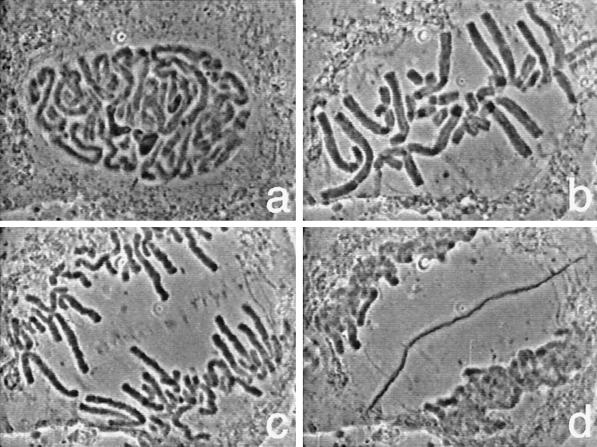
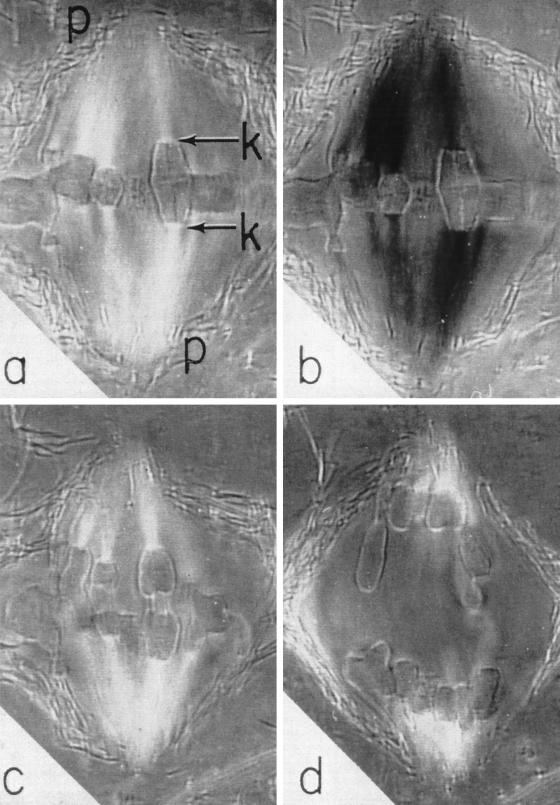
The first sequence shows an endosperm cell from the African blood lily, Haemanthus katherinae, undergoing mitosis (Figure 1). This sequence, captured by A.S. Bajer and J. Molé-Bajer using phase-contrast microscopy, was observed in cells that had been flattened between a layer of agar and gelatin to improve their visibility (Bajer and Molè-Bajer, 1956, 1986). The sequence vividly displays the chromosomes as they condense and align on the metaphase plate (Figure 1b). In the meantime the three large, dark nucleoli (Figure 1a) disappear. Then the chromosomes split and move apart in anaphase (Figure 1c). Finally the chromosomes become decondensed as they are packaged into two daughter nuclei in telophase (Figure 1d). Between the nuclei, small dancing vesicles appear (Figure 1c), align, and fuse with each other to form the cell plate (Figure 1d). The cell plate eventually gives rise to the cell walls and separates the plant cell into two.
Figure 1.
Mitosis and cell plate formation in a flattened endosperm cell of the African blood lily, Haemanthus katherinae, observed with phase contrast microscopy.
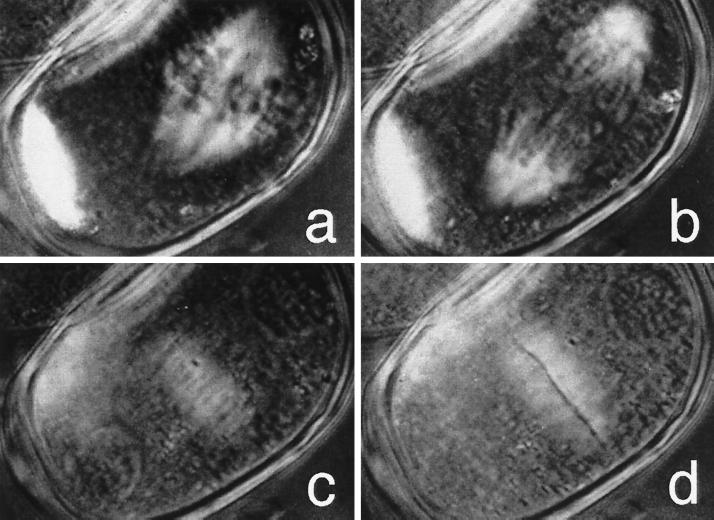
In the next sequence, we see the pollen mother cell of an Easter lily, Lilium longiflorum, undergoing mitosis and cell division (Figure 2). These cells synchronously undergo the first of their two divisions to form four pollen grains when the flower bud is exactly 22.4 mm long (Figure 3). A bud of this length was collected and centrifuged at ∼1800 × g for 3 min to displace the highly light-scattering granules and to make the other contents of the cell more visible. After excising an anther from the centrifuged flower bud in seven-eighths-strength frog Ringer’s solution, the cells were observed between crossed polarizers in the presence of a compensator (Figure 4). Observed with a polarizing microscope in this manner, regions of the cell where molecules are regularly aligned, i.e., birefringent regions, become highlighted (Figures 2, 5, and 6).
Figure 2.
Mitosis and cell plate formation in centrifuged pollen mother cell of the Easter lily, Lilium longiflorum, observed with polarization microscopy. Reproduced from The Journal of Cell Biology, vol 130, 687–700, 1995, by copyright permission of The Rockefeller University Press.
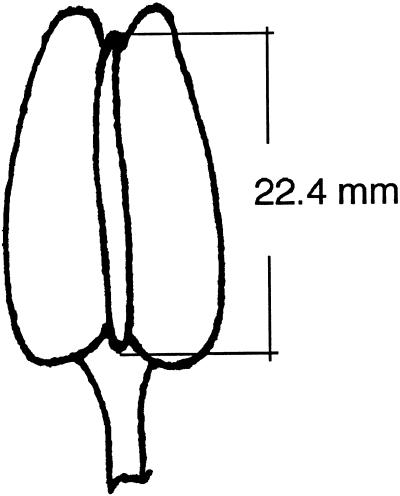
Figure 3.
Length of flower bud of Lilium longiflorum in which pollen mother cells undergo their first division (after Erickson, 1948).
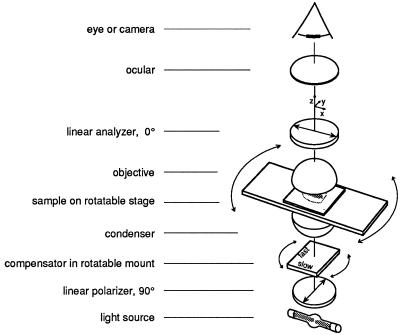
Figure 4.
Schematic of a polarizing microscope with crossed polarizers and a compensator. Reproduced from The Journal of Cell Biology, vol 139, 985–994, 1997, by copyright permission from The Rockefeller University Press.
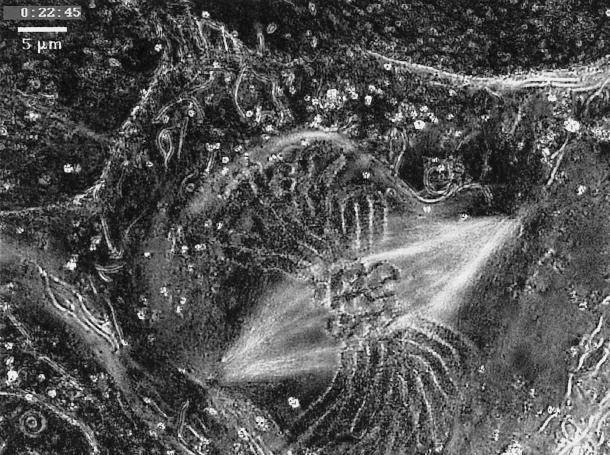
Figure 5.
Primary spermatocyte of Pardalophora apiculata observed with a rectified polarizing microscope (from Nicklas, 1971). Reproduced from Advances in Cell Biology, vol 2, 225–298, copyright 1971 Appleton-Century-Crofts.
Figure 6.
Mitosis in tissue-cultured lung cell of a newt, Taricha granulosa, recorded with the new Pol-Scope. Reproduced from The Journal of Cell Biology, vol 139, 985–994, 1997, by copyright permission of The Rockefeller University Press.
As with the Bajer’s endosperm cell series, this series on the pollen mother cell mitosis was initially time-lapse recorded on 16-mm ciné film. The elapsed time from breakdown of the nuclear envelope to formation of the cell plate was ∼2 h. The ciné records were transferred to video some 40 years later.
The polarizing microscope view of the pollen mother cells distinctly shows the spindle fibers that were not visible with phase-contrast microscopy (for polarizing microscope images of Haemanthus endosperm cells, see Inoué and Bajer, 1961; Inoué, 1964). Phase-contrast microscopy clearly shows the chromosome and nucleoli because of their higher refractive index, but not the spindle fibers that lead the chromosomes apart to the spindle poles or the phragmoplast fibers that bring the vacuoles to the cell plate. The refractive index of these fibers is too close to that of the surrounding cytoplasm. They nevertheless show clearly in a well-tuned polarizing microscope, because the fibers are birefringent, being made up of a bundle of regularly aligned molecular filaments. This sequence, taken by Inoué in 1950, demonstrated, for the very first time, the reality of spindle fibers and fibrils in living cells (Inoué, 1953, 1964) as well as the highly dynamic, labile nature of the molecular filaments (later identified as microtubules).
The microtubules disassembled reversibly when cells were exposed to cold, to high hydrostatic pressure, or to antimitotic drugs such as colchicine (reviewed in Inoué, 1964, 1981). During slow depolymerization of microtubules by these agents, metaphase-arrested chromosomes were pulled to a spindle pole anchored to the cell surface. After removal of the depolymerizing agent, growing spindle fibers pushed the chromosomes toward the metaphase plate. Thus arose the notion that chromosome movement toward the metaphase plate was associated with (and powered by) assembly and growth of microtubules, whereas movement of the chromosomes toward the spindle poles was associated with (and powered by) disassembly and shortening of the microtubules attached to the kinetochore of each sister chromosome (recent evidence and discussions summarized in Inoué and Salmon, 1995).
These polarized light microscopy studies on the birefringence of dividing cells demonstrated the assembly properties of microtubules and their dynamic function in living cells long before microtubules themselves were discovered or their assembly properties were characterized in vitro (reviewed in Inoué, 1981).
The microtubules that make up the spindle fibers and their shortening in anaphase can be seen more distinctly in the sequence of high-resolution images of a grasshopper spermatocyte (Pardalophora apiculata; Figure 5) taken by Nicklas (1971) with a rectified polarizing microscope. Rectification provides a higher-resolution image, restoring the needed extinction and correcting for the image error found in conventional polarizing microscopes when high–numerical aperture lenses are used (Inoué and Hyde, 1957).
The final video sequence shows a dividing newt (Taricha granulosa) lung epithelial cell (Figure 6) recorded by R. Oldenbourg, P.T. Tran, and E.D. Salmon with the new Pol-Scope. With the Pol-Scope, each image is generated by an image-processing computer from four video images taken in rapid succession at different settings of two electronically driven liquid crystal compensators. In the images thus displayed by the computer, the brightness of each pixel is strictly proportional to the birefringence of the specimen point and independent of orientation of the birefringence axis (Oldenbourg, 1996). Thus, in addition to providing displays with exquisitely high resolution and definition, Oldenbourg’s new Pol-Scope provides highly sensitive, dynamic image information on molecular alignment that is strictly quantitative.
In addition to being of historic interest, considered use of polarized light microscopy should continue to reveal much regarding the behavior of molecular and fine structural dynamics, noninvasively, in dividing, developing, and otherwise actively functioning living cells (Oldenbourg, 1998).
Supplementary Material
ACKNOWLEDGMENTS
Several of the video sequences accompanying this article were reproduced, with permission, from Video Supplement 2 of the journal Cell Motility and the Cytoskeleton. In the journal see “Cellular Motile Processes: Molecules and Mechanisms” (1990. Cell Motil. Cytoskeleton 17, 356–372) and the accompanying VHS videotape edited by Jean M. Sanger and Joseph W. Sanger for extensive additional material.
Footnotes
REFERENCES
- Bajer A, Molè-Bajer J. Ciné-micrographic studies on mitosis in endosperm. II. Chromosome, cytoplasm and Brownian movements. Chromosoma. 1956;7:558–607. [Google Scholar]
- Bajer A, Molè-Bajer J. Reorganization of microtubules in endosperm cells and cell fragments of the higher plant Haemanthus in vivo. J Cell Biol. 1986;102:263–281. doi: 10.1083/jcb.102.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RO. Cytological and growth correlations in the flower bud and anther of Lilium longiflorum. Am J Bot. 1948;35:729–739. [Google Scholar]
- Inoué S. Polarization optical studies of the mitotic spindle. I. The demonstration of spindle fibers in living cells. Chromosoma. 1953;5:487–500. doi: 10.1007/BF01271498. [DOI] [PubMed] [Google Scholar]
- Inoué S. Primitive Motile Systems in Cell Biology. R.D. Allen and N. Kamiya, New York: Academic Press; 1964. Organization and function of the mitotic spindle; pp. 549–598. [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol (special issue, Discovery in Cell Biology) 1981;91:131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S, Bajer A. Birefringence in endosperm mitosis. Chromosoma. 1961;12:48–63. doi: 10.1007/BF00328913. [DOI] [PubMed] [Google Scholar]
- Inoué S, Hyde WL. Studies on depolarization of light at microscope lens surfaces. II. The simultaneous realization of high resolution and high sensitivity with the polarizing microscope. J Biophys Biochem Cytol. 1957;3:831–838. doi: 10.1083/jcb.3.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. In: In: Advances in Cell Biology. Prescott DM, Goldstein L, editors. Vol. 2. E.H. McConkey, New York: Appelton-Century-Crofts; 1971. pp. 225–298. [Google Scholar]
- Oldenbourg R. A new view on polarization microscopy. Nature. 1996;381:811–812. doi: 10.1038/381811a0. [DOI] [PubMed] [Google Scholar]
- Oldenbourg R. Methods in Cell Biology: Structure, Composition and Function of the Mitotic/Meiotic Spindle. C. Rieder, New York: Academic Press; 1998. Polarized light microscopy of spindles. (in press). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.