIC core.
3.10.3. Reduction-Substitution
The reduction/substitution route involves the use of a reducing chelator, such as bidentate 1,2-bis[bis(2-ethyoxyethyl)phosphino]ethane [189, 190], or a monodentate ligand, such as triphenylphosphine-3,3’3”-trisulfonate (TPPTS) [167–169]. The reducing ligand/chelator serve for two purposes: reducing 99mTcO4− to a lower oxidation state and acting as ligand/chelator in bonding to the Tc. Examples of this type of reducing chelator or ligands include 1,2-bis[bis(2-ethyoxyethyl)phosphino]ethane in 99mTc-Tetrofosmin [189, 190], and TPPTS in complexes [99mTc(HYNIC-BM)(tricine)(TPPTS)] [167–169]. If TPPTS is replaced by an imine-N containing heterocycle, SnCl2 has to be used to reduce 99mTcO4− [170]. TPPTS has also been used as a reducing agent for preparation of Tc-nitrido complexes [99mTcN(dithiocarbamate)2] [121, 122].
3.11. BFCs for 186/188Re-Labeling of Biomolecules
Rhenium is the group II congener of technetium. The coordination chemistry of rhenium is very similar (not identical) to that of technetium due to their periodic relationship. As a consequence, the BFCs developed for the 99mTc-labeling can be used for the 186/188Re-labeling of biomolecules [191–193]. As a matter of fact, many therapeutic rhenium radiopharmaceuticals have been developed on the basis of 99mTc imaging agents. Despite of their similarities, there are also significant differences in 99mTc and 186/188Re. For example, a stronger reducing agent is needed to reduce Re(VII) in 186/187ReO4− to a lower oxidation state due to slow reduction rate. The ternary ligand system (Figure 10: HYNIC-BM, tricine and TPPTS) works well for the 99mTc-labeling of small biomolecules [167–181]; but it has very limited success for the 186/188Re-labeling of the same biomolecules due to slow redox chemistry of rhenium. To avoid this problem, the preformed chelate approach is often the choice for successful 186/188Re-labeling of antibodies and their fragments [194–196]. A peptide-based BFC has also been successfully used for 186Re-labeling of monoclonal antibodies [197]. The chemistry of rhenium in nuclear medicine has been reviewed by Blower, Griffiths and their coworkers [192, 193].
Figure 10.
BFCs for 67/68Ga and 111In-labeling of biomolecules. The R group may be a biomolecule or a linker attached to the biomolecule.
4. BFCs for Radiolabeling of Biomolecules with Gallium and Indium Radionuclides
Current interest in the coordination chemistry of gallium stems, at least in large part, from potential applications of 68Ga-labeled biomolecules as PET imaging agents. 111In is a gamma emitter with the γ-photon energy of 173 (89%) and 247 keV (95%) and is widely used (second only to 99mTc) in gamma scintigraphy. The coordination chemistry and radiochemistry related to gallium and indium radionuclides have been reviewed recently [6, 7, 13].
4.1. Gallium and Indium Chemistry
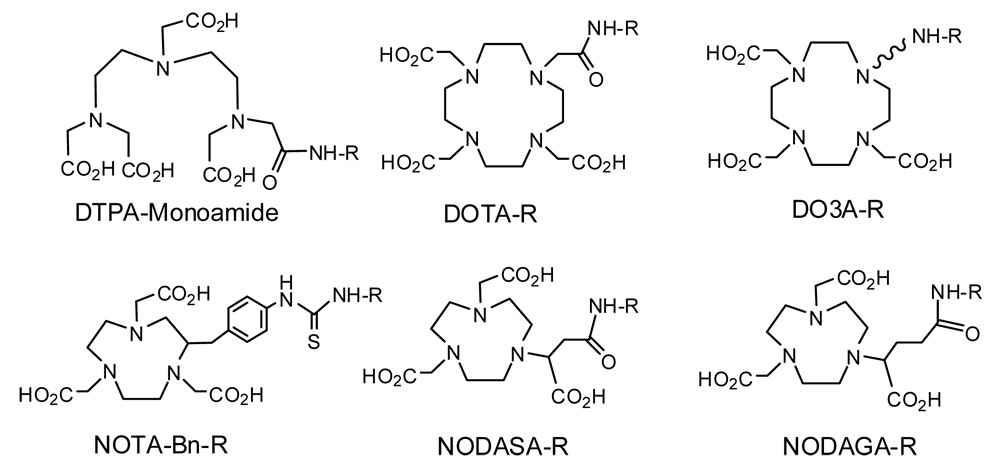
Both gallium and indium are group IIIB metals. The most prevalent oxidation state of gallium and indium in aqueous solution is +3. Due to their high charge density, Ga(III) and In(III) prefer hard donors, such as amine-N and carboxylate-O atoms. Because of the small size, Ga(III) is often six-coordinated [198–203]. Both Ga(III) and In(III) are similar to Fe(III) with respect to their coordination chemistry and biological properties. Since they are highly charged cations, hydrolysis of Ga(III) and In(III) at pH >4 remains a significant challenge during radiolabeling. Another challenge is the ligand exchange with transferrin after 68Ga and 111In radiopharmaceuticals are injected into biological system. It is no surprising that the BFCs for target-specific 68Ga and 111In radiopharmaceuticals are dominated by polydentate chelators (Figure 10) with hard donors, such as amine-N and carboxylate-N atoms. Among these macrocyclic and acyclic BFCs, NODASA (1,4,7- triazacyclononane-N-succinic acid-N’,N”-diacetic acid) and NODAGA (1,4,7- triazacyclononane-N-glutamic acid-N’,N”-diacetic acid) are particularly useful for chelation of 68Ga due to the perfect fit between the size of Ga(III) and coordination cavity formed by the N3O3 donor atoms [201–205]. The coordination cavity of DOTA (1,4,7,10- tetraazacyclododecane-1,4,7,10-tetraacetic acid) is too big for Ga(III), and only six (N4O2) out of N4O4 donors are used in bonding to Ga(III) [206]. As a result, the thermodynamic stability constant of Ga(NOTA) (log K = 30.98; NOTA = 1,4,7-triazacyclononane-1,4,7-triacetic acid) is much higher than that of Ga(DOTA) (log K = 21.33) [207].
4.2. Differences between Gallium and Indium
Despite their similarities, Ga(III) and In(III) are different in their size and charge density. This difference is often reflected by their different coordination chemistry with DTPA (diethylenetriaminepentaacetic acid) and DOTA derivatives. For example, Ga(III) has an ionic radius of 0.65 Å [208]. The coordination number of Ga(III) is 6 in its complexes, such as [Ga(Brbad)]ClO4·DMSO (H2Brbad = 1,10-bis(2-hydroxy-5-bromobenzyl)-1,4,7,10-tetra-azadecane) [209], Ga(DOTA-D-Phe-NH2) (DOTA-d-PheNH2 = 1,4,7,10-tetraazacyclododecane-4,7,10-tricarboxymethyl-1-yl-acetyl-d-Phe-NH2) [206], Ga(NODASA) [204], Ga(HDOTA) [210], and Ga(DO3A-xy-TPP)+ (DO3A-xy-TPP = triphenyl(4-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl)methyl)benzyl)phosphonium) [211]. In(III) has an ionic radius of 0.92 Å [208], and is either seven-coordinated in its complexes, such as In(DO3A-xy-TPP)+ [211] and In(DO3A) (DO3A = 1,4,7,10-tetraazacyclododecane-1,4,7-triacetate) [212], or 8-coordinated in In(DTPA)2− [213], In(DOTA-D-Phe-NH2) [208], In(DOTA-AA) (DOTA-AA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(p-aminoanilide)) [214], and In(DTPA-BA2) (BA = benzylamine) [215]. The structural difference has been attributed to the higher tumor uptake of 67Ga-DOTATOC than that of 111In-DOTATOC, and much lower kidney uptake of 67Ga-DOTATOC than that of 111In-DOTATOC [206].
4.3. BFCs for 68Ga and 111In-Labeling of Biomolecules
DTPA, DOTA and NOTA derivatives (Figure 10) are often used for the 68Ga and 111In-labeling of small biomolecules [204, 205, 216–222]. Among different BFCs, NODASA and NODAGA are particularly useful for 68Ga-labeling due to the high hydrophilicity and stability of their 68Ga chelates, and their higher 68Ga-labeling efficiency than that of the corresponding DOTA analogs [204, 217]. The fast and efficient radiolabeling is especially critical for the 68Ga-labeled small biomolecules due to its short half-life (t1/2 = 68 min). It is important to note that free 68Ga and 111In tend to localize in liver and lungs due to their strong binding capability to transferrin while 90Y and lanthanide isotopes are readily deposited on the bone [223].
5. BFCs for Radiolabeling of Biomolecules with Copper Radionuclides
5.1. Why Copper Radionuclides?
Copper has several radionuclides, such as 60Cu, 61Cu, 62Cu, 64Cu and 67Cu. The rich coordination chemistry of copper in combination with diverse nuclear properties of its radionuclides offers many opportunities for development of diagnostic (60Cu, 61Cu, 62Cu and 64Cu) and therapeutic (64Cu and 67Cu) radiotracers. The coordination chemistry of Cu(II) with various acyclic and macrocyclic chelators is well understood. In addition, the availability of 62Zn-62Cu generators and 64Cu with high specific activity makes it more feasible to develop target-specific radiopharmaceuticals with copper radionuclide. Copper radionuclide production, coordination chemistry, radiochemistry, and nuclear medicine applications have been reviewed exhaustively [6, 7, 13, 72, 224–228].
5.2. Copper Chemistry
Copper is a first-row transition metal. Its chemistry in aqueous solution is restricted to two oxidation states: Cu(I) and Cu(II). Cu(I) has the d10 configuration. Its complexes remain stable in aqueous solution only when chelators contain soft donors, such as phosphine-P and thioether-S. The coordination geometry for Cu(I) complexes are almost always tetrahedron. Cu(II) has the d9 configuration. The coordination number is 4, 5 or 6 depending on denticity of the chelator. The 4-coordinated Cu(II) complexes are normally square-planar while the square-pyramid coordination geometry is often seen in 5-coordinated Cu(II) complexes. In six-coordinated Cu(II) complexes, the two apical donor atoms are weakly bonded to the Cu(II) in a distorted octahedral arrangement, due to John-Teller distortion. Because of the d9 configuration, Cu(II) complexes are often kinetically labile with respect to ligand dissociation. Therefore, the design of BFCs (Figure 11) for copper radionuclides has been focused on macrocyclic chelators that are able to form Cu(II) complexes with both high thermodynamic stability and kinetic inertness [224–228]. BFCs for radiolabeling of biomolecules with copper radionuclide have been reviewed recently [225].
Figure 11.
BFCs for labeling of biomolecules with copper radionuclides. The R group may be a biomolecule or a linker attached to the biomolecule.
5.3. BFCs for Copper Radionuclides
Meares and coworkers reported the first use macrocyclic chelators, such as cyclam and cyclen (Figure 11), for 67Cu-labeling of monoclonal antibodies [229]. Since then, many DOTA and TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid) derivatives have been successfully used for the 64/67Cu-labeling of biomolecules [79–86, 230–237], including antibodies and small peptides. The 64/67Cu-labeled antibody conjugates often have high liver uptake and long liver retention, which was attributed to the transfer of 64/67Cu from the 64/67Cu-TETA chelate to ceruloplasim and/or superoxide dismutase in the liver [238 The cross-bridged cyclam derivatives (Figure 11) are developed to improve the stability of their 64/67Cu chelates. The 64Cu-labeling requires extensive incubation time at >75 °C to complete chelation of 64Cu [239, 240]. For small molecules, heating at elevated temperatures will not be a significant challenge as long as it can improve the radiolabeling efficiency. CB-TE2A (Figure 11: CB-TE2A = 2,2′-(1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diyl)diacetic acid) has also been used for 64Cu-labeling of bombesin peptides [241, 242]. It was found that the 64Cu-labeled CB-TE2A-bombesin conjugates have better in vivo stability than their DOTA analogs. SarAr (Figure 11: SarAr = N1-(4-aminobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1,8-diamine) is a relatively new BFC, and has been conjugated to the whole and fragmented B72.3 murine antibody [225, 245]. It was claimed that the resultant immunoconjugate was radiolabeled quantitatively using slight molar excess (<10%) of 64Cu. The 64Cu-labeling was reported to be significantly faster than other macrocyclic BFCs under the same reaction conditions [245]. It must be noted that such a high 64Cu-labeling efficiency is truly remarkable, and is extremely intriguing. However, small biomolecules should be used to demonstrate if 64Cu is indeed attached to the SarAr BFC or simply bonded to amino acid resides (histidine and cysteine) of the whole or fragmented B72.3 murine antibody. Recently, Prasanphanich and coworkers reported the use of 64Cu-labeled NOTA and DOTA-bombesin conjugates for imaging gastrin-releasing peptide receptor-positive tumors [246]. It has been clearly demonstrated that the NOTA chelator has high 64Cu-labeling efficiency, and its 64Cu chelate has very high solution stability. Results from both biodistribution and microPET imaging studies indicate that the 64Cu-labeled NOTA-bombesin conjugates have very little or no in vivo dissociation of 64Cu from the radiotracer [246].
6. BFCs for Radiolabeling of Biomolecules of Yttrium and Lanthanide Radionuclides
6.1. Requirements for Therapeutic Radiopharmaceuticals
While diagnostic radiotracers rely on high T/B ratios in a short period of time, the success of tumor radiotherapy depends on high concentration of radionuclide in tumor for a long duration [3–7, 12, 15, 16]. Thus, an ideal therapeutic radiopharmaceutical must have the following characteristics: high tumor uptake, high tumor-to-background ratio, long tumor residence time, and fast clearance. High tumor uptake and fast renal clearance are important to improve the T/B ratio and to reduce radiation burden to normal organs, such as kidneys and bone marrow. The radiopharmaceutical must have high RCP ≥ 90% and high solution stability. Since the radiopharmaceutical is manufactured in a centralized facility, it must retain its chemical and biological integrity during storage and transportation. This requires that the BFC form a metal chelate with high thermodynamic stability and kinetic inertness. Once again, the coordination chemistry of BFCs plays a significant role in the development of therapeutic radiopharmaceuticals [4, 6, 12].
6.2. Fundamentals of Yttrium and Lanthanide Chemistry
Yttrium and lanthanide metals favor the +3 oxidation state. Due to their similar charge, ionic radii (Figure 12) and coordination chemistry, yttrium is often treated as a “pseudo-lanthanide” metal. Since the 4f electrons are inner electrons, shielded from external influences by overlying 5s2, 5p6 and 6s2- electron shells, and are normally not involved in the bonding, interactions between “hard”-donor atoms, such as amine-N and carboxylate-O, and lanthanide metal ions are predominately ionic. Free Y(III) and Ln(III) ions are coordinated by a number of water molecules in aqueous solution. The metal chelate formation involves replacement of water molecules by a polydentate chelator. Due to their large size, coordination numbers of Y(III) and Ln(III) ions are typically between 7 and 10. While few six coordinate species are known, coordination numbers of 8 and 9 are very common in Y(III) and Ln(III) complexes with polydentate chelators [247, 248].
Figure 12.
Ionic radii (Å) for trivalent yttrium and lanthanide ions (data from ref. 208).
6.2.1. Hydrolysis and Precipitation
One of the characteristics of Y(III) and Ln(III) cations in aqueous solution is their easy precipitation with commonly occurring anions like hydroxide, phosphate or carbonate. Both phosphate and carbonate are able to compete for Y(III) and Ln(III) with the BFC-BM conjugate. The effect of metal hydroxide formation may not play a significant role in the release of radionuclide from radiometal chelate, mainly due to the presence of more phosphate and carbonate anions in the blood circulation. The high affinity of Y(III) and Ln(III) for phosphate anions may also explain their affinity for the bone.
6.2.2. Thermodynamic Stability
Once the radiopharmaceutical is injected into the blood stream, its concentration may become so low that dissociation of the radiometal from its metal chelate will eventually become favored. Loss of radiometal may result in accumulation of radioactivity in non-target organs. It has been reported that 90Y and lanthanide isotopes are readily deposited on the bone [223]. If free 90Y is injected in a human subject, about 50% of the injected dose will localize in the bone, 25% of the injected dose will go to liver, 10% of the injected dose is evenly distributed in many other organs while only 15% of the injected dose is excreted via renal system. Thus, the BFC must form a metal chelate with high thermodynamic stability to retain its chemical integrity in competition with natural chelators, such as tranferrin. However, high thermodynamic stability is not the sole requirement because it only reflects the direction, not the rate, of the dissociation reaction. As a matter of fact, the solution stability of a radiopharmaceutical in the blood is predominantly determined by the kinetic inertness, not thermodynamic stability, of the metal chelate.
6.2.3. Kinetic Inertness
The term kinetic inertness refers to the rate of dissociation of the radionuclide from a metal chelate. Dissociation kinetics plays a significant role for the in vivo stability of radiopharmaceuticals. While fast dissociation kinetics are characteristic of metal complexes of acyclic BFCs [249–259], an accumulated body of literature has shown that metal complexes of macrocyclic chelators are much more kinetically inert [260–267]. This has been demonstrated by the high solution stability of 90Y-labeled DOTA-BM conjugates even though the stability constant of Y(DOTA)− is comparable to that of Y(DTPA)2− [247, 248]. If DOTA is the BFC, acid-catalyzed dissociation of radionuclide from its metal chelate should be minimal in blood circulation. Recently, McMurry and coworkers studied 88Y-labeled antibodies with acyclic BFCs, and found that acid-catalyzed dissociation is not the dominant pathway for in vivo release of 88Y [257]. There are many receptors in the bone marrow or on the bone surface for radiotracers to bind. Some small radiometal chelates of polyaminocarboxylates, such as HEDTA (N-hydroxyethylethylenediamine-N,N,’N’-triacetic acid), often show very high bone uptake [268–271]. Therefore, accumulation of radioactivity in the bone may not be caused by loss of radionuclide from its metal chelate. The overwhelming importance on the acid-catalyzed dissociation of radionuclide found in the literature is, in some way, oversimplified. Biological studies in different animal models remain the most appropriate method for evaluating in vivo stability of the radiopharmaceutical.
It is important to note that the choice of kinetic characteristics for the metal chelate of a BFC is also dependent on pharmacokinetics of radiotracer. Radiolabeled antibodies have long biological half-lives in blood circulation and at the tumor site. Since they are often metabolized in liver, the radiometal chelate must have extremely high thermodynamic stability and kinetic inertness to withstand the competition from metal ions and native chelators, such as transferrin, in the blood circulation, and to tolerate the hepatobiliary metabolism. For the radiolabeled small biomolecules, their biological half-lives in the blood are much shorter than that of the radiolabeled antibodies and antibody fragments. The requirement of kinetic inertness for the BFC radiometal chelate may not be as demanding. The main goal in choosing a successful BFC is to minimize the in vivo dissociation of radionuclide from the radiometal chelate in radiopharmaceuticals.
6.3. BFCs for 90Y and Lanthanide Radionuclides
There are several requirements for an ideal BFC in chelation of 90Y and lanthanide radionuclides [5, 12]. The BFC must form a metal chelate with high thermodynamic stability and kinetic inertness in order to keep the metal chelate intact under physiological conditions. Decomposition of the metal chelate produces free metal ion, which may deposit on the bone and cause bone marrow toxicity. The BFC must form a metal chelate with minimum isomerism since the tumor uptake of a radiopharmaceutical depends not only on the receptor binding affinity of targeting biomolecules but also on the physical and chemical properties of both biomolecule and metal chelate. Formation of isomers may have a significant impact on physical and biological properties of the radiopharmaceutical. The BFC should have high hydrophilicity to improve blood clearance and renal excretion of the labeled and unlabeled BFC-BM conjugate. Fast renal clearance of unlabeled BFC-BM will minimize its competition with the radiolabeled BFC-BM bioconjugate for receptors. In addition, the BFC has to be able to withstand radiolysis because a large dose of β-radiation can produce free radicals and result in a significant amount of decomposition of the metal chelate during the manufacturing process and transportation.
6.3.1. Denticity Requirement
The denticity requirement of a BFC is largely dependent on the size and coordination geometry preference of the metal ion. Yttrium and lanthanide metal ions are large and need 8 – 9 donor atoms to complete the coordination sphere [12, 247, 248]. It is not surprising that most of BFCs (Figure 13) contain at least eight donor atoms. It should be noted that the denticity requirement for 90Y and lanthanide radiopharmaceuticals is different from that for MRI contrast agents. For MRI contrast agents, the chelator is most likely hepta- or octadentate, leaving at least one site open for water coordination to enhance the proton relaxation rates. For therapeutic radiopharmaceuticals, higher denticity may provide the enhanced thermodynamic stability and the improved kinetic inertness, particularly when extra donors are incorporated into a chelating arm attached to the macrocyclic framework.
Figure 13.
Selected acyclic and macrocyclic BFCs for the radiolabeling of biomolecules with yttrium and lanthanide radionuclides.
6.3.2. Selectivity of BFCs
The high selectivity for Y(III) and Ln(III) ions can be achieved by using macrocyclic BFCs with 8 or more donor atoms. In this respect, DOTA derivatives are particularly useful for 90Y and lanthanide therapeutic radiopharmaceuticals. The macrocyclic framework is well organized so that they form metal complexes with extremely high thermodynamic stability and kinetic inertness. The low pKa values (2 – 5) of carboxylic groups result in less competition from protons and minimum acid-assisted demetallation. The acetate chelating arms have low molecular weight so that the contribution of BFC to overall molecular weight of the BFC-BM conjugate is minimized. The high hydrophilicity of acetate chelating arms will favor faster clearance from blood, liver and kidneys. Recently, Brechbiel et al reported 2,2′-(2-(4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl)ethylazanediyl)diacetic acid (Figure 13: BCNOTA) as the BFC for the 86Y-chelation. It was found that BCNOTA has very high radiolabeling efficiency and forms the 86Y complex with high solution stability [272].
6.3.3. Attachment Position of Biomolecules
For illustration purpose, DOTA will be used as an example. Generally, there are three approaches to attach a biomolecule to a BFC. In the first approach, the attachment of the targeting biomolecule is at one of carbon atoms of the macrocycle (Figure 13). In the second approach, the targeting biomolecule is attached to one of four acetate chelating arms (Figure 13). In both cases, conjugation of the biomolecule does not lead to a significant change in thermodynamic stability and kinetic inertness of the metal chelate as compared to those of the DOTA chelate. In the third approach, the targeting biomolecule is conjugated to one of four acetate groups via a CO-NH amide bond (Figure 10 and Figure 13). Compared to the carboxylate-oxygen, the carbonyl-oxygen is a relatively weak donor for Y(III) and Ln(III) ions. This is consistent with the significantly lower thermodynamic stability of the Y(III) complex (e.g. KY-DOTA-monoamide ~ 21.8; KY-DOTA = 23.5) [273]. However, the kinetic inertness of the Y(III) complex remains relatively unchanged as evidenced by the high solution stability of 90Y-labeled DOTA-BM conjugates [263–267, 274, 275].
6.3.4. Conjugation Groups
A number of conjugation techniques have been developed for modification of biomolecules. The conjugation groups for attachment of a BFC to the biomolecule include anhydride, bromo- or iodoacetamide, isothiocyanate, N-hydroxysuccinimide (NHS) ester, and maleimide. These conjugation groups are electrophiles, which require a nucleophile functionality in the biomolecule. In some instances, the biomolecule of interest contains only electrophilic functionality (e.g. carboxylic acid), further synthetic elaboration is needed. In these cases, a bis-nucleophilic reagent, such as ethylenediamine or propylenediamine, is often used to convert the electrophilic functionality into a nucleophilic group. These reactive groups, whether they are naturally part of the biomolecule or artificially introduced, can serve as "handles" for conjugation of a BFC. Selection of conjugation group is largely dependent on the “handle” in biomolecules. Very often the “handle” is a primary amine or a thiol group. The functional groups reactive towards primary amines include DTPA dianhydride, NHS-activated esters, and isothiocyanates while maleimide is very reactive to thiols.
6.3.4.1. DTPA Anhydride
DTPA dianhydride is commercially available and reacts readily with primary amines to form the DTPA-biomolecule conjugate [216, 217, 249]. The reaction can be performed in both aqueous and non-aqueous media. There are two possible products: DTPA-monoamide and DTPA-bisamide (Chart IV). The cross-linking between two macromolecules (antibodies and antibody fragments) is disadvantageous, and may have dramatic impact of the biological and immunogenic properties of the DTPA-biomolecule conjugate. For small biomolecules, however, the cross-linking may prove to be beneficial for the improved receptor binding kinetics because simultaneous binding of two biomolecules on adjacent receptor sites will result in a slow dissociation of the receptor ligand. Asymmetric anhydrides of DTPA and DOTA have also been used to prepare their bioconjugates [249, 273, 274]. However, the yield is often very low [273].
Chart IV.
Conjugation Groups for Acyclic and Macrocyclic BFCs.
6.3.4.2. NHS-Ester
The NHS-esters (Chart IV) have intermediate reactivity toward amines, with high selectivity for aliphatic amines. The optimum pH for reaction in aqueous systems is 8.0 – 9.0. Virtually any molecule that contains a carboxylic group can be converted into its NHS ester, making NHS-activated ester groups among the most powerful and the most commonly used conjugation groups for antibodies and small biomolecules. Water-soluble NHS-activated ester has also been used for conjugation of biomolecules to DOTA [80, 275, 276].
6.3.4.3. Isothiocynates
Like NHS esters, isothiocyanates (Chart IV) are amine-reactive groups with intermediate reactivity, and form thiourea bonds with primary amines from proteins or small biomolecules. They are somewhat more stable in water than the NHS esters and react with amines in aqueous solution at pH 9.0 – 9.5. Isothiocyanates may not be suitable for modifying biomolecules sensitive to alkaline pH conditions. Aromatic isothiocyanates are often used to conjugate biomolecules onto DTPA and DOTA analogs [252–255, 260, 277–279].
6.3.4.4. Maleimide
Maleimide (Chart IV) is a thiol-reactive group [276, 280], and reacts selectively with a thiol to form a thioether bond without any interference from histidine and other reactive groups. The optimum pH for the reaction is near 7.0. At pH higher than 8.0, maleimides may hydrolyze to form non-reactive maleamic acids. Since many biomolecules contain no thiol groups, the use of maleimide as a conjugation group is in some way limited.
6.4. Radiolabeling of DTPA-BM Conjugates
A major advantage of using DTPA analogs as BFCs is their extremely high radiolabeling efficiency under mild conditions, but the kinetic lability of their metal chelates often results in dissociation of radiometal from the metal chelate. Stimmel et al studied the 90Y, 153Sm and 177Lu-chelation properties of DOTA and DTPA analogs [261, 262], and found that the 90Y-chelation efficiency of acyclic BFCs (Figure 13: DTPA, nitro-CHX-A-DTPA, and nitro-MX-DTPA) was much higher than that of macrocyclic BFCs, and that trace metal contamination had minimal effect on radiolabeling.
6.5. Radiolabeling of DOTA-BM Conjugates
The advantage of using DOTA analogs as BFCs is the kinetic inertness of their radiometal chelates. However, the radiolabeling kinetics of DOTA-based BFCs is normally very slow, and more dependent on radiolabeling conditions [220, 261–267], including DOTA-BM concentration, pH, reaction temperature and heating time, buffering agent and buffer concentration, and presence of other metal ions, such as Zn(II) and Fe(III). At room temperature, the radiolabeling yield of the DOTA-BM conjugate is very low. Therefore, heating at the elevated temperatures (>50 °C) is needed for successful radiolabeling [220, 261–267]. The high temperature radiolabeling may not cause any significant degradation of radiolabeled small biomolecules; but it often causes a significant loss of immunoreactivity of radiolabeled antibodies. In spite of the high solution stability of their radiometal chelates, slow radiolabeling kinetics remains a major obstacle for the wide use of DOTA analogs as BFCs in target-specific radiopharmaceuticals. Hopefully, the successful use of BCNOTA (Figure 13) will help overcome this problem for the 90Y-labeled monoclonal antibodies [272].
6.6. Differences between 90Y and 111In-Labeled Biomolecules
Many acyclic and macrocyclic BFCs have been used for 90Y and 111In-labeling of biologically active molecules. While the 90Y-labeled BFC-BM bioconjugates are used for radiotherapy, the 111In-labeled BFC-BM bioconjugates are often used as surrogates for imaging and dosimetry determination since 90Y has no γ-emission. 111In has a half-life (t1/2 = 2.8 days) almost identical to that of 90Y (t1/2 = 2.7 days). Although In(III) and Y(III) share very similar coordination chemistry, results from recent literature have shown different biodistribution patterns between 90Y and 111In-labeled BFC-BM bioconjugates [282–284]. Before using 111In-labeled BFC-BM bioconjugates as imaging surrogates, several critical questions need to be addressed. Are the 90Y and 111In-labeled BFC-BM bioconjugates biologically equivalent? What are the factors contributing to their differences, if any? How does the radiometal chelate and PKM linkers affect biological properties of the radiolabeled BFC-BM bioconjugates?
6.6.1. Structure Differences
In(III) and Y(III) are trivalent metal cations. The main difference is their size. As a result, In(III) and Y(III) often have different coordination chemistry with DTPA and DOTA derivatives. For example, Y(III) has an ionic radius of 1.02 Å [208], which fits perfectly to the cavity of DOTA. Y(III) complexes with DOTA derivatives are eight-coordinated and maintain their rigid structure in solution [206, 214, 220]. In(III) has an ionic radius of 0.92 Å [208]. The coordination number of In(III) is 6 or 7 [200, 206, 209–212]. Only a few eight-coordinated In(III) complexes are known [212–215]. Due to its smaller size, In(III) does not fit to the cavity of DOTA. Although In(III) is shown to be eight-coordinated in solid state of In(DOTA-AA) [214], the carbonyl-oxygen may become dissociated in solution. As a result, In(DOTA-BA) and Y(DOTA-BA) show significant differences in their solution properties as demonstrated by their variable temperature 1H NMR spectra. Y(DOTA-BA) becomes fluxional only at > 60 °C while In(DOTA-BA) is fluxional at room temperature [220].
6.8.2. Differences in Lipophilicity
Onthanks et al reported the 111In and 90Y complexes of a DOTA-conjugated integrin αvβ3 antagonist TA138 [57]. By a reversed phase HPLC method, it was found that the retention time of 111In-TA138 is ~4.5 min shorter than that of 90Y-TA138. Since the only difference in 111In-TA138 and 90Y-TA138 is the radiometal, different HPLC retention times strongly suggest that In(III) and Y(III) do not share the same coordination sphere in solution. Similar lipophilicity differences were observed for the 111In and 90Y-labeled DOTA-BA [220], and cyclic RGD peptide DTPA conjugates [216, 281]. It is believed that the lipophilicity differences between 111In and 90Y-labeled DOTA-BM and DTPA-BM conjugates are caused by structural differences between In(III) and Y(III) chelates in solution. It must be emphasized that the lipophilicity difference between 111In and 90Y-labeled bioconjugates depends largely on biomolecules. For small biomolecules, this difference is detectable. For 111In and 90Y-labeled antibodies, the radiometal chelate is only a small portion of the radiolabeled bioconjugate; thereby it would be difficult to detect the difference in their lipophilicity.
6.6.3. Biological Equivalence
Onthanks et al reported the bioequivalence between 111In-TA138 and 90Y-TA138 using the c-neu Oncomouse® tumor model [57]. Despite their significant differences in lipophilicity [57], biodistribution data showed that 111In-TA138 and 90Y-TA138 are biologically equivalent with respect to their uptake in tumors and other organs, such as liver, spleen, bone and kidneys. Thus, 111In-TA138 is useful as an imaging surrogate for 90Y-TA138, and should be able to accurately predict the biodistribution characteristics and radiation dosimetry of 90Y-TA138, the radiopharmaceutical useful for tumor radiotherapy.
Mäcke and coworkers [206] reported the solution stability, somatostatin receptor binding and biodistribution characteristics of the 90Y and 111In-labeled DOTA-D-Phe1-Tyr3-Octreotide (DOTATOC). The solution stability of 111In-DOTATOC and 90Y-DOTATOC in the blood serum is very high with half-lives for radiometal exchange being 1850 h and 2100 h, respectively [206]. This suggests that there is no significant release of radionuclide from 111In-DOTATOC and 90Y-DOTATOC. The IC50 values for 111In-DOTATOC (2.57±0.2 nM) and 90Y-DOTATOC (2.2±0.3 nM) indicated that there was no difference in the receptor binding affinity of 111In-DOTATOC and 90Y-DOTATOC. Biodistribution data of 111In-DOTATOC and 90Y-DOTATOC in nude mice bearing the AR4-2J tumor xenografts also showed that there was no significant difference in kidney and tumor uptake for 111In-DOTATOC and 90Y-DOTATOC at 4 h and 24 h post-injection. These data clearly demonstrated that 111In-DOTATOC and 90Y-DOTATOC are biologically equivalent even though In(III) and Y(III) do not share the same coordination chemistry in their radiometal chelate [206, 220]. It should be noted that the metal chelate is only a part of 111In or 90Y-labeled DOTA-BM conjugate. 111In and 90Y chelates may have different solution structures, which causes a slight difference in lipophilicity between the 111In-DOTA-BM and 90Y-DOTA-BM. Ultimately, it will be the biological equivalence that determines whether the 111In-labeled DOTA-BM conjugate can be used to accurately predict the radiation dosimetry of its 90Y analog.
7. Conclusions
There is a tremendous effort in the development of target-specific radiopharmaceuticals for early detection of diseases and radiotherapy of cancer. This effort relies heavily on the use of radiolabeled receptor ligands. Because of their high specificity and selectivity, the radiolabeled receptor ligands offer advantages over traditional perfusion radiotracers. Identification of targets and receptor ligands is critical for successful development of receptor-based radiotracers. Eventually, the challenge remains to be fundamentals of bioconjugate chemistry for target-specific delivery of radionuclide to the diseased tissues. There is no such an easy task that one can simply attach a radiometal chelate onto the selected biomolecule without significantly changing its receptor binding affinity and biodistribution characteristics.
Coordination chemistry plays a significant role in the design of BFCs, radiolabeling kinetics, solution stability, modification of pharmacokinetics, and formulation development. Because the receptor population in the diseased organ or tissues is often limited in numbers, using a large amount of BFC-BM bioconjugate may block receptor binding of the radiolabeled BFC-BM bioconjugate. Thus, selection of an appropriate BFC and optimization of its radiolabeling efficiency should be a significant part of the radiopharmaceutical development. The choice of BFC depends on the nature and oxidation state of radiometal, and requires a good understanding of its coordination chemistry of the radiometal to be labeled. The main objective is to select an efficient bifunctional coupling or chelating system that forms the radiometal chelate with very high thermodynamic stability and kinetic inertness. The ultimate goal is to develop a new generation of target-specific radiopharmaceuticals that will satisfy the unmet medical need for early detection of diseases or systemic radiotherapy of cancers.
List of Abbreviations for Common Chelators
- BCNOTA
2,2′-(2-(4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl)ethylazanediyl)diacetic acid
- CB-TE2A
2,2′-(1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diyl)diacetic acid
- DADS
N2S2 diamidedithiols
- DO3A
1,4,7,10-tetraazacyclododecane-1,4,7-triacetate
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DOTA-AA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(p-aminoanilide)
- DOTA-D-PheNH2
1,4,7,10-tetraazacyclododecane-4,7,10-tricarboxymethyl-1-yl-acetyl-D-Phenylalanine-amide)
- DTPA
diethylenetriaminepentaacetic acid
- HEDTA
N-hydroxyethylethylenediamine-N,N,’N’-triacetic acid
- HYNIC
6-hydrazinonicotinamide
- MAMA
monoamidemonoaminedithiols
- map
2,3-bis(mercaptoacetamido)propanoate
- mapt
4,5-bis(thioacetamido)pentanoate
- NOTA
1,4,7-triazacyclononane-1,4,7-triacetic acid
- NODAGA
1,4,7-triazacyclononane-N-glutamic acid-N’,N”-diacetic acid
- NODASA
1,4,7-triazacyclononane-N-succinic acid-N’,N”-diacetic acid
- SarAr
N1-(4-aminobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1,8-diamine
- TETA
1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banerjee S, Pillai MRA, Ramamoorthy N. Evolution of Tc-99m in diagnostic radiopharmaceuticals. Semin. Nucl. Med. 2001;31:260–277. doi: 10.1053/snuc.2001.26205. [DOI] [PubMed] [Google Scholar]
- 2.Jain D. Technetium-99m labeled myocardial perfusion imaging agents. Semin. Nucl.Med. 1999;29:221–236. doi: 10.1016/s0001-2998(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 3.Jurisson SS, Lydon JD. Potential technetium small molecule radiopharmaceuticals. Chem. Rev. 1999;99:2205–2218. doi: 10.1021/cr980435t. [DOI] [PubMed] [Google Scholar]
- 4.Liu S. Ether and crown ether-containing cationic 99mTc complexes useful as radiopharmaceuticals for heart imaging. Dalton Trans. 2007:1183–1193. doi: 10.1039/b618406e. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Edwards DS. Fundamentals of receptor-based diagnostic metalloradiopharmaceuticals. Topics in Current. Chem. 2002;222:259–278. [Google Scholar]
- 6.Liu S. The role of coordination chemistry in development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 2004;33:1–18. doi: 10.1039/b309961j. [DOI] [PubMed] [Google Scholar]
- 7.Reichert DE, Lewis JS, Anderson CJ. Metal complexes as diagnostic tools. Coord.Chem. Rev. 1999;184:3–66. [Google Scholar]
- 8.McEwan AJB. Unsealed source therapy of painful bone metastases: an update. Semin.Nucl. Med. 1997;27:165–182. doi: 10.1016/s0001-2998(97)80046-3. [DOI] [PubMed] [Google Scholar]
- 9.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem. Rev. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Edwards DS, Barrett JA. 99mTc-labeling of highly potent small peptides. Bioconj. Chem. 1997;8:621–636. doi: 10.1021/bc970058b. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Edwards DS. 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem. Rev. 1999;99:2235–2268. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Edwards DS. Bifunctional chelators for target specific therapeutic lanthanide radiopharmaceuticals. Bioconj. Chem. 2001;12 doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CJ, Welch MJ. Radiometal labeled agents (non-technetium) for diagnostic imaging. Chem. Rev. 1999;99:2219–2234. doi: 10.1021/cr980451q. [DOI] [PubMed] [Google Scholar]
- 14.Heeg MJ, Jurisson SS. The role of inorganic chemistry in the development of radiometal agents for cancer therapy. Acc. Chem. Res. 1999;32:1053–1060. [Google Scholar]
- 15.Illidge TM, Brock S. Radioimmunotherapy of cancer: using monoclonal antibodies to target radiotherapy. Current Pharmaceutical Design. 2000;6:1399–1418. doi: 10.2174/1381612003399257. [DOI] [PubMed] [Google Scholar]
- 16.Vriesendorp HM, Quadri SM, Borchardt PE. Tumor therapy with radiolabeled antibodies: optimization of therapy. BioDrugs. 1998;10:275–293. doi: 10.2165/00063030-199810040-00003. [DOI] [PubMed] [Google Scholar]
- 17.Potamianos S, Varvarigou AD, Archimandritis SC. Radioimmunoscintigraphy and radioimmunotherapy in cancer: Principles and applications. Anticancer Res. 2000;20:925–948. [PubMed] [Google Scholar]
- 18.Wun T, Kwon DS, Tuscano JM. Radioimmunotherapy: potential as a therapeutic strategy in non-Hodgekin’s lymphoma. BioDrugs. 2001;15:151–162. doi: 10.2165/00063030-200115030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Witzig TE. The use of ibritumomab tiuxetan radioimmunotherapy for patients with relapsed B-cell non-Hodgekin’s lymphoma. Semi. Oncol. 2000;27 suppl. 12:74–78. [PubMed] [Google Scholar]
- 20.Culy CR, Lamb HM. 131I Tositumomab. BioDrugs. 2000;14:195–202. doi: 10.2165/00063030-200014030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Chatal JF, Hoefnagel CA. Radionuclide therapy. Lancet. 1999;354:931–935. doi: 10.1016/S0140-6736(99)06002-X. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu H, Tenhunen M. Physical and biological targeting of radiotherapy. Acta Oncologica. 1999;38 Suppl. 13:75–83. doi: 10.1080/028418699432806. [DOI] [PubMed] [Google Scholar]
- 23.Britton KE. Towards the goal of cancer-specific imaging and therapy. Nucl. Med.Commun. 1997;18:992–1007. doi: 10.1097/00006231-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC. Regulatory peptides receptors as molecular targets for cancer diagnosis and therapy. Q. J. Nucl. Med. 1997;41:63–70. [PubMed] [Google Scholar]
- 25.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localization and therapy with radiopeptides. Current Med. Chem. 2000;7:971–994. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 26.Lister-James J, Moyer BR, Dean RT. Small peptides radiolabeled with Tc-99m. Q. J.Nucl. Med. 1996;40:221–233. [PubMed] [Google Scholar]
- 27.Blok D, Feitsma RIJ, Vermeij P, Pauwel EJK. Peptide radiopharmaceuticals in nuclear medicine. Eur. J. Nucl. Med. 1999;26:1511–1519. doi: 10.1007/s002590050488. [DOI] [PubMed] [Google Scholar]
- 28.Okarvi SM. Recent developments in Tc-99m-labelled peptide-based radiopharmaceuticals: an overview. Nucl. Med. Commun. 1999;20:1093–1112. doi: 10.1097/00006231-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J.Nucl. Med. 2000;41:1704–1713. [PubMed] [Google Scholar]
- 30.Boerman OC, Oyen WJG, Corstens FHM. Radiol-labeled receptor-binding peptides:A new class of radiopharmaceuticals. Semi. Nucl. Med. 2000;30:195–208. doi: 10.1053/snuc.2000.7441. [DOI] [PubMed] [Google Scholar]
- 31.Ercan MT, Caglar M. Radiopharmaceuticals for the visualization of infectious and inflammatory lessons. Current Pharmaceutical Design. 2000;6:1159–1177. doi: 10.2174/1381612003399914. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Edwards DS. New Radiopharmaceuticals for imaging infection and inflammation. Drugs of the Future. 2001;26:375–382. [Google Scholar]
- 33.Liu S. HYNIC derivatives as bifunctional coupling agents for 99mTc-labeling of small biomolecules. Topics in Current Chem. 2005;252:193–216. [Google Scholar]
- 34.Liu S, Robinson SP, Edwards DS. Radiolabeled integrin αvβ3 antagonists as radiopharmaceuticals for tumor radiotherapy. Topics in Current Chem. 2005;252:193–216. [Google Scholar]
- 35.Signore A, Annovazzi A, Chianelli M, Coretti F, Van de Wiele C, Watherhouse RN, Scopinaro F. Peptide radiopharmaceuticals for diagnosis and therapy. Eur. J. Nucl. Med. 2001;28:1555–1565. doi: 10.1007/s002590100583. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman TJ, Quinn TP, Volkert WA. Radiometallated receptor-avid peptide conjugates for specific in vivo targeting of cancer cells. Nucl. Med. Biol. 2001;28:527–539. doi: 10.1016/s0969-8051(01)00209-8. [DOI] [PubMed] [Google Scholar]
- 37.Langer M, Beck-Sichinger AG. Peptides as carriers for tumor diagnosis and treatment. Curr. Med. Chem.–Anti-Cancer Agents. 2001;1:71–93. doi: 10.2174/1568011013354877. [DOI] [PubMed] [Google Scholar]
- 38.Weiner RE, Thakur ML. Radiolabeled peptides in the diagnosis and therapy of oncological diseases. Appl. Radiat. Isot. 57;2002:749–763. doi: 10.1016/s0969-8043(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 39.de Jong M, Kwekkeboom D, Valkema R, Krenning EP. Radiolabelled Peptides for tumor therapy: current status and future directions. Eur. J. Nucl. Med. 2003;30:463–469. doi: 10.1007/s00259-002-1107-8. [DOI] [PubMed] [Google Scholar]
- 40.Fichna J, Janecka A. Synthesis of target-specific radiolabeled peptides for diagnostic imaging. Bioconj. Chem. 2003;14:3–17. doi: 10.1021/bc025542f. [DOI] [PubMed] [Google Scholar]
- 41.Behr TM, Gotthardt M, Barth A, Béhé M. Imaging tumors with peptide-based radioligands. Q. J. Nucl. Med. 2001;45:189–200. [PubMed] [Google Scholar]
- 42.Liu S, Robinson SP, Edwards DS. Integrin αvβ3 directed radiopharmaceuticals for tumor imaging. Drugs of the Future. 2003;28:551–564. [Google Scholar]
- 43.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3-targeted radiotracers for tumor imaging. Molecular Pharmaceuticals. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 44.Quadri SM, Vriesendorp HM. Effects of linker chemistry on pharmacokinetics of radioimmunoconjugates. Q. J. Nucl. Med. 1998;42:250–261. [PubMed] [Google Scholar]
- 45.Williams LE, Lewis MR, Bebb GG, Clarcke KG, Odom-Maryon TL, Shively JE, Raubitscheck AA. Biodistribution of 111In- and 90Y-labeled DOTA and maleimidocysteineamido-DOTA conjugated to chimeric anticarcinoembryonic antigen antibody in xenograft-bearing nude mice: comparison of stable and chemically labile linker systems. Bioconj. Chem. 1998;9:87–93. doi: 10.1021/bc970137n. [DOI] [PubMed] [Google Scholar]
- 46.Arano Y, Matsushima H, Tagawa M, Koizumi M, Endo K, Konish J, Yokoyama A. A novel bifunctional metabolizable linker for the conjugation of antibodies with radionuclides. Bioconj. Chem. 1991;2:71–76. doi: 10.1021/bc00008a001. [DOI] [PubMed] [Google Scholar]
- 47.Smith-Jones PM, Stolz B, Albert R, Knecht H, Bruns C. Synthesis, biodistribution and renal handling of various chelate-somatostatin conjugates with metabolizable linking groups. Nucl. Med. Biol. 1997;24:761–769. doi: 10.1016/s0969-8051(97)00112-1. [DOI] [PubMed] [Google Scholar]
- 48.Studer M, Meares CF. A convenient and flexible approach for introducing linkers on bifunctional chelating agents. Bioconj. Chem. 1992;3:420–423. doi: 10.1021/bc00017a011. [DOI] [PubMed] [Google Scholar]
- 49.Li M, Meares CF. Synthesis, metal chelate stability studies, and enzyme digestion of a peptide-linked DOTA derivative and its corresponding radiolabeled immunoconjugates. Bioconj. Chem. 1993;4:275–283. doi: 10.1021/bc00022a005. [DOI] [PubMed] [Google Scholar]
- 50.Peterson JJ, Meares CF. Enzymatic cleavage of peptide-linked radiolabeles from immunoconjugates. Bioconj. Chem. 1999;10:553–557. doi: 10.1021/bc990010t. [DOI] [PubMed] [Google Scholar]
- 51.Peterson JJ, Meares CF. Cathepsin substrate as cleavable peptide linkers in bioconjugates, selected from a fluorescence quench combinatorial library. Bioconj.Chem. 1998;9:618–626. doi: 10.1021/bc980059j. [DOI] [PubMed] [Google Scholar]
- 52.Haubner R, Wester HJ, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. RGD-peptides for tumor targeting: biological evaluation of radioiodinated analogs and introduction of a novel glycosylated peptide with improved biokinetics. J. Labelled Compd. Radiopharm. 1997;40:383–385. [Google Scholar]
- 53.Haubner R, Wester HJ, Reuning U, Senekowisch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor imaging. J. Nucl. Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 54.Haubner R, Wester HJ, Burkhart F, Senekowisch-Schmidtke R, Weber WA, Goodman SL, Kessler H, Schwaiger M. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J. Nucl. Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 55.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowisch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 56.Harris TD, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars CE, Edwards DS, Onthanks D, Silva P, Yalamanchili P, Robinson SP, Lazewatsky J, Barrett JA, Bozarth J. Design, synthesis and evaluation of radiolabeled integrin αvβ3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biotherapy & Radiopharmaceuticals. 2003;18:627–641. doi: 10.1089/108497803322287727. [DOI] [PubMed] [Google Scholar]
- 57.Onthank DC, Liu S, Silva PJ, Barrett JA, Harris TD, Robinson SP, Edwards DS. 90Y and 111In complexes of a DOTA-conjugated integrin αvβ3 receptor antagonist: different but biologically equivalent. Bioconj. Chem. 2004;15:235–241. doi: 10.1021/bc034108q. [DOI] [PubMed] [Google Scholar]
- 58.Harris TD, Kalogeropoulos S, Nguyen T, Dwyer G, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Robinson S, Lazewatsky J, Barrett JA. Structure-activity relationships of 111In- and 99mTc-labeled quinolin-4-one peptidomimetics as ligands for the vitronectin receptor: potential tumor imaging agents. Bioconj. Chem. 2006;17:1294–1313. doi: 10.1021/bc060063s. [DOI] [PubMed] [Google Scholar]
- 59.Harris TD, Cheesman E, Harris AR, Sachleben R, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Silva P, Robinson S, Lazewatsky J, Rajopadhye M, Barrett JA. Radiolabeled divalent peptidomimetic vitronectin receptor antagonists as potential tumor radiotherapeutic and imaging Agents. Bioconj. Chem. 2007;18:1266–1279. doi: 10.1021/bc070002+. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl. Med. Biol. 2004;31:11–19. doi: 10.1016/j.nucmedbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, Bremner R, Bading JR, Conti PS. Integrin αvβ3-targeted imaging of lung cancer. Neoplasia. 2005;7:271–279. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, He Z, Hsieh W, Kim Y, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconj. Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dijkgraaf I, Liu S, Kruijtzer JAW, Soede AC, Oyen WJG, Liskamp RMJ, Corstens FHM, Boerman OC. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD Peptide. Nucl. Med. Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Ruth TJ, Pate BD, Robertson R, Porter JK. Radionuclide production for biosciences. Nucl. Med. Biol. 1989;16:323–336. [Google Scholar]
- 65.Ehrhardt GJ, Ketring AR, Ayers LM. Reactor-produced radionuclides at the University of Missouri Research Reactior. Appl. Radiat. Isot. 1998;49:295–297. doi: 10.1016/s0969-8043(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 66.Karelin YA, Toporov YG. RIAR reactor produced radionuclides. Appl. Radiat. Isot. 1998;49:299–304. [Google Scholar]
- 67.Knapp FF, Jr, Mirzadeh S, Beets AL, O’Doherty M, Blower PJ, Verdera ES, Gaudiano JS, Kropp J, Gihlke J, Palmedo H, Biersack HJ. Reactor-produced radioisotopes from ORNL for bone pain palliation. Appl. Radiat. Isot. 1998;49:309–315. doi: 10.1016/s0969-8043(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 68.Schubiger PA, Alberto R, Smith A. Vehicles, chelators, and radionuclides: choosing the “building blocks” of an effective therapeutic radioimmunoconjugate. Bioconj. Chem. 1996;7:165–179. doi: 10.1021/bc950097s. [DOI] [PubMed] [Google Scholar]
- 69.Finn R. Chemistry applied to iodine radionuclides. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. New York: John Wiley & Sons; 2003. pp. 423–440. [Google Scholar]
- 70.Snider SE, Kilbourn MR. Chemistry of fluorine-18 radiopharmaceuticals. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. New York: John Wiley & Sons; 2003. pp. 195–227. [Google Scholar]
- 71.Welch MJ, McCarthy TJ. The potential role of generator-produced radiopharmaceuticals in clinical PET. J. Nucl. Med. 2000;41:315–317. [PubMed] [Google Scholar]
- 72.Anderson CJ, Green MA, Yashi YF. Chemistry of copper radionuclides and radiopharmaceutical products. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. New York: John Wiley & Sons; 2003. pp. 402–422. [Google Scholar]
- 73.Bormans G, Janssen A, Adriaens P, Crombez D, Witsenboer A, Degoeij J, Mortelmans L, Verbruggen A. 62Zn/62Cu generator for the routine production of 62Cu-PTSM. Appl. Radiat. Isot. 1992;43:1437–1441. [Google Scholar]
- 74.Haynes NG, Lacy JL, Nayak N, Martin CS, Dai D, Mathias CJ, Green MA. Performance of a 62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J. Nucl. Med. 2000;41:309–314. [PubMed] [Google Scholar]
- 75.Lim JK, Mathias CJ, Green MA. Mixed bis(thiosemicarbazone) ligands for the preparation of copper radiopharmaceuticals: synthesis and evaluation of tetradentate ligands containing two dissimilar thiosemicarbazone functions. J. Med. Chem. 1997;40:132–136. doi: 10.1021/jm9605703. [DOI] [PubMed] [Google Scholar]
- 76.Ackerman LJ, West DX, Mathias CJ, MA Green M. Synthesis and evaluation of copper radiopharmaceuticals with mixed bis(thiosemicarbazone) ligands. Nucl. Med.Biol. 1999;26:551–554. doi: 10.1016/s0969-8051(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 77.Mathias CJ, Green MA, Morrison WB, Knapp DW. Evaluation of Cu-PTSM as a tracer of tumor perfusion: comparison with labeled microsphere in spontaneous canine neoplasms. Nucl. Med. Biol. 1994;21:83–87. doi: 10.1016/0969-8051(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 78.Mathias CJ, Bergmann SR, Green MA. Species-dependent binding of copper(II) bis(thiosemicarbazone) Radiopharmaceuticals to serum albumin. J. Nucl. Med. 1995;36:1451–1455. [PubMed] [Google Scholar]
- 79.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol.Imag. Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET imaging of glioma αvβ3 integrin expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 81.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconj. Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 82.Chen X. Multimodality imaging of tumor integrin αvβ3 expression. Mini-Rev. Med.Chem. 2006;6:227–234. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- 83.Anderson CJ, Pajeau TS, Edwards EB, Sherman ELC, Rogers BE, Welch MJ. In vitro and in vivo evaluation of copper-64-octreotide conjugates. J. Nucl. Med. 1995;36:2315–2325. [PubMed] [Google Scholar]
- 84.Anderson CJ, Jones LA, Bass LA, Sherman ELC, McCarthy DW, Cutler PD, Lanahan MV, Cristel ME, Lewis JS, Schwarz SW. Radiotherapy, toxicity and dosimetry of In vitro and in vivo evaluation of copper-64 TETA-octrotide in tumor bearing rats. J. Nucl. Med. 1998;39:1944–1951. [PubMed] [Google Scholar]
- 85.Lewis JS, Srinivasan A, Schmidt MA, Anderson CJ. In vitro and in vivo evaluation of 64Cu-TETA- Tyr3-octreotate. A new somatostatin analog with improved target tissue uptake. Nucl. Med. Biol. 1999;26:267–273. doi: 10.1016/s0969-8051(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 86.Lewis JS, Lewis MR, Cutler PD, Srinivasan A, Schmidt MA, Schwarz SW, Morris MM, Miller JP, Anderson CJ. Radiotherapy and dosimetry of 64Cu-TETA- Tyr3-octreotate in a somatostatin receptor-positive, tumor-bearing rat model. Clin. Cancer Res. 1999;5:3608–3616. [PubMed] [Google Scholar]
- 87.Tisato F, Porchia M, Bolzati C, Refoso F, Vittadini A. The preparation of substitution-inert 99Tc metal-fragments: promising candidates for the design of new 99mTc radiopharmaceuticals. Coord. Chem. Rev. 2006;250:2034–2045. [Google Scholar]
- 88.Hansen L, Marzilli LG, Taylor A. The influence of stereoisomerism on the pharmacokinetics of Tc radiopharmaceuticals. Q. J. Nucl. Med. 1998;42:280–293. [PubMed] [Google Scholar]
- 89.Klingensmith WC, III, Fritzberg AR, Spitzer VM, Johnson DL, Kuni CC, Williamson MR, Washer G, Weil R., III Clinical evaluation of Tc-99m N,N’-bis(mercaptoacetyl)-2,3-diaminopropanoate as a replacement for I-131 hippurate: concise communication. J. Nucl. Med. 1984;25:42–48. [PubMed] [Google Scholar]
- 90.Rao TN, Adhikesavalu D, Camerman A, Fritzberg AR. Technetium(V) and rhenium(V) complexes of 2,3-bis(mercaptoacetamido)propanoate. Chelate ring stereochemistry and influence on chemical and biological properties. J. Am. Chem. Soc. 1990;112:5798–5804. [Google Scholar]
- 91.Liu S, Edwards DS. New N2S2 diamidedithiol and N3S triamidethiols as bifunctional chelating agents for labeling small peptides with technetium-99m. In: Nicolini M, Banoli G, Mazzi U, editors. Technetium and Rhenium in Chemistry and Nuclear Medicine. Vol. 4. Padova: SGEditorali; 1995. pp. 383–393. [Google Scholar]
- 92.Eshima D, Taylor A, Jr, Fritzberg AR, Kasina S, Hansen L, Sorenson JF. Animal evaluation of technetium-99m triamide mercaptide complexes as potential renal imaging agents. J. Nucl. Med. 1987;28:1180–1186. [PubMed] [Google Scholar]
- 93.Vanbilloen HP, De Roo MJ, Verbruggen AM. Complexes of technetium-99m with tetrapeptides containing one alanyl and three glycyl moieties. Eur. J. Nucl. Med. 1996;23:40–48. doi: 10.1007/BF01736988. [DOI] [PubMed] [Google Scholar]
- 94.Fritzberg AR, Abrams PG, Beaumier PL, Kasina S, Morgan AC, Rao TN, Reno JM, Sanderson JA, Srinivasan A, Wilbur DS, Vanderheyden JL. Specific and stable labeling of antibodies with technetium-99m with a diamide dithiolate chelating agent. Proc. Natl. Acad. Sci. USA. 1988;85:4025–4029. doi: 10.1073/pnas.85.11.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eary JF, Schroff RW, Abrams PG, Fritzberg AR, Morgan AC, Kasina S, Reno JM, Srinivasan A, Woodhouse CS, Wilbur DS, Natale RB, Collins C, Stehlin JS, Mitchell M, Nelp WB. Successful imaging of malignant melanoma with technetium-99m-labeled monoclonal antibodies. J. Nucl. Med. 1989;30:25–32. [PubMed] [Google Scholar]
- 96.Kasina S, Rao TN, Srinivasan A, Sanderson JA, Fitzner JN, Reno JM, Beaumier PL, Fritzberg AR. Development and biological evaluation of a kit for preformed chelate technetium-99m radiolabeling of an antibody Fab fragment using a diamide dimercaptide chelating agent. J. Nucl. Med. 1991;32:1445–1451. [PubMed] [Google Scholar]
- 97.Liu S, Edwards DS, Looby RJ, Harris AR, Poirier MJ, Rajopadhye M, Bourque JP, Carroll TR. Labeling cyclic IIb/IIIa receptor antagonists with 99mTc by the preformed chelate approach: effects of chelators on properties of [99mTc]chelator-peptide conjugate. Bioconj. Chem. 1996;7:196–202. doi: 10.1021/bc9500958. [DOI] [PubMed] [Google Scholar]
- 98.Barrett JA, Damphousse DJ, Heminway SJ, Liu S, Edwards DS, Looby RJ, Carroll TR. Biological evaluation of 99mTc-labeled cyclic GPIIb/IIIa receptor antagonists in the canine arteriovenous shunt and deep vein thrombosis models: effects of chelators on biological properties of [99mTc]chelator-peptide conjugates. Bioconj. Chem. 1996;7:203–208. doi: 10.1021/bc9500960. [DOI] [PubMed] [Google Scholar]
- 99.Rajopadhye M, Edwards DS, Bourque PJ, Carroll TR. Synthesis and technetium-99m labeling of cyclic GPIIb/IIIa receptor antagonists conjugated to 4,5-bis(mercaptoacetamido)pentanoic acid (mapt) Bioorg. & Med. Chem. Lett. 1996;6:1737–1740. [Google Scholar]
- 100.Lever SZ, Baidoo KE, Mahmood A. Structure proof of syn/anti isomerism in N-alkylated diaminedithiol (DADT) complexes of technetium. Inorg. Chim. Acta. 1990;176:183–184. [Google Scholar]
- 101.Walovitch RC, Cheesman EH, Maheu LJ, Hall KM. Studies of the retention mechanism of the brain perfusion imaging agent 99mTc-Bicisate (99mTc-ECD) J. Cerebral Blood Flow and Metabolism. 1994;14:S4–S11. [PubMed] [Google Scholar]
- 102.Harris TD, Edwards DS, Platts SH. Synthesis and characteristics of isomers L,L, D,D, and D,L of Tc-99m-ECD in monkeys. J. Nucl. Med. 1992;33:979–980. [Google Scholar]
- 103.Oya S, Kung MP, Frederick D, Kung HF. New bisaminoethanethiol (BAT) ligands which form two interconvertable Tc-99m complexes. Nucl. Med. Biol. 1995;22:749–757. doi: 10.1016/0969-8051(95)00021-o. [DOI] [PubMed] [Google Scholar]
- 104.Kung HF, Guo YZ, Yu CC, Billings J, Subramanyam V, Calabrese JC. New brain perfusion imaging agents based on 99mTc-bis(aminoethanethiol) complexes: stereoisomers and biodistribution. J. Med. Chem. 1989;32:433–437. doi: 10.1021/jm00122a024. [DOI] [PubMed] [Google Scholar]
- 105.Francesconi LC, Graczyk G, Wehrli S, Shaikh SN, McClinton D, Liu S, Zubieta J, Kung HF. Synthesis and characterization of neutral MVO (M = Tc, Re) Amine-thiol complexes containing a pendant phenylpiperidine group. Inorg. Chem. 1993;32:3114–3124. [Google Scholar]
- 106.Mach RH, Kung HF, Guo YZ, Yu CC, Subramanyam V, Calabrese JC. Synthesis, characterization and biodistribution of neutral and lipid-soluble 99mTc-PAT-HM and 99mTc-TMR for brain imaging. Nucl. Med. Biol. 1989;16:829–837. doi: 10.1016/0883-2897(89)90168-2. [DOI] [PubMed] [Google Scholar]
- 107.Baidoo KE, Lever SZ. Synthesis of a diaminedithiol bifunctional chelating agent for incorporation of technetium-99m into biomolecules. Bioconj. Chem. 1990;1:132–137. doi: 10.1021/bc00002a007. [DOI] [PubMed] [Google Scholar]
- 108.Eisenhut M, Lehmann WD, Becker W, Behr T, Elser H, Strittmatter W, Baum RP, Valerius T, Repp R, Deo Y. Bifunctional NHS-BAT ester for antibody conjugation and stable technetium-99m labeling: conjugation chemistry, immunoreactivity and kit formulation. J. Nucl. Med. 1996;37:362–370. [PubMed] [Google Scholar]
- 109.O'Neil JP, Wilson SR, Katzenellenbogen JA. Preparation and structural characterization of monoamine-monoamide bis(thiol) oxo complexes of technetium(V) and rhenium(V) Inorg. Chem. 1994;33:319–323. [Google Scholar]
- 110.DiZio JP, Fiacshi R, Davison A, Jones AG, Katzenellenbogen JA. Progestin-rhenium complexes: metal labeled steroids with high receptor binding affinity, potential receptor-directed agents for diagnostic imaging or therapy. Bioconj. Chem. 1991;2:353–366. doi: 10.1021/bc00011a011. [DOI] [PubMed] [Google Scholar]
- 111.O’Neil JP, Carlson KE, Anderson CJ, Welch MJ, Katzenellenbogen JA. Progestin radiopharmaceuticals labeled with technetium and rhenium: synthesis, binding affinity,and in vivo distribution of a new progestin N2S2-metal conjugate. Bioconj. Chem. 1994;5:182–193. doi: 10.1021/bc00027a002. [DOI] [PubMed] [Google Scholar]
- 112.DiZio JP, Anderson CJ, Davison A, Ehrhardt GJ, Carlson KE, Welch MJ, Katzenellenbogen JA. Technetium- and rhenium-labeled progestins: synthesis, receptor binding and in vivo distribution of an 11β-substituted progestin labeled with technetium-99 and rhenium-186. J. Nucl. Med. 1992;33:558–569. [PubMed] [Google Scholar]
- 113.Meegalla S, Plüssl K, Kung MP, Chumpradit S, Stevenson DA, Frederick D, Kung HF. Tc-99m-labeled tropanes as dopamine transporter imaging agents. Bioconj. Chem. 1996;7:421–429. doi: 10.1021/bc960029l. [DOI] [PubMed] [Google Scholar]
- 114.Rajagopalan R, Grummon GD, Bugaj J, Hallemann LS, Webb EG, Marmion ME, Vanderheyden JL, Srinivasan A. Preparation, characterization, and biological evaluation of technetium(V) and rhenium(V) complexes of novel heterocyclic tetradentate N3S ligands. Bioconj. Chem. 1997;8:407–415. doi: 10.1021/bc9700401. [DOI] [PubMed] [Google Scholar]
- 115.Wong E, Fauconnier T, Bennett S, Valliant J, Nguyen T, Lau F, Lu LFL, Pollak A, Bell RA, Thornback JR. Rhenium(V) and Technetium(V) Oxo Complexes of an N2N'S Peptidic Chelator: Evidence of Interconversion between the Syn and Anti Conformations. Inorg. Chem. 1997;36:5799–5808. doi: 10.1021/ic9706562. [DOI] [PubMed] [Google Scholar]
- 116.Lister-James J, Knight LC, Maurer AH, Bush LR, Moyer BR, Dean RT. Thrombus imaging with technetium-99m-labeled, activated platelet receptor binding peptide. J. Nucl. Med. 1996;37:775–781. [PubMed] [Google Scholar]
- 117.Muto P, Lastoria S, Varrella P, Vergara E, Salvatore M, Morgano G, Lister-James J, Bernardy JD, Dean RT, Wencker D, Borer JS. Detecting deep venous thrombosis with technetium-99m-labeled synthetic peptide P280. J. Nucl. Med. 1995;36:1384–1391. [PubMed] [Google Scholar]
- 118.Pearson DA, Lister-James J, McBride WJ, Wilson DM, Martel LJ, Civitello ER, Dean RT. Thrombus imaging using technetium-99m labeled high potency GPIIb/IIIa receptor antagonists. Chemistry and initial biological studies. J. Med. Chem. 1996;39:1372–1382. doi: 10.1021/jm950112e. [DOI] [PubMed] [Google Scholar]
- 119.Lister-James J, Vallabhajosula S, Moyer BR, Pearson DA, McBride BJ, De Rosch MA, Bush LR, Machac J, Dean RT. Pre-clinical evaluation of technetium-99m platelet receptor-binding peptide. J. Nucl. Med. 1997;38:105–111. [PubMed] [Google Scholar]
- 120.Marchi A, Marvelli L, Rossi R, Magon L, Bertolasi V, Ferretti V, Gilli P. Nitridoand oxo-technetium(V) chelate complexes with N2S2 ligands: Synthesis and crystal structures. J. Chem. Soc., Dalton Trans. 1992:1485–1490. [Google Scholar]
- 121.Duatti A, Marchi A, Pasqualini R. Formation of the Tc≡N multiple bond from the reaction of ammonium pertechnetate with S-methyl dithiocarbazate and its application to the preparation of technetium-99m radiopharmaceuticals. J. Chem. Soc., Dalton Trans. 1990:3729–3733. [Google Scholar]
- 122.Pasqualini R, Duatti A. Synthesis and characterization of the new neutral myocardial imaging agent [99mTcN(noet)2] (noet = N-ethyl-N-ethoxydithiocarbamato) J. Chem. Soc., Chem. Commun. 1992:1354–1355. [Google Scholar]
- 123.Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M. Bis(dithiocarbamato) nitrido technetium-99m radiopharmaceuticals: a class of neutral myocardial imaging agents. J. Nucl. Med. 1994;35:334–341. [PubMed] [Google Scholar]
- 124.Bolzati C, Boschi A, Duatti A, Prakash S, Uccelli L. Geometrically controlled selective formation of nitrido technetium(V) asymmetrical heterocomplexes with bidentate ligands. J. Am. Chem. Soc. 2000;122:4510–4511. [Google Scholar]
- 125.Bolzati C, Boschi A, Uccelli L, Tisato F, Refosco F, Cagnolini A, Duatti A, Prakash S, Bandoli G, Vittadini A. Chemistry of the strong electrophilic metal fragment [99mTc(N)(PXP)]2+ (PXP = diphosphine ligand). A novel tool for the selective labeling of small molecules. J. Am. Chem. Soc. 2002;124:11468–11479. doi: 10.1021/ja0200239. [DOI] [PubMed] [Google Scholar]
- 126.Bolzati C, Refosco F, Cagnolini A, Tisato F, Boschi A, Duatti A, Uccelli L, Dolmella A, Marotta E, Tubaro M. Synthesis, solution-state and solid-state structural characterization of monocationic nitrido heterocomplexes [M(N) (DTC)(PNP)]+ (M = 99Tc and Re; DTC = dithiocarbamate; PNP = heterodiphosphane) Eur. J. Inorg. Chem. 2004:1902–1913. [Google Scholar]
- 127.Boschi A, Bolzati C, Benini E, Malago E, Uccelli L, Duatti A, Piffanelli A, Refosco F, Tisato F. A novel approach to the high specific-activity labeling of small peptides with the techentium-99m fragment [99mTc(N)(PNP)]2+ (PNP = diphosphine ligand) Bioconj. Chem. 2001;12:1035–1042. doi: 10.1021/bc0155162. [DOI] [PubMed] [Google Scholar]
- 128.Boschi A, Uccelli L, Duatti A, Bolzati C, Refosco F, Tisato F, Malagnoli R, Baradli PG, Borea PA. Asymmetrical nitrido Tc-99m heterocomplexes as potential imaging agents for benzodiazepine receptors. Bioconj. Chem. 2003;14:1279–1288. doi: 10.1021/bc034124n. [DOI] [PubMed] [Google Scholar]
- 129.Boltzati C, Muhmood A, Malago E, Uccelli L, Boschi A, Jones AG, Refosco F, Duatti A, Tisato F. The [99mTc(N)(PNP)]2+ metal fragment: a technetium-nitrido synthon for use with biologically active molecule. The N-(2-methoxyphenyl)piperazylcysteine analogues as examples. Bioconj. Chem. 2003;14:1231–1242. doi: 10.1021/bc034100g. [DOI] [PubMed] [Google Scholar]
- 130.Alberto R, Schibli R, Egli A, Schubiger PA, Abram U, Kaden TA. A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(H2O)3(CO)3]+ from [99mTcO4]− in aqueous solution and its reaction with bifunctional ligand. J. Am. Chem. Soc. 1998;120:7987–7988. [Google Scholar]
- 131.Alberto R, Ortner K, Wheatley N, Schibli R, Schubiger PA. Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [99mTc(H2O)3(CO)3]+ J. Am. Chem. Soc. 2001;123:3135–3136. doi: 10.1021/ja003932b. [DOI] [PubMed] [Google Scholar]
- 132.Schibli R, Schwarzbach R, Alberto R, Ortner K, Schmalle H, Dumas C, Egli A, Schubiger PA. Steps toward high specific activity labeling of biomolecules for therapeutic application: preparation of precursor [188Re(H2O)3(CO)3]+ and synthesis of tailor-made bifunctional ligand systems. Bioconj. Chem. 2002;13:750–756. doi: 10.1021/bc015568r. [DOI] [PubMed] [Google Scholar]
- 133.Alberto R, Schibli R, Abram U, Egli A, Knapp FF, Schubiger PA. Potential of the “[M(CO)3]+” (M = Re, Tc) moiety for the labeling of biomolecules. Radiochim. Acta. 1977;79:99–103. [Google Scholar]
- 134.Häfliger P, Mundwiler S, Ortner K, Spingler B, Alberto R, Andócs G, Balogh L, Bodo K. Structure, stability, and biodistribution of cationic [M(CO)3]+ (M = Re, 99Tc, 99mTc) complexes with tridentate amine ligands. Synth. React. Inorg. Met.-Org. Chem. 2005;35:27–34. [Google Scholar]
- 135.Rattat D, Eraets K, Cleynhens B, Knight H, Fonge H, Verbruggen A. Comparison of tridentate ligands in competition experiments for their ability to form a [99mTc(CO)3]+ complex. Tetrahedron Lett. 2004;45:2531–2534. [Google Scholar]
- 136.Wei LH, Banerjee SR, Levadala MK, Babich JW, Zubieta J. Complexes of the fac-{Re(CO)3}+ core with tridentate ligands derived from arylpiperazines. Inorg. Chim. Acta. 2004;357:1499–1516. [Google Scholar]
- 137.Pak JK, Benny P, Spingler B, Ortner K, Alberto R. Nε functionalization of metal and organic protected L-Histidine for a highly efficient, direct labeling of biomolecules with [99mTc(H2O)3(CO)3]+ Chem. Eur. J. 2003;9:2053–2061. doi: 10.1002/chem.200204445. [DOI] [PubMed] [Google Scholar]
- 138.Kim Y, He Z, Hsieh W, Liu S. Synthesis, characterization and X-ray crystal structure of [Re(PNP)(CO)3]Br·2CH3OH: model compound for a new class of cationic 99mTc radiotracers. Inorg. Chim. Acta. 2006;359:2479–2488. [Google Scholar]
- 139.Schubiger PA, Grünberg J, Ametamey SM, Honer M, Garcia-Garayoa E, Bläuenstein P, Waibel R, Novak-Hofer I, Schibli R. Radiopharmaceuticals: from molecular imaging to targeted radionuclide therapy. Chimia. 2004;58:731–735. [Google Scholar]
- 140.Stichelberger A, Waibel R, Dumas C, Schubiger PA, Schibli R. Versatile synthetic approach to new bifunctional chelating agents tailor made for labeling with the fac-[M(CO)3]+ core (M = Tc, 99mTc, Re): synthesis, in vitro, and in vivo behavior of the model complex [M(APPA)(CO)3] (APPA = [(5-amino-pentyl)-pyridin-2-yl-methylamino]- acetic acid) Nucl. Med. Biol. 2003;30:465–470. doi: 10.1016/s0969-8051(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 141.Alberto R, Schibli R, Schubiger PA, Abram U, Pietzsch HJ, Johannsen B. First application of fac-[99mTc(H2O)3(CO)3]+ in bioorganometallic chemistry: design, structure, and in vitro affinity of 5-HT1A receptor ligand labeled with 99mTc. J. Am. Chem. Soc. 1999;121:6076–6077. [Google Scholar]
- 142.La Bella R, Garcia-Garayoa E, Langer M, Bläuenstein P, Beck-Sickinger AG, Schubiger PA. In vitro and in vivo evaluation of a 99mTc(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl. Med. Biol. 2002;29:553–560. doi: 10.1016/s0969-8051(02)00314-1. [DOI] [PubMed] [Google Scholar]
- 143.Schibli R, La Bella R, Alberto R, Garcia-Garayoa E, Ortner K, Abram U, Schubiger PA. Influence of the denticity of ligand systems on the in vitro and in vivo behavior of 99mTc(I)-tricarbonyl complexes: a hint for the future functionalization of biomolecules. Bioconj. Chem. 2000;11:345–351. doi: 10.1021/bc990127h. [DOI] [PubMed] [Google Scholar]
- 144.Waibel R, Alberto R, Willuda J, Finnern R, Schibli R, Stichelberger A, Egli A, Abram U, Mach JP, Plückthun A, Schubiger PA. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat.Biotechnol. 1999;17:897–901. doi: 10.1038/12890. [DOI] [PubMed] [Google Scholar]
- 145.Banerjee SR, Maresca KP, Francesconi L, Valliant J, Babich JW, Zubieta J. New directions in the coordination chemistry of 99mTc: a reflection on technetium core structures and a strategy for new chelate design. Nucl. Med. Biol. 2005;32:1–20. doi: 10.1016/j.nucmedbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 146.Fani M, Psimadas D, Zikos C, Xanthopoulos S, Loudos GK, Bouziotis P, Varvarigou AD. Comparative evaluation of linear and cyclic 99mTc-RGD peptides for targeting of integrins in tumor angiogenesis. Anticancer Res. 2006;26:431–434. [PubMed] [Google Scholar]
- 147.Zhang X, Chen X. Preparation and characterization of 99mTc(CO)3-BPy-RGD complex as αvβ3 integrin receptor-targeted imaging agent. Appl. Radiat. Isot. 2007;65:70–78. doi: 10.1016/j.apradiso.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 148.Alves S, Correia JDG, Gano L, Rold TL, Prasanphanich A, Haubner R, Rupprich M, Alberto R, Decristoforo C, Snatos I, Smith CJ. In vitro and in vivo evaluation of a novel 99mTc(CO)3-pyrazolyl conjugate of cyclo-(Arg-Gly-Asp-d-Tyr-Lys) Bioconj. Chem. 2007;18:530–537. doi: 10.1021/bc060234t. [DOI] [PubMed] [Google Scholar]
- 149.Alberto R, Schibli R, Waibel R, Abram U, Schubiger PA. Basic aqueous chemistry of [M(H2O)3(CO)3]+ (M = Re, Tc) directed towards radiopharmaceutical application. Coord. Chem. Rev. 1999;190–192:901–919. [Google Scholar]
- 150.Schibli R, Schubiger PA. Current use and future potential of organometallic radiopharmaceuticals. Eur. J. Nucl. Med. 2002;29:1529–1542. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 151.Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990;31:2022–2028. [PubMed] [Google Scholar]
- 152.Schwartz DA, Abrams MJ, Hauser MM, Gaul FE, Larsen SK, Rauh D, Zubieta J. Preparation of hydrazino-modified proteins and their use for the synthesis of 99mTc-protein conjugates. Bioconj. Chem. 1991;2:333–336. doi: 10.1021/bc00011a007. [DOI] [PubMed] [Google Scholar]
- 153.Ultee ME, Bridger GJ, Abrams MJ, Longley CB, Burton CA, Larsen S, Henson GW, Padmanabhan S, Gaul FE, Schwartz DA. Tumor imaging with technetium-99m-labeled hydrazinonicotinamide-Fab’ conjugates. J. Nucl. Med. 1997;38:133–138. [PubMed] [Google Scholar]
- 154.Babich JW, Solomon H, Pike MC, Kroon D, Graham W, Abrams MJ, Tompkins RG, Rubin RH, Fischman AJ. Technetium-99m labeled hydrazine nicotinamide derivatized chemotactic peptide analogs for imaging focal sites of bacterial infection. J. Nucl. Med. 1993;34:1967–1974. [PubMed] [Google Scholar]
- 155.Babich JW, Fischman AJ. Effect of "co-ligand" on the biodistribution of 99mTc-labeled hydrazino nicotinic acid derivatized chemotactic peptides. Nucl. Med. Biol. 1995;22:25–30. doi: 10.1016/0969-8051(94)00081-t. [DOI] [PubMed] [Google Scholar]
- 156.Babich JW, Graham W, Barrow SA, Fischman AJ. Comparision of the infection imaging properties of a 99mTc labeled chemotactic peptide with 111In IgG. Nucl. Med.Biol. 1995;22:643–648. doi: 10.1016/0969-8051(94)00138-a. [DOI] [PubMed] [Google Scholar]
- 157.Babich JW, Coco WG, Barrow SA, Fischman AJ, Femia FJ, Zubieta J. 99mTc-labeled chemotactic peptides: influence of coligands on distribution of molecular species and infection imaging properties. Synthesis and structural characterization of model complexes with the {Re(η2-HNNC5H4N)(η1-HNNC5H4N)} core. Inorg. Chim. Acta. 2000;309:123–136. [Google Scholar]
- 158.Decristoforo C, Mather SJ. Preparation, 99mTc-labeling, and in vitro characterization of HYNIC and N3S modified RC-160 and [Tyr3]Octreotide. Bioconj. Chem. 1999;10:431–438. doi: 10.1021/bc980121c. [DOI] [PubMed] [Google Scholar]
- 159.Decristoforo C, Melendez L, Sosabowski JK, Mather SJ. 99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: preclinical evaluation and comparison with 111In-Octreotide. J. Nucl. Med. 2000;41:1114–1119. [PubMed] [Google Scholar]
- 160.Decristoforo C, Mather SJ. 99mTc-labeled peptide-HYNIC conjugates: effect of lipophilicity and stability on biodistribution. Nucl. Med. Biol. 1999;26:389–396. doi: 10.1016/s0969-8051(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 161.Decristoforo C, Mather SJ. Technetium-99m somatostatin analogues: effect of labeling methods and peptide sequence. Eur. J. Nucl. Med. 1999;26:869–876. doi: 10.1007/s002590050461. [DOI] [PubMed] [Google Scholar]
- 162.Bangard M, Béhé M, Guhlke S, Otte R, Bender H, Maecke HR, Birsack HJ. Detection of somatostatin receptor-positive tumours using the new 99mTc-tricine-HYNIC-D-Phe1-Tyr3-octreotide: first results in patients and comparison with 111In-DTPA-D-Phe1-Tyr3-octreotide. Eur. J. Nucl. Med. 2000;27:628–637. doi: 10.1007/s002590050556. [DOI] [PubMed] [Google Scholar]
- 163.Decristoforo C, Mather SJ, Cholewinski W, Donnemiller E, Riccabona G, Moncayo R. 99mTc-EDDA/HYNIC-TOC: a new 99mTc-labeled radiopharmaceutical for imaging somatostatin receptor-positive tumors: first clinical results and intrapatient comparison with 111In-labeled octreotide derivatives. Eur. J. Nucl. Med. 2000;27:1318–1325. doi: 10.1007/s002590000289. [DOI] [PubMed] [Google Scholar]
- 164.Laverman P, Dams ETM, Oyen WJG, Storm G, Koenders EB, Prevost R, van der Meer JWM, Corstens FHM, Boerman OC. A novel method to label liposomes with 99mTc by the hydrazinonicotinyl derivative. J. Nucl. Med. 1999;40:192–197. [PubMed] [Google Scholar]
- 165.Zhang Y, Liu N, Zhu ZH, Rusckowski M, Hnatowich DJ. Influence of different chelators (HYNIC, MAG3 and DTPA) on tumor cell accumulation and mouse biodistribution of technetium-99m labeled antisense DNA. Eur. J. Nucl. Med. 2000;27:1700–1707. doi: 10.1007/s002590000343. [DOI] [PubMed] [Google Scholar]
- 166.Hnatowich DJ, Winnard P, Jr, Virzi F, Fogarasi M, Santo T, Smith CL, Cantor CR, M Rusckowski M. Technetium-99m labeling of DNA oligonucleotides. J. Nucl. Med. 1995;36:2306–2314. [PubMed] [Google Scholar]
- 167.Liu S, Edwards DS, Looby RJ, Harris AR, Poirier MJ, Barrett JA, Heminway SJ, Carroll TR. Labeling a hydrazinonicotinamide-modified cyclic IIb/IIIa receptor antagonist with 99mTc using aminocarboxylates as co-ligands. Bioconj. Chem. 1996;7:63–71. doi: 10.1021/bc950069+. [DOI] [PubMed] [Google Scholar]
- 168.Edwards DS, Liu S, Ziegler MC, Harris AR, Crocker AC, Heminway SJ, Barrett JA, Bridger GJ, Abrams MJ, Higgins JD. RP463: A stabilized technetium-99m complex of a hydrazino nicotinamide conjugated chemotactic peptide for infection imaging. Bioconj. Chem. 1999;10:884–891. doi: 10.1021/bc990049y. [DOI] [PubMed] [Google Scholar]
- 169.Edwards DS, Liu S, Barrett JA, Harris AR, Looby RJ, Ziegler MC, Heminway SJ, Carroll TR. A new and versatile ternary ligand system for technetium radiopharmaceuticals: water soluble phosphines and tricine as coligands in labeling a hydrazino nicotinamide-modified cyclic glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconj. Chem. 1997;8:146–154. doi: 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]
- 170.Liu S, Edwards DS, Harris AR. A novel ternary ligand system for technetium radiopharmaceuticals: imine-N containing heterocycles as coligands in labeling a hydrazinonicotinamide-modified cyclic platelet glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconj. Chem. 1998;9:583–595. [Google Scholar]
- 171.Liu S, Edwards DS, Harris AR, Heminway SJ, Barrett JA. Technetium complexes of a hydrazinonicotinamide-conjugated cyclic peptide and 2-hydrazinopyridine:Synthesis and characterization. Inorg. Chem. 1999;38:1326–1335. doi: 10.1021/ic980973o. [DOI] [PubMed] [Google Scholar]
- 172.Liu S, Ziegler MC, Edwards DS. Radio-LC-MS for the characterization of 99mTc-labeled bioconjugates. Bioconj. Chem. 2000;11:113–117. doi: 10.1021/bc990111r. [DOI] [PubMed] [Google Scholar]
- 173.Brouwers AH, Laverman P, Boerman OC, Oyen WJG, Barrett JA, Harris TD, Edwards DS, Corstens FHM. A 99Tcm-labeled leukotriene B4 receptor antagonist for scintigraphic detection of infection in rabbits. Nucl. Med. Commun. 2000;21:1043–1051. doi: 10.1097/00006231-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 174.Liu S, Harris AR, Ziegler MC, Edwards DS, Williams NE. 99mTc-labeling of a hydrazinonictotinamide-conjugated LTB4 receptor antagonist useful for imaging infection and inflammation. Bioconj. Chem. 2002;13:881–886. doi: 10.1021/bc010120b. [DOI] [PubMed] [Google Scholar]
- 175.Liu S, Edwards DS, Ziegler MC, Harris AR, Hemingway SJ, Barrett BA. 99mTc-Labeling of a hydrazinonictotinamide-conjugated vitronectin receptor antagonist. Bioconj. Chem. 2001;12:624–629. doi: 10.1021/bc010012p. [DOI] [PubMed] [Google Scholar]
- 176.Liu S, Hsieh W, Kim YS, SI Mohammed SI. S. I. Effect of coligands on biodistribution characteristics of ternary ligand 99mTc complexes of a HYNIC-conjugated cyclic RGDfK dimer. Bioconj. Chem. 2005;16:1580–1588. doi: 10.1021/bc0501653. [DOI] [PubMed] [Google Scholar]
- 177.Liu S, Hsieh W, Jiang Y, Kim Y, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for non-invasive imaging integrin αvβ3-positive breast cancer. Bioconj. Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 178.Liu S, He Z, Hsieh W, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconj. Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconj. Chem. 2006;17:1069–1076. doi: 10.1021/bc060055b. [DOI] [PubMed] [Google Scholar]
- 180.Liu S, Edwards DS, Harris AR, Ziegler MC, Poirier MJ, Ewels BA, DiLuzio WR, Hui P. Towards developing a non-SnCl2 formulation for RP444: a new radiopharmaceutical for thrombus imaging. J. Pharm. Sci. 2001;90:114–123. doi: 10.1002/1520-6017(200102)90:2<114::aid-jps2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 181.Edwards DS, Liu S, Harris AR, Ewels BA. 99mTc-labeling hydrazones of a hydrazinonicotinamide conjugated cyclic peptide. Bioconj. Chem. 1999;10:803–807. doi: 10.1021/bc990022e. [DOI] [PubMed] [Google Scholar]
- 182.Delmon-moingeon LI, Mahmood A, Davison A, Jones AG. Strategies for labeling monoclonal antibodies and antibody-like molecules with technetium-99m. J. Nucl. Biol.Med. 1991;35:47–59. [PubMed] [Google Scholar]
- 183.Rhodes BA. Direct labeling of proteins with 99mTc. Nucl. Med. Biol. 1991;18:667–676. doi: 10.1016/0883-2897(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 184.Eckelman WC, Steigman J. Direct labeling with 99mTc. Nucl. Med. Biol. 1991;18:3–7. doi: 10.1016/0883-2897(91)90039-n. [DOI] [PubMed] [Google Scholar]
- 185.Griffiths GL, Goldenberg DM, Jones AL, Hansen HJ. Radiolabeling of monoclonal antibodies and fragments with technetium and rhenium. Bioconj. Chem. 1992;3:91–99. doi: 10.1021/bc00014a001. [DOI] [PubMed] [Google Scholar]
- 186.Zamora PO, Rhodes BA. Imidazoles as well as thiolates in proteins bind technetium-99m. Bioconj. Chem. 1992;3:493–498. doi: 10.1021/bc00018a005. [DOI] [PubMed] [Google Scholar]
- 187.Liu S, Edwards DS, Harris AR, Singh PR. 99mTc-labeling kinetics of four thiol-containing chelators and 2-hydrazinopyridine: factors influencing their radiolabeling efficiency. Appl. Radiat. Isot. 1997;48:1103–1111. [Google Scholar]
- 188.Schwochau K. Technetium radiopharmaceuticals-fundamentals, synthesis, structure, and development. Angew. Chem. Int. Ed. Eng. 1994;33:2258–2267. [Google Scholar]
- 189.Kelly JD, Forster AM, Archer CM, Booker FS, Canning LR, Chiu KW, Edwards B, Gill HK, McPartlin M, Nagle KR, Latham IA, Pickett RD, Storey AE, Webbon PM. Technetium-99m-tetrofosmin as a new radiopharmaceutical for myocardial perfusion imaging. J. Nucl. Med. 1993;34:222–227. [PubMed] [Google Scholar]
- 190.Higley B, Smith FW, Smith T, Gemmell HG, Gupta PD, Gvozdanovic DV, Graham D, Hinge D, Davidson J, Lahiri A. Technetium-99m 1,2-bis[bis(2-ethyoxyethyl)phosphino]ethane: human biodistribution, dosimetry and safety of a new myocardial perfusion imaging agent. J. Nucl. Med. 1993;34:30–38. [PubMed] [Google Scholar]
- 191.Najafi A, Alauddin MM, Sosa A, Ma GQ, Chen DCP, Epstein AL, Siegel ME. The evaluation of 186Re-labeled antibodies using N2S4 chelate in vitro and in vivo using tumor-bearing nude mice. Nucl. Med. Biol. 1992;19:205–212. doi: 10.1016/0883-2897(92)90009-n. [DOI] [PubMed] [Google Scholar]
- 192.Blower PJ, Prakash S. The chemistry of rhenium in nuclear medicine. Perspectives Bioinorg. Chem. 1999;4:91–143. [Google Scholar]
- 193.Visser GWM, Gerretsen M, Herscheid JDM, Snow GB, van Dongen G. Labeling of monoclonal antibodies with rhenium-186 using the MAG3 chelate for radioimmunotherapy of cancer: a technical protocol. J. Nucl. Med. 1993;34:1953–1963. [PubMed] [Google Scholar]
- 194.van Gog FB, Visser GWM, Klok R, van der Schors R, Snow GB, van Dongen G. Monoclonal antibodies labeled with rhenium-186 using the MAG3 chelate: relationship between the number of chelated groups and biodistribution characteristics. J. Nucl. Med. 1996;37:352–362. [PubMed] [Google Scholar]
- 195.Chen JQ, Strand SE, Tennvall J, Lindrgren L, Hindorf C, Sjögren HO. Extracorporeal immunoadsorption compared to avidin chase: enhancement of tumor-to-normal tissue ratio for biotinylated rhenium-188-chimeric BR96. J. Nucl. Med. 1997;38:1934–1939. [PubMed] [Google Scholar]
- 196.Beaumier PL, Venkatesan P, Vanderheyden JL, Burgua WD, Kunz LL, Fritzberg AR, Abrams PJ, Morgan AC., Jr Rhenium-186 radioimmunotherapy of small-cell lung carcinoma xenografts in nude mice. Cancer Res. 1991;51:676–681. [PubMed] [Google Scholar]
- 197.Ram S, Buchshaum DJ. A peptide-based bifunctional chelating agent for 99mTc and 186Re labeling of monoclonal antibodies. Cancer. 1994;73:769–773. doi: 10.1002/1097-0142(19940201)73:3+<769::aid-cncr2820731304>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 198.Liu S, Wong E, Rettig SJ, Orvig C. Hexadentate N3O3 amine phenol ligands for group 13 Metal Ions: Evidence for intrastrand and interstrand hydrogen bonds in polydentate tripodal amine phenols. Inorg. Chem. 1993;32:4268–4276. [Google Scholar]
- 199.Liu S, Wong E, Karunaratne V, Rettig SJ, Orvig C. Highly flexible chelating ligands for group 13 metals. Design, synthesis and characterization of hexadentate (N3O3) tripodal amine phenol ligand complexes of aluminum, gallium and indium. Inorg. Chem. 1993;32:1756–1765. [Google Scholar]
- 200.Liu S, Rettig SJ, Orvig C. Polydentate ligand chemistry of group 13 metals: Effects of the size and donor-selectivity of metal ions on structures and properties of aluminum, gallium and indium complexes with potentially heptadentate (N4O3) amine phenol ligands. Inorg. Chem. 1992;31:5400–5407. [Google Scholar]
- 201.Wong E, Caravan P, Liu S, Rettig SJ, Orvig C. Selectivity of potentially hexadentate amine phenols for Ga3+ and In3+ in aqueous solution. Inorg. Chem. 1996;35:715–724. [Google Scholar]
- 202.Cox JL, Craig AS, Helps IM, Jankowski KJ, Parker D, Eaton MAW, Millican AT, Millar K, Beeley NRA, Boyce BA. Synthesis of C- and N-functionalized derivatives of 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), 1,4,7,10-tetraazacyclododecane- 1,4,7,10-tetrayltetra-acetic acid (DOTA), and diethylenetriaminepenta-acetic acid (DTPA): bifunctional complexing agents for the derivatization of antibodies. J. Chem. Soc., Perkin Trans. 1990:2567–2576. [Google Scholar]
- 203.Broan CJ, Cox JL, Craig AS, Kataky R, Parker D, Harrison A, Randall AM, Ferguson G. Structure and solution stability of indium and gallium complexes of 1,4,7-triazacyclononane-1,4,7-triacetate and yttrium complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayltetraacetate and related ligands: kinetically stable complexes for use in imaging and radioimmunotherapy. X-Ray molecular structure of the indium and gallium complexes of 1,4,7-triazacyclononane-1,4,7-triacetic acid. J.Chem. Soc., Perkin Trans. 1991:87–99. [Google Scholar]
- 204.André J, Maecke H, Zehnder M, Macko L, Akyel K. 1,4,7-triazanonane-1-succinic acid-4,7-diacetic acid (NODASA): a new bifunctional chelator for radio gallium-labeling of biomolecules. Chem. Commun. 1998;12:1301–1302. [Google Scholar]
- 205.Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, Reubi JC, Maecke HR. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67Ga/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconj. Chem. 2002;13:530–541. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 206.Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, Hennig M. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumor-targeting properties and potential for receptor-mediated internal radiotherapy. Chem. Eur. J. 1999;5:1974–1981. [Google Scholar]
- 207.Delgado R, Frausto da Siliva JJR. Metal complexes of cyclic tetra azatetraacetic acids. Talanta. 1982;29:815–822. doi: 10.1016/0039-9140(82)80251-8. [DOI] [PubMed] [Google Scholar]
- 208.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–767. [Google Scholar]
- 209.Wong E, Liu S, Lügger T, Hahn FE, Orvig C. Hexadentate N4O2 amine phenol complexes of gallium and indium. Inorg. Chem. 1995;34:93–101. [Google Scholar]
- 210.Viola NA, Rarig RS, Jr, Ouellette W, Doyle RP. Synthesis, structure and thermal analysis of the gallium complex of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid (DOTA) Polyhedron. 2006;25:3457–3462. [Google Scholar]
- 211.Yang CT, Li YX, Liu S. Synthesis and structural characterization of complexes of a DO3A-conjugated triphenylphosphonium cation with diagnostically important metal ions. Inorg. Chem. ASAP. doi: 10.1021/ic7010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Riesen A, Kaden TA, Ritter W, Mäcke HR. Synthesis and X-ray structural characterization of seven coordinate macrocyclic In3+ complexes with relavance to radiopharmaceutical applications. J. Chem. Soc., Chem. Commun. 1989:460–462. [Google Scholar]
- 213.Maecke HR, Riesen A, Ritter W. The molecular structure of indium-DTPA. J. Nucl.Med. 1989;30:1235–1239. [PubMed] [Google Scholar]
- 214.Liu S, He Z, Hsieh W, Fanwick PE. Synthesis, characterization, and crystal structure of In(DOTA-AA) (AA = p-aminoanilide): a model compounds for 111In-labeled DOTA-biomolecule conjugates. Inorg. Chem. 2003;42:8831–8837. doi: 10.1021/ic0349914. [DOI] [PubMed] [Google Scholar]
- 215.Hsieh W, Liu S. Synthesis, characterization, and structures of indium and yttrium complexes In(DTPA-BA2) and Y(DTPA-BA2)(CH3OH) (BA= benzylamine): models for 111In- and 90Y-labeled DTPA-biomolecule conjugates. Inorg. Chem. 2004;43:6006–6014. doi: 10.1021/ic049973g. [DOI] [PubMed] [Google Scholar]
- 216.Liu S, Edwards DS. Synthesis and characterization of two 111In labeled DTPA-peptide conjugates. Bioconj. Chem. 2001;12:630–634. doi: 10.1021/bc010013h. [DOI] [PubMed] [Google Scholar]
- 217.Eisenwiener KP, Powell P, Maecke HR. A convenient synthesis of novel bifunctional pro-chelators for coupling to bioactive peptides for radiometal labeling. Bioorg. Med.Chem. Lett. 2000;10:2133–2135. doi: 10.1016/s0960-894x(00)00413-3. [DOI] [PubMed] [Google Scholar]
- 218.Janssen M, Oyen WJG, Massuger LFAG, Frielink C, Dijkgraaf I, Edwards DS, Rajopadyhe M, Corsten FHM, OC Boerman OC. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biotherapy & Radiopharmaceuticals. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 219.Jia B, Liu Z, Liu ZF, Yu ZL, Yang Z, Zhao HY, He Z, Liu S, Wang F. Linker effects on biological properties of 111In-labeled DTPA conjugates of a cyclic RGDfK dimer. Bioconj. Chem. doi: 10.1021/bc7002988. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu S, Pietryka J, Ellars CE, Edwards DS. Comparison of yttrium and indium complexes of DOTA-BA and DOTA-MBA: models for 90Y- and 111In-labeled DOTA-biomolecule conjugates. Bioconj. Chem. 2002;13:902–913. doi: 10.1021/bc010134h. [DOI] [PubMed] [Google Scholar]
- 221.Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin MG, Orsi F, Maecke HR, Jermann E, Robertson C, Fiorenza M, Tosi G, Paganelli G. Biokinetics and dosemitry in patients administered with 111In-DOTAo,-D-Phe1, Tyr3]octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur. J. Nucl. Med. 1999;26:877–886. doi: 10.1007/s002590050462. [DOI] [PubMed] [Google Scholar]
- 222.De Jong M, Bakker WH, Krenning EP, Breeman WAP, van der Pluijm ME, Bernard B, Visser TJ, Jermann E, Béhé M, Powell P, Mäcke H. Yttrium-90 and indium-111 labeling, receptor binding and biodistribution of [DOTAo,-D-Phe1, Tyr3]octreotide, apromising somatostatin analogue for radionuclide therapy. Eur. J. Nucl. Med. 1997;24:368–371. doi: 10.1007/BF00881807. [DOI] [PubMed] [Google Scholar]
- 223.Ando A, Ando I, Hiraki T, Hisda K. Relation between the location of elements in the periodic table and various organ-uptake rates. Nucl. Med. Biol. 1989;16:57–80. doi: 10.1016/0883-2897(89)90216-x. [DOI] [PubMed] [Google Scholar]
- 224.Blower PJ, Lewis JS, Zweit J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl. Med. Biol. 1996;23:957–980. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- 225.Smith SV. Molecular imaging with copper-64. J. Inorg. Biochem. 2004;98:1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 226.Morphy JR, Parker D, Alexander R, Bains A, Carne AF, Eaton MAW, Harrison A, Millican A, Phipps A, Rhind SK, Titmas R, Weatherby D. Antibody labeling with functionalized cyclam macrocycles. J. Chem. Soc., Chem. Commun. 1988:156–158. [Google Scholar]
- 227.Parker D, Morphy R, Jankowski K, Cox J. Implementation of macrocycle conjugated antibodies for tumor targeting. Pure & Appl. Chem. 1989;61:1637–1641. [Google Scholar]
- 228.Li WP, Meyer LA, Anderson CJ. Radiopharmaceuticals for positron emission tomography imaging of somatostatin receptor positive tumors. Topics Curr. Chem. 2005;252:179–192. [Google Scholar]
- 229.Meares CF. Chelating agents for the binding of metal ions to antibodies. Nucl. Med.Biol. 1986;13:311–318. doi: 10.1016/0883-2897(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 230.Moi MK, Meares CF, McCall MJ, Cole WC, DeNardo SJ. Copper chelates as probes of biological systems: stable copper complexes with macrocyclic bifunctional chelating agent. Anal. Chem. 1985;148:249–253. doi: 10.1016/0003-2697(85)90653-0. [DOI] [PubMed] [Google Scholar]
- 231.Li WP, Lewis JS, Kim J, Bugaj JE, Johnson MA, Erion JL, Anderson CJ. DOTA-d-Tyr1-Octreotate: a somatostatin analogue for labeling with metal and halogen radionuclides for cancer imaging and therapy. Bioconj. Chem. 2002;13:721–728. doi: 10.1021/bc015590k. [DOI] [PubMed] [Google Scholar]
- 232.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of α-MSH. J. Med. Chem. 2005;48:2985–2992. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 233.Biddlecombe GB, Rogers BE, de Visser M, Parry JJ, de Jong M, Erion JL, Lewis JS. Molecular imaging of gastrin-releasing peptide receptor-positive tumors in mice using 64Cu- and 86Y-DOTA-(Pro1, Tyr4)-Bombesin(1–14) Bioconj. Chem. 2007;18:724–730. doi: 10.1021/bc060281l. [DOI] [PubMed] [Google Scholar]
- 234.Parry JJ, Andrews R, Rogers BE. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res. Treat. 2007;101:175–183. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- 235.Parry JJ, Kelly TS, Andrews R, Rogers BE. in vitro and in vivo evaluation of 64Culabeled DOTA-Linker-Bobesin(7–14) analogues containing different amino acid linker moiety. Bioconj. Chem. 2007;18:1110–1117. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- 236.Sprague JE, Kitaura H, Zou W, Ye Y, Achilefu S, Weilbaecher KN, Taitebaum SL, Anderson CJ. Noninvasive imaging of osteoclasts in pyrathyroid hormone-induced osteolysis using a 64Cu-labeled RGD peptide. J. Nucl. Med. 2007;48:311–318. [PMC free article] [PubMed] [Google Scholar]
- 237.Anderson CJ. Metabolism of radiometal labeled proteins and peptides: what are the real radiopharmaceuticals in vivo? Cancer Biotherapy and Radiopharm. 2001;16:451–455. doi: 10.1089/10849780152752056. [DOI] [PubMed] [Google Scholar]
- 238.Deshpande SV, DeNardo SJ, Meares CF, McCall MJ, Adams GP, Moi MK, DeNardo GL. Copper-67-labeled monoclonal antibody Lym-1, a potential radiopharmaceutical for cancer therapy: Labeling and biodistribution in RAJI tumored mice. J. Nucl. Med. 1988;29:217–225. [PubMed] [Google Scholar]
- 239.Cole WC, DeNardo SJ, Meares CF, McCall MJ, DeNardo GL, Esptein AL, O’Brien HA, Moi MK. Comparative serum stability of radiochelates for antibody radiopharmaceuticals. J. Nucl. Med. 1987;28:83–90. [PubMed] [Google Scholar]
- 240.Bartnikas TB, Gitlin JD. Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J. Biol. Chem. 2003;278:33602–33608. doi: 10.1074/jbc.M305435200. [DOI] [PubMed] [Google Scholar]
- 241.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 242.Boswell CA, McQuade P, Weisman GR, Wong EH, Anderson CJ. Optimization of labeling and metabolite analysis of copper-64-labeled azamacrocyclic chelators by radio-LC-MS. Nucl. Med. Biol. 2005;32:29–38. doi: 10.1016/j.nucmedbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 243.Sprague JE, Peng Y, Fiamengo AL, Wooden KS, Southwick EA, Wiseman GR, Wong EH, Golden JA, Rhengold AL, Anderson CJ. Synthesis, characterization and in vivo studies of Cu(II)-64-labeled cross-bridges tetraazamacrocycle-amide complexes as models of peptide conjugate imaging agents. J. Med. Chem. 2007;50:311–318. doi: 10.1021/jm070204r. [DOI] [PubMed] [Google Scholar]
- 244.Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu Bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. J. Nucl. Med. 2007;48 doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 245.Di Bartolo N, Sargeson AM, Smith SV. New 64Cu PET imaging agents for personalised medicine and drug development using the hexa-aza cage. SarAr. Org.Biomol. Chem. 2006;4:3350–3357. doi: 10.1039/b605615f. [DOI] [PubMed] [Google Scholar]
- 246.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MJ, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin- releasing peptide receptor-expressing tissues. PNAS. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lauffer RB. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and Design. Chem. Rev. 1987;87:901–927. [Google Scholar]
- 248.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 249.Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconj. Chem. 1992;3:342–345. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- 250.Brechbiel MW, Gansow OA. Backbone-substituted DTPA ligands for 90Y radioimmunotherapy. Bioconj. Chem. 1991;2:187–194. doi: 10.1021/bc00009a008. [DOI] [PubMed] [Google Scholar]
- 251.Pulukkody KP, Norman TJ, Parker D, Royle L, Broan CJ. Synthesis of charged and uncharged complexes of gadolinium and yttrium with cyclic polyazaphosphinic acid ligands for in vivo applications. J. Chem. Soc., Perkin Trans. 1993:605–620. [Google Scholar]
- 252.Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(pnitrobenzyl)-trans-CyDTPA. Bioorg. & Med. Chem. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 253.Camera L, Kinuya S, Garmestani K, Wu C, Brechbiel MW, Pai LH, McMurry TJ, Gansow OA, Pastan I, Paik CH, Carrasquillo JA. Evaluation of the serum stability and in vivo biodistribution of CHX-DTPA and other ligands for yttrium labeling of monoclonal antibodies. J. Nucl. Med. 1994;35:882–889. [PubMed] [Google Scholar]
- 254.Cummins CH, Rutter EW, Jr, Fordyce WA. A convenient synthesis of bifunctional chelating agents based on diethylenetriaminepentaacetic acid and their coordination chemistry with yttrium(III) Bioconj. Chem. 1991;2:180–186. doi: 10.1021/bc00009a007. [DOI] [PubMed] [Google Scholar]
- 255.Williams MA, Rapoport H. Synthesis of enantiometrically pure diethylenetriaminepentaacetic acid analogues. L-Phenylalanine as the educt for substitution at the central acetic acid. J. Org. Chem. 1993;58:1151–1158. [Google Scholar]
- 256.Jackson GE, Wynchank S, Woundenberg M. Gadolinium(III) complex equilibria: the implications for Gd(III) MRI contrast agents. Magnetic Resonance Med. 1990;16:57–66. doi: 10.1002/mrm.1910160107. [DOI] [PubMed] [Google Scholar]
- 257.McMurry TJ, Pippin CG, Wu C, Deal KA, Brechbiel MW, Mirzadeh S, Gansow OA. Physical parameters and biological stability of yttrium(III) diethylenetriaminepentaacetic acid derivative conjugates. J. Med. Chem. 1998;41:3546–3549. doi: 10.1021/jm980152t. [DOI] [PubMed] [Google Scholar]
- 258.Sherry AD, Cacheris WP, Kuan KT. Stability constants for Gd3+ binding to model DTPA-conjugates and DTPA-proteins: implications for their use as magnetic resonance contrast agents. Magnetic Resonance Med. 1988;8:180–190. doi: 10.1002/mrm.1910080208. [DOI] [PubMed] [Google Scholar]
- 259.Wu C, Brechbiel MW, Gansow OA, Kobayashi H, Carrasquillo J, Pastan I. Stability of the four 2-(p-nitrobenzyl)-trans-CyDTPA 88Y complexes. Radiochim. Acta. 1997;79:123–126. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 260.Moi MK, Meares CF, DeNardo SJ. The peptide way to macrocyclic bifunctional chelating agents: synthesis of 2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecane-N,N’N”,N’”-tetraacetic acid and study of its yttrium(III) complex. J. Am. Chem. 1988;110:6266–6267. doi: 10.1021/ja00226a063. [DOI] [PubMed] [Google Scholar]
- 261.Stimmel JB, Stockstill ME, Kull FC., Jr Yttrium-90 chelation properties of tetraazatetraacetic acid macrocycles, diethylenetriaminepentaacetic acid analogues, and a novel terpyridine acyclic chelator. Bioconj. Chem. 1995;6:219–225. doi: 10.1021/bc00032a010. [DOI] [PubMed] [Google Scholar]
- 262.Stimmel JB, Kull FC., Jr Sammarium-153 and lutetium-177 chelation properties of selected macrocyclic and acyclic ligands. Nucl. Med. Biol. 1998;25:117–125. doi: 10.1016/s0969-8051(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 263.Liu S, Ellars CE, Edwards DS. Ascorbic acid: useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. Bioconj. Chem. 2003;14:1052–1056. doi: 10.1021/bc034109i. [DOI] [PubMed] [Google Scholar]
- 264.Liu S, Edwards DS. Stabilization of 90Y-labeled DOTA-biomolecule conjugates using gentisic and ascorbic acid. Bioconj. Chem. 2001;12:554–558. doi: 10.1021/bc000145v. [DOI] [PubMed] [Google Scholar]
- 265.Liu S, Cheung E, Rajopadyhe M, Ziegler MC, Edwards DS. 90Y- and 177Lu-labeling of a DOTA-conjugated vitronectin receptor antagonist for therapy. Bioconj. Chem. 2001;12:559–568. doi: 10.1021/bc000146n. [DOI] [PubMed] [Google Scholar]
- 266.Kukis DL, DeNardo SJ, DeNardo GL, O’Donnell RT, Meares CF. Optimized conditions for chelation of yittrium-90-DOTA immunoconjugates. J. Nucl. Med. 1998;39:2105–2110. [PubMed] [Google Scholar]
- 267.Jang YH, Blanco M, Dasgupta S, Keire DA, Shively JE, Goddard WA., III Mechanism and energetics for complexation of 90Y with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), a model for cancer radioimmunotherapy. J. Am. Chem. Soc. 1999;121:6142–6151. [Google Scholar]
- 268.Goeckeler WF, Edwards B, Volkert WA, Holmes RA, Simon J, Wilson D. Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J. Nucl. Med. 1987;28:495–504. [PubMed] [Google Scholar]
- 269.Goeckeler WF, Troutner DE, Volkert WA, Edwards B, Simon J, Wilson D. 153Sm radiotherapeutic bone agents. Nucl. Med. Biol. 1986;13:479–482. doi: 10.1016/0883-2897(86)90028-0. [DOI] [PubMed] [Google Scholar]
- 270.Kim WD, Kiefer GE, Maton F, McMillan K, Muller RN, Sherry AD. Relaxometry, luminescence measurements, electronphorisis, and animal biodistribution of lanthanide(III) complexes of some polyaza macrocyclic acetates containing pyridine. Inorg. Chem. 1995;34:2233–2243. [Google Scholar]
- 271.Atkins HL, Mausner LF, Srivastava SC, Meinken GE, Straub RF, Cabahug CJ, Weber DA, Wong CT, Sacker DF, Madajewicz S, Park TL, Meek AG. Biodistribution of 117mSn4+ DTPA for palliative therapy of painful osseous metastases. Radiology. 1993;186:279–283. doi: 10.1148/radiology.186.1.7677974. [DOI] [PubMed] [Google Scholar]
- 272.Chong HS, Garmestani K, Ma DS, Milenic DE, Overstreet T, Brechbiel MW. Synthesis and biological evaluation of novel macrocyclic ligands with pendent donor groups as potential yttrium chelators for radioimmunotherapy with improved complex formation kinetics. J. Med. Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 273.Sherry AD, Brown RD, III, Gerades CFG, Koenig SH, Kuan KT, Spiller M. Synthesis and characterization of the gadolinium(3+) complex of DOTA-propylamide: a model DOTA-protein conjugate. Inorg. Chem. 1989;28:620–622. [Google Scholar]
- 274.Sieving PF, Watson A, Rocklage SM. Preparation and characterization of paramagnetic polychelates and their protein conjugates. Bioconj. Chem. 1990;1:65–71. doi: 10.1021/bc00001a008. [DOI] [PubMed] [Google Scholar]
- 275.Lewis MR, Raubitschek A, Shively JE. A facile, water soluble method for modification of proteins with DOTA. Use of elevated temperature and optimized pH to achieve high specific activity and high chelate stability in radiolabeled immunoconjugates. Bioconj. Chem. 1994;5:565–576. doi: 10.1021/bc00030a012. [DOI] [PubMed] [Google Scholar]
- 276.Lewis MR, Shively JE. Maleimidocycteineamido-DOTA derivatives: New reagents for radiometal chelate conjugation to antibody sulfhydryl groups undergo pH-dependent cleavage reactions. Bioconj. Chem. 1998;9:72–86. doi: 10.1021/bc970136v. [DOI] [PubMed] [Google Scholar]
- 277.Corson DT, Meares CF. Efficient multigram synthesis of the bifunctional chelating agent (S)-1-p-isothiocyanatobenzyl-diethylene-tetraaminepentaacetic acid. Bioconj.Chem. 2000;11:292–299. doi: 10.1021/bc990125x. [DOI] [PubMed] [Google Scholar]
- 278.DeNardo SJ, Zhong GR, Salako Q, Li M, DeNardo GL, Meares CF. Pharmacokinetics of chimeric L6 conjugated to indium-111- and yttrium-90-DOTA-peptide in tumor bearing mice. J. Nucl. Med. 1995;36:829–836. [PubMed] [Google Scholar]
- 279.Brechbiel MW, Gansow OA, Atcher RW, Schlom J, Simpson DE, Esteban J, Colcher D. Synthesis of 1-(p-isothiocyanatobenzyl) derivatives of DTPA and EDTA. Antibody labeling and tumor-imaging studies. Inorg. Chem. 1986;25:2772–2781. [Google Scholar]
- 280.McCall MJ, Diril H, Meares CF. Simplified method for conjugating macrocyclic bifunctional chelating agents to antibodies via 2-iminothiolane. Bioconj. Chem. 1990;1:222–226. doi: 10.1021/bc00003a007. [DOI] [PubMed] [Google Scholar]
- 281.Liu S, Cheung E, Rajopadyhe M, Williams NE, Overoye KL, Edwards DS. Isomerism and solution dynamics of 90Y-labeled DTPA-biomolecule conjugates. Bioconj.Chem. 2001;12:84–91. doi: 10.1021/bc000071n. [DOI] [PubMed] [Google Scholar]
- 282.Carrasquillo JA, White JD, Paik CH, Raubitschek A, Le N, Rotman M, Brechbiel MW, Gansow OA, Top LE, Perentesis P, Reynolds JC, Nelson DL, Waldmann TA. Similarities and differences in 111In- and 90Y-labeled 1B4M-DTPA antiTac monoclonal antibody distribution. J. Nucl. Med. 1999;40:268–276. [PubMed] [Google Scholar]
- 283.Camera L, Kinuya S, Garmestani K, Brechbiel MW, Wu C, Pai LH, McMurry TJ, Gansow OA, Pastan I, Paik CH, JA Carrasquillo JA. Comparative biodistribution of indium- and yttrium-labeled B3 monoalonal antibody conjugated to either 2-(p-SCN-Bz)-6-methyl-DTPA (1B4M-DTPA) or 2-(p-SCN-Bz-)-1,4,7,10-tetraazacyclododecane tetraacetic acid (2B-DOTA) Eur. J. Nucl. Med. 1994;21:640–646. doi: 10.1007/BF00285586. [DOI] [PubMed] [Google Scholar]
- 284.Rösch F, Herzog H, Stolz B, Brockmann J, Köhle M, Mühlensiepen H, Marbach P, Müller-Gärtner HW. Uptake kinetics of the somatostatin receptor ligand [86Y]DOTA-d-Phe1-Tyr3-octreotide ([86Y]SMT487) using positron emission tomography in non-human primates and calculation of radiation doses of the 90Y-labeled analogue. Eur. J. Nucl. Med. 1999;26:358–366. doi: 10.1007/s002590050398. [DOI] [PubMed] [Google Scholar]