Abstract
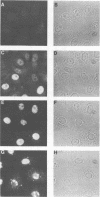
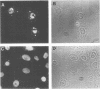
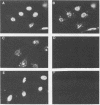
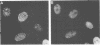
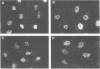
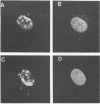
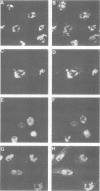
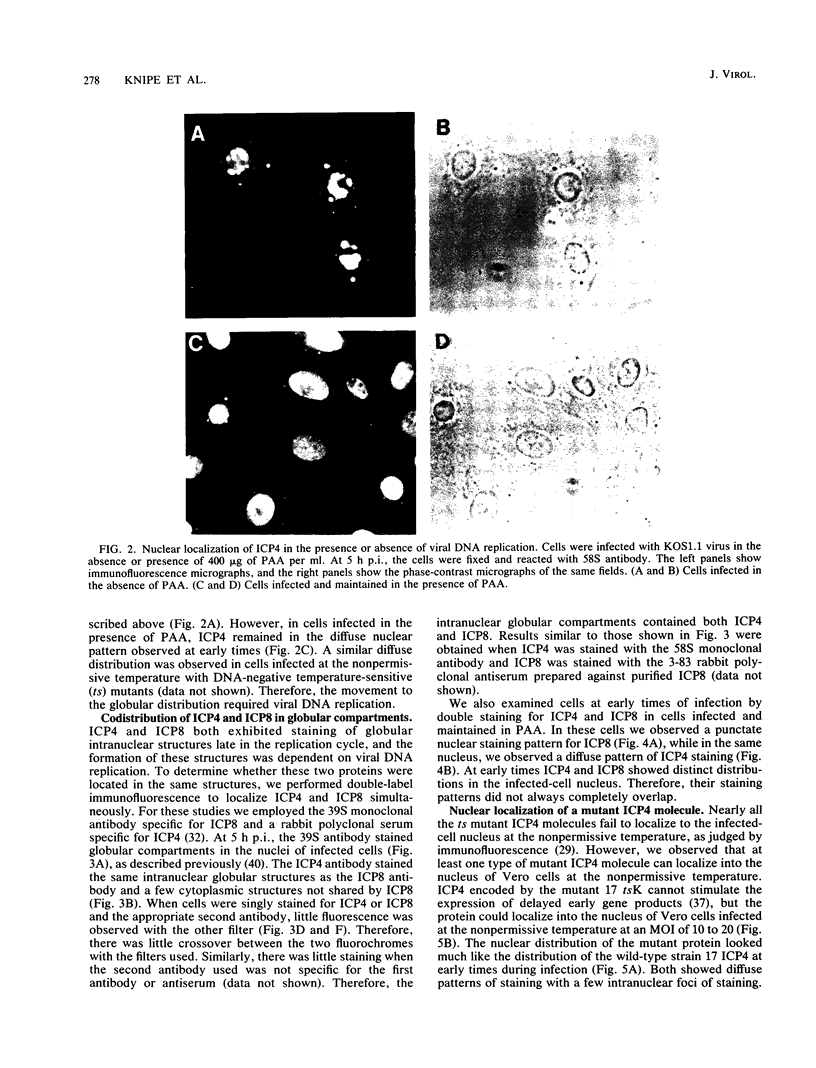
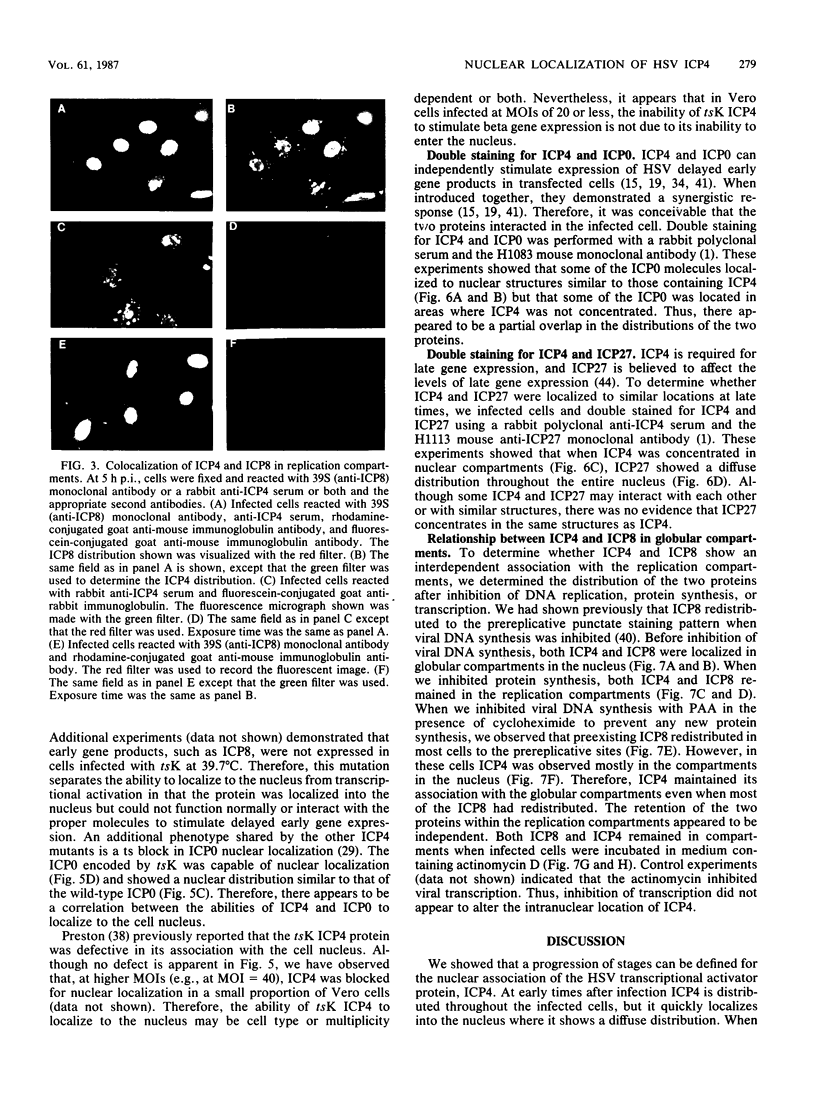
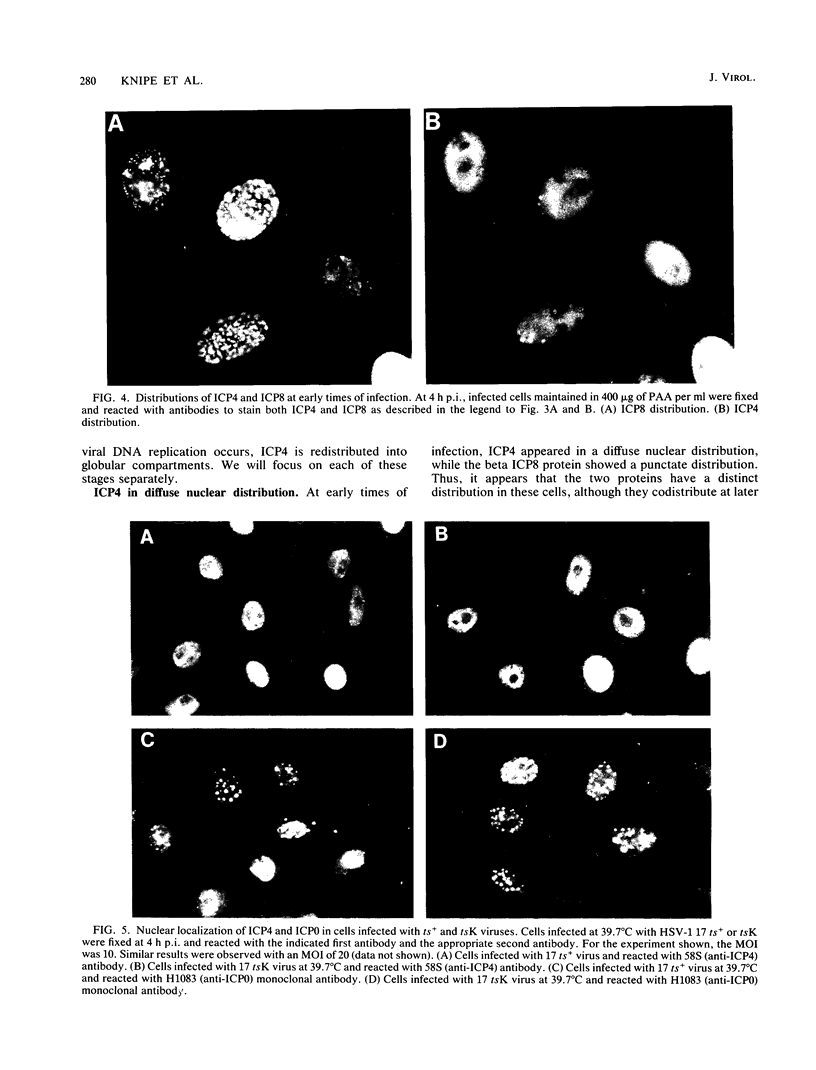
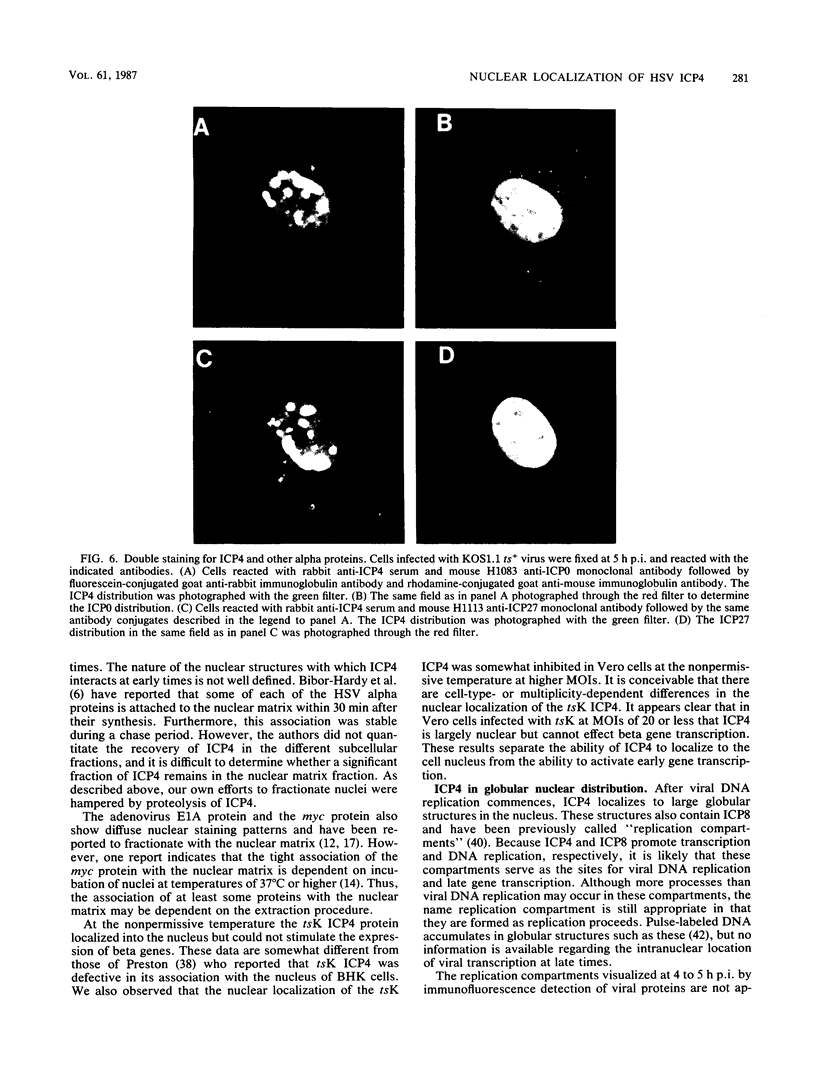
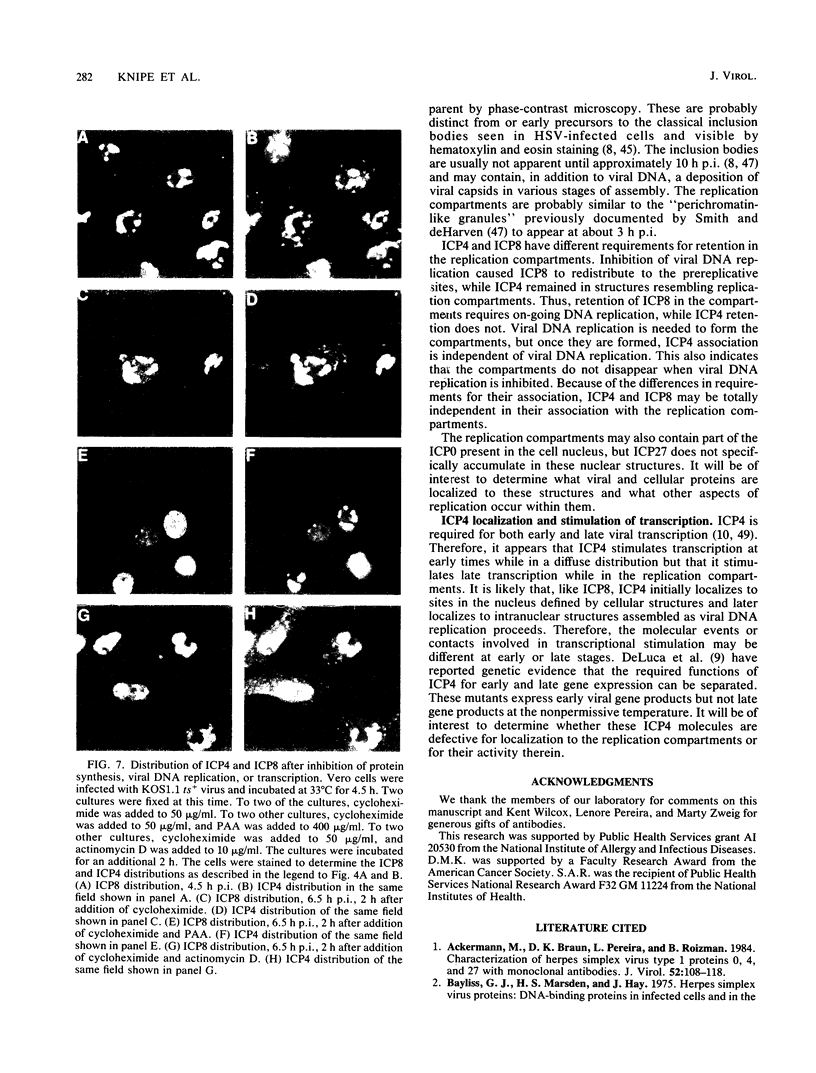
The nuclear localization of the herpes simplex virus transcriptional activator protein ICP4 was studied by indirect immunofluorescence. At early times after viral infection, ICP4 quickly localized to a diffuse intranuclear distribution. ICP4 later concentrated in globular compartments within the nucleus. The redistribution to the compartments was dependent on viral DNA replication. Double staining for ICP4 and ICP8, the early major DNA-binding protein, revealed that both were found in the same intranuclear globular compartments at late times. These were previously named "replication compartments" (M. P. Quinlan, L. B. Chen, and D. M. Knipe, Cell 36:857-868, 1984). Because ICP4 and ICP8 are known to function in transcriptional activation and DNA replication, respectively, both DNA replication and late transcription may occur in these compartments. The association of ICP4 and ICP8 with the replication compartments appeared to be independent in that the retention of ICP8 in the compartments required ongoing viral DNA synthesis, while the association of ICP4 was independent of viral DNA synthesis once the compartments were formed. Because ICP4 shows a different distribution at early and late times, stimulation of transcription by ICP4 may involve different molecular events or contacts during these two periods of the replicative cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Braun D. K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984 Oct;52(1):108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Beard P., Faber S., Wilcox K. W., Pizer L. I. Herpes simplex virus immediate early infected-cell polypeptide 4 binds to DNA and promotes transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4016–4020. doi: 10.1073/pnas.83.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Hoffmann P., Brown L., Feldman L., Blankenship M. L. Partial characterization of temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Oct 30;122(2):251–267. doi: 10.1016/0042-6822(82)90225-2. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bibor-Hardy V., Bernard M., Simard R. Nuclear matrix modifications at different stages of infection by herpes simplex virus type 1. J Gen Virol. 1985 May;66(Pt 5):1095–1103. doi: 10.1099/0022-1317-66-5-1095. [DOI] [PubMed] [Google Scholar]
- Brady J., Bolen J. B., Radonovich M., Salzman N., Khoury G. Stimulation of simian virus 40 late gene expression by simian virus 40 tumor antigen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2040–2044. doi: 10.1073/pnas.81.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROUSE H. V., CORIELL L. L., BLANK H., SCOTT T. F. M. Cytochemical studies on the intranuclear inclusion of herpes simplex. J Immunol. 1950 Jul;65(1):119–128. [PubMed] [Google Scholar]
- DeLuca N. A., Courtney M. A., Schaffer P. A. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J Virol. 1984 Dec;52(3):767–776. doi: 10.1128/jvi.52.3.767-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Coen D. M., McKnight S. L. Promoter domains required for expression of plasmid-borne copies of the herpes simplex virus thymidine kinase gene in virus-infected mouse fibroblasts and microinjected frog oocytes. Mol Cell Biol. 1985 Aug;5(8):1940–1947. doi: 10.1128/mcb.5.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElKareh A., Murphy A. J., Fichter T., Efstratiadis A., Silverstein S. "Transactivation" control signals in the promoter of the herpesvirus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1002–1006. doi: 10.1073/pnas.82.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Hancock D. C. Studies on the interaction of the human c-myc protein with cell nuclei: p62c-myc as a member of a discrete subset of nuclear proteins. Cell. 1985 Nov;43(1):253–261. doi: 10.1016/0092-8674(85)90030-3. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Nevins J. R. Localization of the adenovirus E1Aa protein, a positive-acting transcriptional factor, in infected cells infected cells. Mol Cell Biol. 1983 May;3(5):829–838. doi: 10.1128/mcb.3.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. J., Powell K. L. DNA-binding properties of a herpes simplex virus immediate early protein. J Virol. 1982 Dec;44(3):1084–1087. doi: 10.1128/jvi.44.3.1084-1087.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Hughes R. G., Jr, Munyon W. H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975 Aug;16(2):275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Ruyechan W. T., Honess R. W., Roizman B. Molecular genetics of herpes simplex virus: the terminal a sequences of the L and S components are obligatorily identical and constitute a part of a structural gene mapping predominantly in the S component. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4534–4538. doi: 10.1073/pnas.76.9.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Smith J. L. A mutant herpesvirus protein leads to a block in nuclear localization of other viral proteins. Mol Cell Biol. 1986 Jul;6(7):2371–2381. doi: 10.1128/mcb.6.7.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Spang A. E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982 Jul;43(1):314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Metzler D. W., Wilcox K. W. Isolation of herpes simplex virus regulatory protein ICP4 as a homodimeric complex. J Virol. 1985 Aug;55(2):329–337. doi: 10.1128/jvi.55.2.329-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Persson R. H., Bacchetti S., Smiley J. R. Cells that constitutively express the herpes simplex virus immediate-early protein ICP4 allow efficient activation of viral delayed-early genes in trans. J Virol. 1985 May;54(2):414–421. doi: 10.1128/jvi.54.2.414-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Abnormal properties of an immediate early polypeptide in cells infected with the herpes simplex virus type 1 mutant tsK. J Virol. 1979 Nov;32(2):357–369. doi: 10.1128/jvi.32.2.357-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Notarianni E. L. Poly(ADP-ribosyl)ation of a herpes simplex virus immediate early polypeptide. Virology. 1983 Dec;131(2):492–501. doi: 10.1016/0042-6822(83)90515-9. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985 May;5(5):957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Atkinson M. A., Hay J. Intranuclear distribution of herpes simplex virus type 2 DNA synthesis: examination by light and electron microscopy. J Gen Virol. 1983 Sep;64(Pt 9):2087–2092. doi: 10.1099/0022-1317-64-9-2087. [DOI] [PubMed] [Google Scholar]
- SCOTT T. F. M., BURGOON C. F., CORIELL L. L., BLANK H. The growth curve of the virus of herpes simplex in rabbit corneal cells grown in tissue culture with parallel observations on the development of the intranuclear inclusion body. J Immunol. 1953 Dec;71(6):385–396. [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Limited proteolysis of herpes simplex virus glycoproteins that occurs during their extraction from vero cells. J Virol. 1984 Apr;50(1):258–262. doi: 10.1128/jvi.50.1.258-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]