Abstract
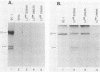
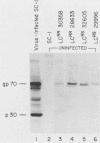
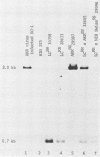
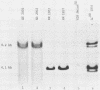
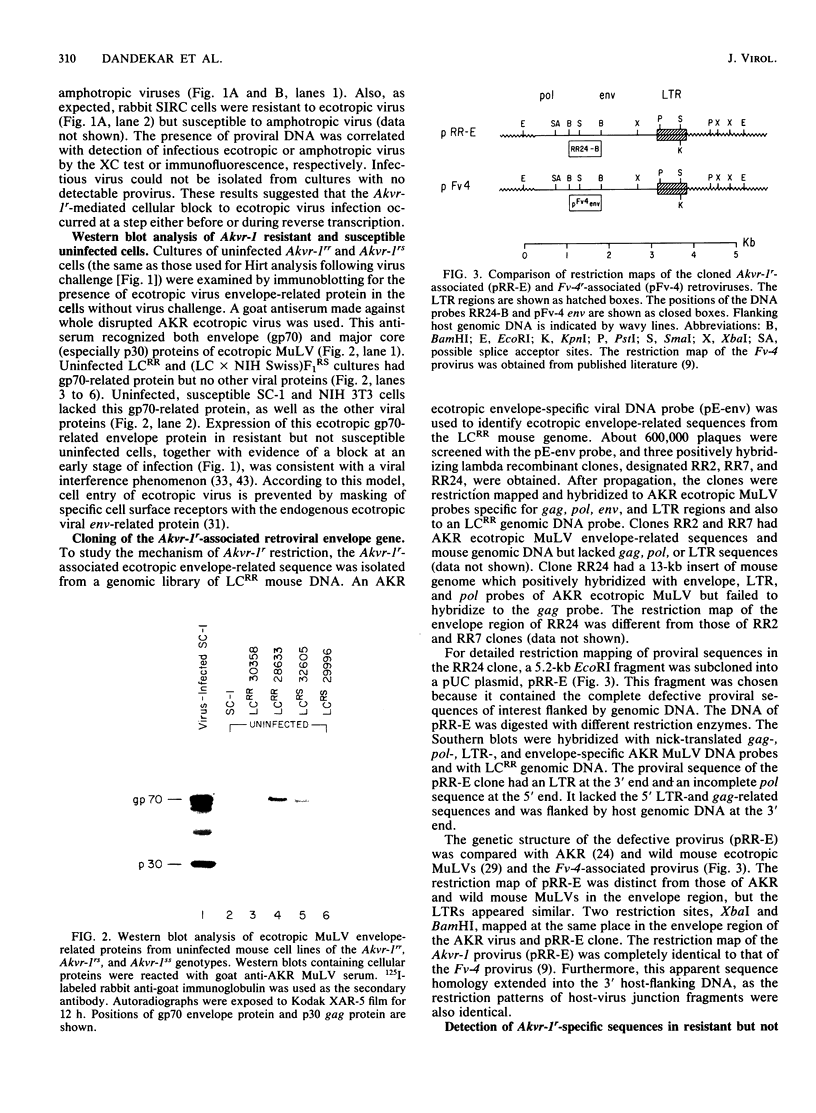
A dominant restriction allele, Akvr-1r, from California wild mice (Mus musculus domesticus) confers resistance to exogenous ecotropic murine leukemia virus (MuLV) infection. The presence of an ecotropic MuLV envelope-related glycoprotein in uninfected virus-resistant cells suggests that viral interference is a possible mechanism for this resistance. We molecularly cloned the ecotropic MuLV envelope-related sequence from the genomic DNA of a wild mouse homozygous for the Akvr-1r locus. The cloned provirus was defective and contained a C-terminal end of the pol gene, a complete envelope gene, and a 3' long terminal repeat. The presence of this provirus was directly correlated with Akvr-1r-mediated virus resistance in cell cultures and hybrid mice. The Akvr-1r provirus restriction map and partial DNA sequence were identical to those of the Fv-4r allele, an ecotropic MuLV resistance locus from Japanese feral mice (M. musculus molossinus), which was previously shown to be allelic with the Akvr-1r gene. The 3' host flanking sequences of Fv-4r and Akvr-1r also had identical restriction maps. These findings indicate that Akvr-1r and Fv-4r are the same gene. It was probably acquired by interbreeding of these feral species in recent times. Conservation of this locus might be favored by the useful function that it performs in protection against ecotropic MuLV infection endemic in both populations of wild mice.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Ruscetti S., Ali I., Haapala D. K., Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982 Nov;123(1):139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. A cell membrane "gp70" associated with Fv-4 gene: immunological characterization, and tissue and strain distribution. Virology. 1984 Feb;133(1):65–76. doi: 10.1016/0042-6822(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sato H., Odaka T. Mapping of the Fv-4 mouse gene controlling resistance to murine leukemia viruses. Int J Cancer. 1981 Aug 15;28(2):237–240. doi: 10.1002/ijc.2910280218. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai K., Ikeda H., Yuasa Y., Suzuki S., Odaka T. Mouse strain resistant to N-, B-, and NB-tropic murine leukemia viruses. J Virol. 1976 Nov;20(2):436–440. doi: 10.1128/jvi.20.2.436-440.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Gromet N. J., Ikeda H., Buckler C. E. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc Natl Acad Sci U S A. 1984 Feb;81(3):834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., Berman E. J., Estes J. D., Gardner M. B. Murine retroviral restriction genes Fv-4 and Akvr-1 are alleles of a single locus. J Virol. 1983 Sep;47(3):649–651. doi: 10.1128/jvi.47.3.649-651.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T., Ikeda H., Moriwaki K., Matsuzawa A., Mizuno M., Kondo K. Genetic resistance in Japanese wild mice (Mus musculus molossinus) to an NB-tropic Friend murine leukemia virus. J Natl Cancer Inst. 1978 Nov;61(5):1301–1306. doi: 10.1093/jnci/61.5.1301. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Pani P. K., Weiss R. A. A dominant epistatic gene which inhibits cellular susceptibility to RSV(RAV-O). J Gen Virol. 1971 Dec;13(3):455–462. doi: 10.1099/0022-1317-13-3-455. [DOI] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B. Resistance to fibroblasts and hematopoietic cells to ecotropic murine leukemia virus infection; an Akvr-1R gene effect. Int J Cancer. 1983 Apr 15;31(4):491–496. doi: 10.1002/ijc.2910310415. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Toth E., Gardner M. B. Characterization of purely ecotropic and amphotropic naturally occurring wild mouse leukemia viruses. Intervirology. 1977;8(6):323–335. doi: 10.1159/000148908. [DOI] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Cheong M. P., Hartley J. W., Huebner R. J. A viral interference test for mouse leukemia viruses. Virology. 1967 Sep;33(1):180–184. doi: 10.1016/0042-6822(67)90111-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Bird S., Weinberg R. A. Evidence for the Asiatic origin of endogenous AKR-type murine leukemia proviruses. J Virol. 1980 Sep;35(3):824–835. doi: 10.1128/jvi.35.3.824-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. FV-4: a new gene affecting the splenomegaly induction by Friend leukemia virus. Jpn J Exp Med. 1975 Dec;45(6):473–478. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965 Aug;26(4):664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Naito Y., Moriwaki K. Unstable resistance of G mouse fibroblasts to ecotropic murine leukemia virus infection. J Virol. 1979 Mar;29(3):1078–1086. doi: 10.1128/jvi.29.3.1078-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Odaka T. Surface antigen expressed in hematopoietic cells derived from Fv-4r mouse strains. J Natl Cancer Inst. 1982 Jun;68(6):1005–1009. [PubMed] [Google Scholar]