Abstract
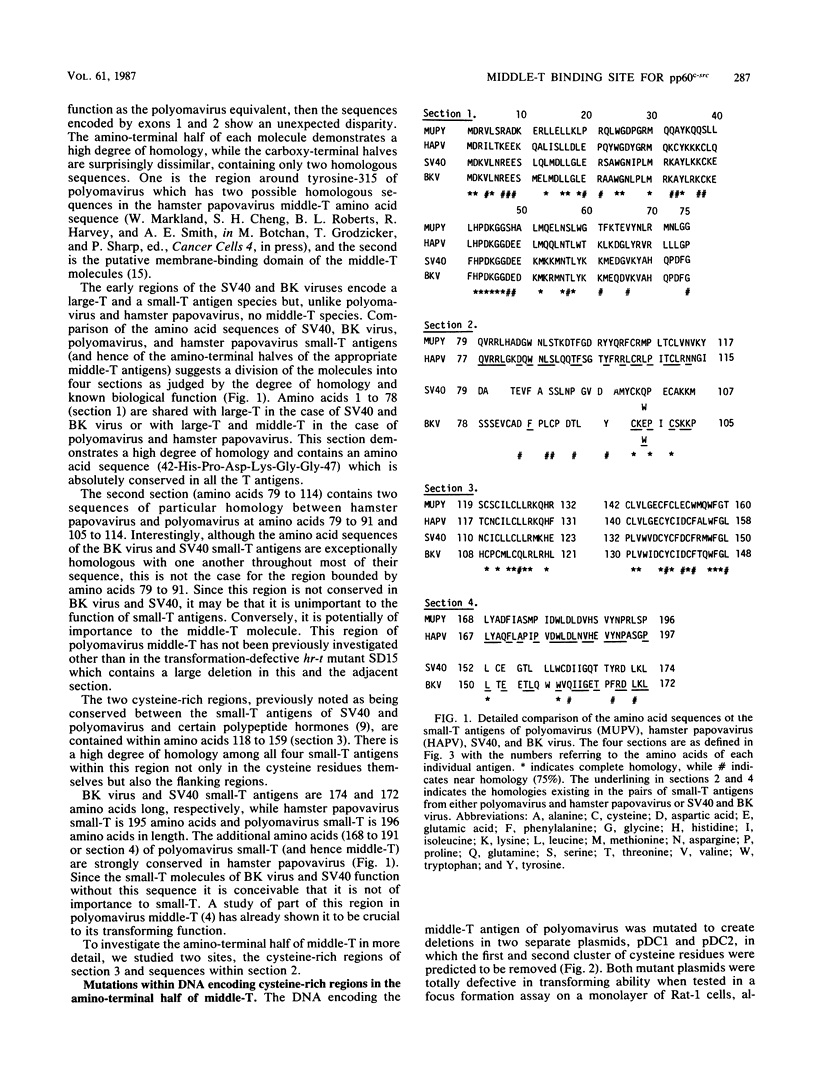
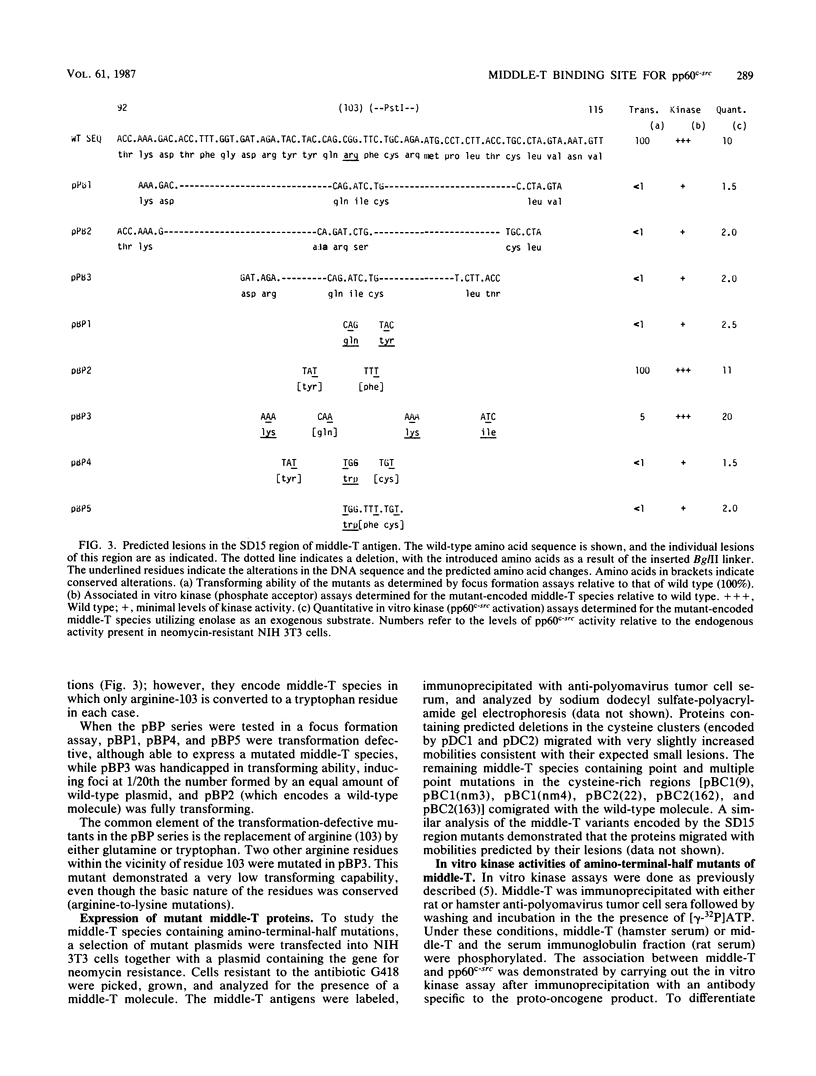
The majority of the carboxy-terminal half of polyomavirus middle-T antigen has been variously mutated and, with the exception of the putative membrane-binding domain (amino acids 394 to 415), was found to be largely dispensible for the transforming activity of the protein. A comparison of the small-T antigen amino acid sequences (equivalent to the region of middle-T encoded by exon 1) of simian virus 40, BK virus, polyomavirus, and a recently described hamster papovavirus highlighted regions of potential interest in mapping functions to the amino-terminal half of polyomavirus middle-T antigen. The regions of interest include amino acids 168 to 191 (previously investigated by this group [S. H. Cheng, W. Markland, A. F. Markham, and A. E. Smith, EMBO J. 5:325-334, 1986]), two cysteine-rich clusters (amino acids 120 to 125 and 148 to 153), and amino acids 92 to 117 (within the limits of the previously described hr-t mutant, SD15). Point mutations, multiple point mutations, and deletions were made by site-specific and site-directed mutagenesis within the cysteine-rich clusters and residues 92 to 117. Studies of the transforming ability of the altered middle-T species demonstrated that this activity is highly sensitive to amino acid changes. All four regions (as defined above) within the amino-terminal half of middle-T have now been studied in detail. The phenotype of the mutants is predominantly transformation defective, and the corresponding variant middle-T species are characterized by being either totally or severely handicapped in the ability to associate actively with pp60c-src. Whether the mutations affect the regions of interaction between middle-T and pp60c-src or simply interfere with the overall conformation of this domain is not known. However, there would appear to be a conformational constraint on this portion of the molecule with regard to its interaction with pp60c-src and by extension to the ability of the middle-T species to transform.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin T. L. The hr-t gene of polyoma virus. Biochim Biophys Acta. 1982 Dec 21;695(2):69–95. doi: 10.1016/0304-419x(82)90018-x. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Benjamin T. L. Identification of DNA sequence changes leading to loss of transforming ability in polyoma virus. J Biol Chem. 1980 Jan 10;255(1):230–235. [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Delmas V., Bastien C., Scherneck S., Feunteun J. A new member of the polyomavirus family: the hamster papovavirus. Complete nucleotide sequence and transformation properties. EMBO J. 1985 May;4(5):1279–1286. doi: 10.1002/j.1460-2075.1985.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Dilworth S. M. Polyomavirus: an overview of its unique properties. Adv Cancer Res. 1983;39:183–268. doi: 10.1016/s0065-230x(08)61036-2. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Oostra B. A., Ely B. K., Smith A. E. Deletion loop mutagenesis: a novel method for the construction of point mutations using deletion mutants. Nucleic Acids Res. 1982 Sep 11;10(17):5161–5171. doi: 10.1093/nar/10.17.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Yaniv M. Deletions of N-terminal sequences of polyoma virus T-antigens reduce but do not abolish transformation of rat fibroblasts. Mol Cell Biol. 1982 Oct;2(10):1238–1246. doi: 10.1128/mcb.2.10.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Cheng S. H., Oostra B. A., Smith A. E. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J Virol. 1986 Jul;59(1):82–89. doi: 10.1128/jvi.59.1.82-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Oostra B. A., Harvey R., Markham A. F., Colledge W. H., Smith A. E. Site-directed mutagenesis of polyomavirus middle-T antigen sequences encoding tyrosine 315 and tyrosine 250. J Virol. 1986 Aug;59(2):384–391. doi: 10.1128/jvi.59.2.384-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Benjamin T. L. 12-O-tetradecanoylphorbol-13-acetate stimulates phosphorylation of the 58,000-Mr form of polyomavirus middle T antigen in vivo: implications for a possible role of protein kinase C in middle T function. J Virol. 1986 May;58(2):239–246. doi: 10.1128/jvi.58.2.239-246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Satake M., Robins T., Ito Y. Isolation and characterization of NIH 3T3 cells expressing polyomavirus small T antigen. J Virol. 1986 Oct;60(1):105–113. doi: 10.1128/jvi.60.1.105-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Raptis L., Boynton A. L., Whitfield J. F. Protein kinase C promotes the phosphorylation of immunoprecipitated middle T antigen from polyoma-transformed cells. Biochem Biophys Res Commun. 1986 May 14;136(3):995–1000. doi: 10.1016/0006-291x(86)90431-6. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol Cell Biol. 1984 May;4(5):817–821. doi: 10.1128/mcb.4.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]