Abstract
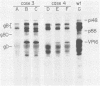
Twenty-two volunteers seronegative for antibodies to herpes simplex virus (HSV) were enrolled in a trial to determine tolerance and immunogenicity of an HSV-2 glycoprotein subunit vaccine. Vaccine was administered at days 0, 28, and 140, and sera were obtained on days 0, 7, 14, 21, 28, 35, 49, 56, 140, 147, and 365 for determination of HSV neutralizing antibody activity and antibody-dependent cell cytotoxicity (ADCC). Sera were also tested by immunoprecipitation of radiolabeled HSV-2-infected cell proteins and polyacrylamide gel electrophoresis to identify the viral proteins which elicited antibody responses in vaccine recipients. After vaccination two male volunteers presented with atypical first-episode genital herpes: patient 1 with a culture-negative genital lesion at day 53 and patient 3 with urethritis at day 68. Seroconversion to wild-type viral proteins not present in the vaccine was detectable by radioimmunoprecipitation-polyacrylamide gel electrophoresis within 10 days in both patients. Two additional volunteers, one a sex contact of patient 1, seroconverted asymptomatically to nonvaccine proteins during the trial. All four vaccine breakthrough patients were indistinguishable from the other volunteers in the time required to develop neutralizing and ADCC antibodies, in the titer of these antibodies, and the time to seroconversion to gB and gD vaccine proteins. However, only one of the four breakthrough patients had antibodies to g80 (a complex of gC-2 and gE) after vaccination as compared with 15 of the other 18 volunteers (P = 0.05). Neither neutralizing antibody nor ADCC titers consistently identified acquisition of wild-type viral infection; therefore, protein-specific serologies were required to detect wild-type antibodies in these four patients. These data underscore the importance of using serologic assays which will distinguish naturally acquired infection from the immune response to vaccination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. P., Rapp F. Concept review of genital herpes vaccines. J Infect Dis. 1982 Mar;145(3):413–421. doi: 10.1093/infdis/145.3.413. [DOI] [PubMed] [Google Scholar]
- Ashley R. L., Corey L. Effect of acyclovir treatment of primary genital herpes on the antibody response to herpes simplex virus. J Clin Invest. 1984 Mar;73(3):681–688. doi: 10.1172/JCI111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley R., Benedetti J., Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985 Oct;17(2):153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- Ashley R., Mertz G., Clark H., Schick M., Salter D., Corey L. Humoral immune response to herpes simplex virus type 2 glycoproteins in patients receiving a glycoprotein subunit vaccine. J Virol. 1985 Nov;56(2):475–481. doi: 10.1128/jvi.56.2.475-481.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. I., Lovett M. A., Bryson Y. J. Serologic analysis of first-episode nonprimary genital herpes simplex virus infection. Presence of type 2 antibody in acute serum samples. Am J Med. 1984 Dec;77(6):1055–1060. doi: 10.1016/0002-9343(84)90188-8. [DOI] [PubMed] [Google Scholar]
- Braun D. K., Pereira L., Norrild B., Roizman B. Application of denatured, electrophoretically separated, and immobilized lysates of herpes simplex virus-infected cells for detection of monoclonal antibodies and for studies of the properties of viral proteins. J Virol. 1983 Apr;46(1):103–112. doi: 10.1128/jvi.46.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Corey L., Adams H. G., Brown Z. A., Holmes K. K. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983 Jun;98(6):958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Buckmaster A., Palfreyman J. W., Hope R. G., Minson A. C. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J Virol. 1984 May;50(2):547–554. doi: 10.1128/jvi.50.2.547-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Buckmaster A., Hancock D., Buchan A., Fuller A., Minson A. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus type 2. J Gen Virol. 1982 Dec;63(2):297–305. doi: 10.1099/0022-1317-63-2-297. [DOI] [PubMed] [Google Scholar]
- Mertz G. J., Peterman G., Ashley R., Jourden J. L., Salter D., Morrison L., McLean A., Corey L. Herpes simplex virus type-2 glycoprotein-subunit vaccine: tolerance and humoral and cellular responses in humans. J Infect Dis. 1984 Aug;150(2):242–249. doi: 10.1093/infdis/150.2.242. [DOI] [PubMed] [Google Scholar]
- Mertz G. J., Schmidt O., Jourden J. L., Guinan M. E., Remington M. L., Fahnlander A., Winter C., Holmes K. K., Corey L. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985 Jan-Mar;12(1):33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Luce C. F., Duffey A. Antibodies to Herpesvirus hominis types 1 and 2 in humans. I. Patients with genital herpetic infections. Am J Epidemiol. 1970 Jun;91(6):539–546. doi: 10.1093/oxfordjournals.aje.a121165. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Roizman B., Warren J., Thuning C. A., Fanshaw M. S., Norrild B., Meignier B. Application of molecular genetics to the design of live herpes simplex virus vaccines. Dev Biol Stand. 1982;52:287–304. [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Stamm W. E., Wagner K. F., Amsel R., Alexander E. R., Turck M., Counts G. W., Holmes K. K. Causes of the acute urethral syndrome in women. N Engl J Med. 1980 Aug 21;303(8):409–415. doi: 10.1056/NEJM198008213030801. [DOI] [PubMed] [Google Scholar]
- Stavraky K. M., Rawls W. E., Chiavetta J., Donner A. P., Wanklin J. M. Sexual and socioeconomic factors affecting the risk of past infections with herpes simplex virus type 2. Am J Epidemiol. 1983 Jul;118(1):109–121. doi: 10.1093/oxfordjournals.aje.a113612. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Zang E. A., Harley E. J., Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981 Sep-Oct;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]