Abstract
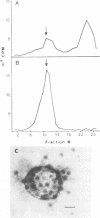
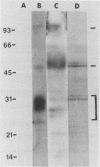
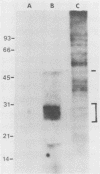
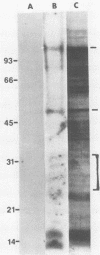
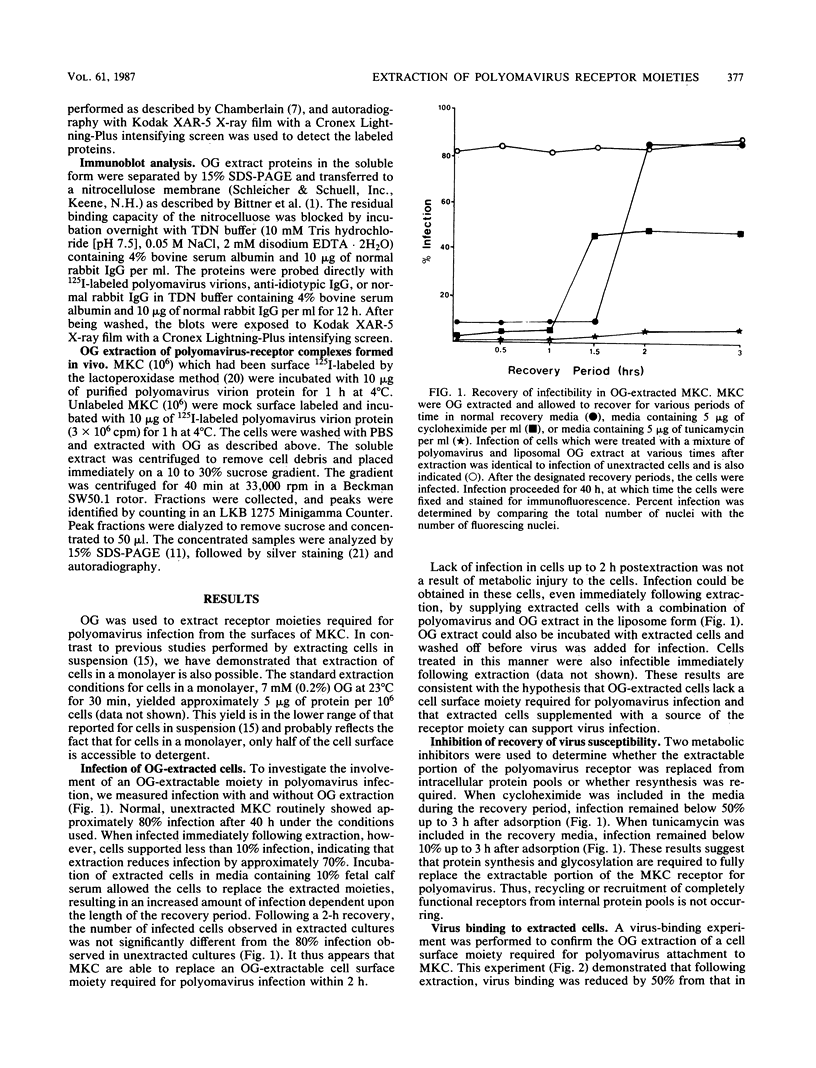
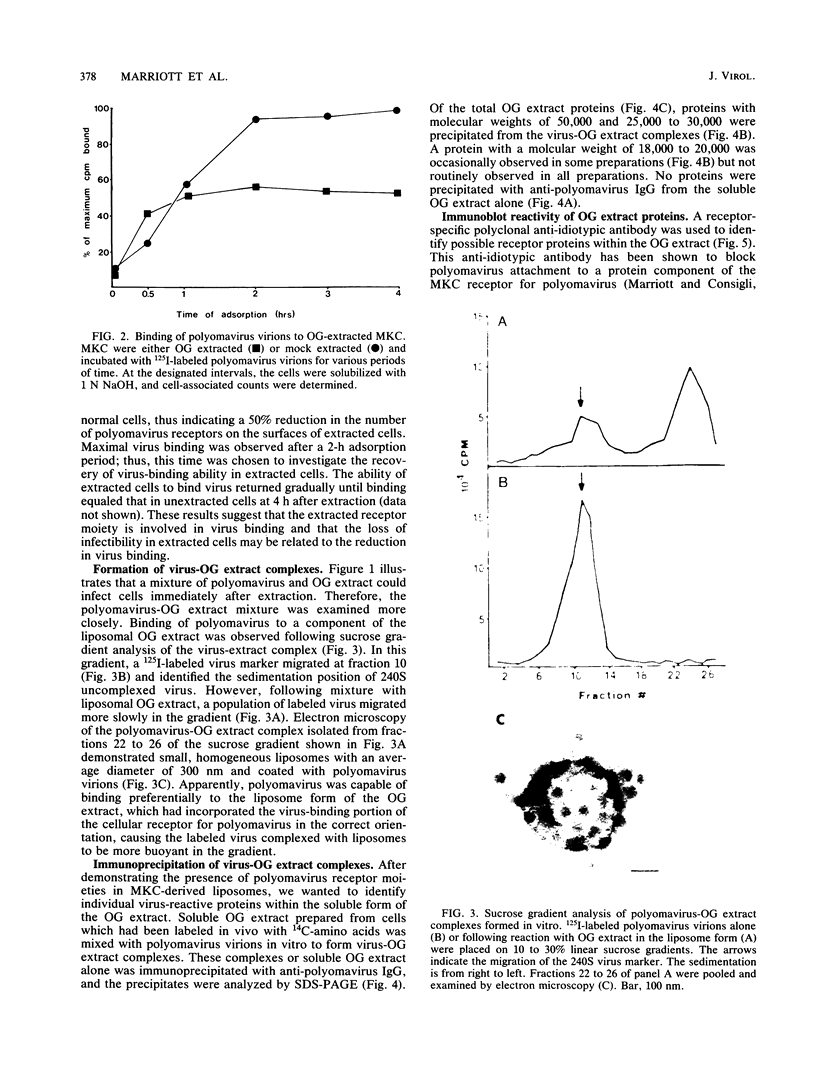
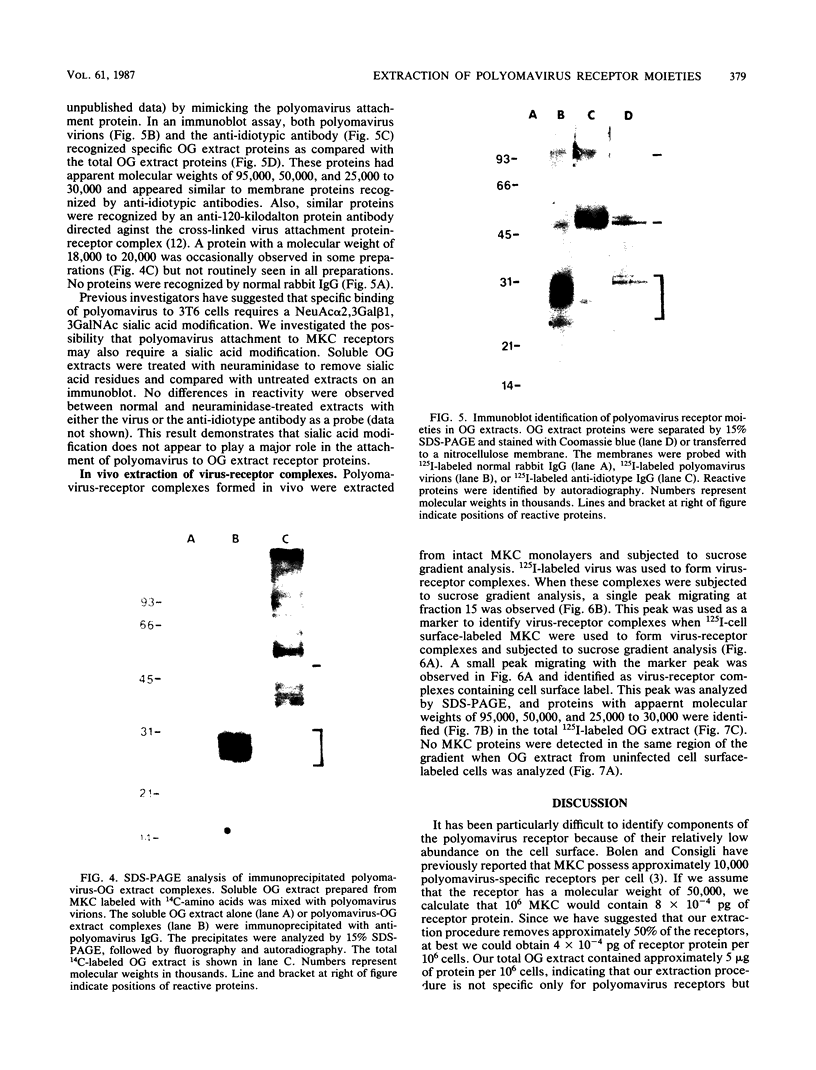
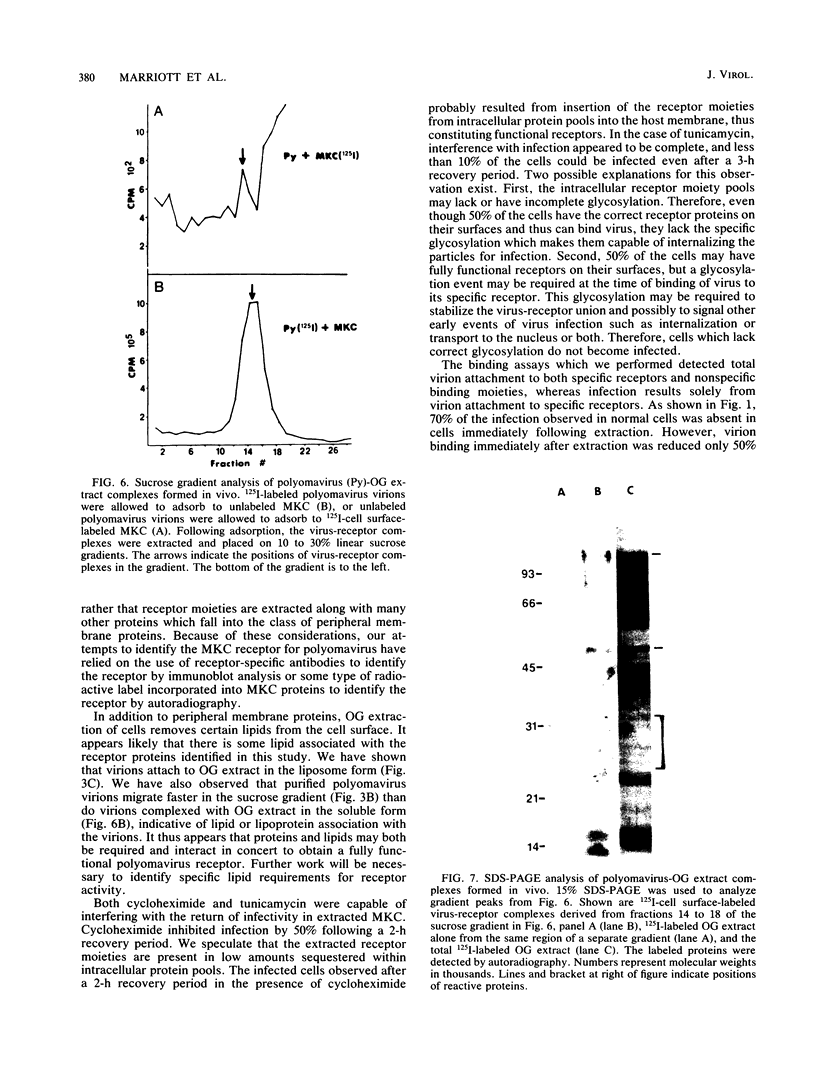
Polyomavirus receptor moieties were extracted from the surfaces of mouse kidney cells with the nonionic detergent octyl-beta-D-glucopyranoside. Following extraction with this detergent, mouse kidney cells were refractory to polyomavirus infection. Binding studies demonstrated that this loss of susceptibility resulted from extraction of a peripheral membrane protein or proteins required for proper virus attachment to and infection of mouse kidney cells. Infection of extracted mouse kidney cells returned following a 2-h recovery period. However, the presence of cycloheximide or tunicamycin in the recovery media interfered with recovery from infection. Cells could be infected immediately after extraction by supplying them with the extracted moieties prior to or concomitant with infection. A complex of polyomavirus and the extracted receptor protein was formed by in vitro incubation and was stable in sucrose gradient analysis. Functional receptor moieties were prepared in the form of liposomes from the detergent extract. The virus-receptor complex was immunoprecipitated with anti-polyomavirus immunoglobulin G, and the portion of the complex contributed by the cell was identified. Immunoblot analysis of the mouse kidney cell detergent extract with a receptor-specific 125I-labeled anti-idiotypic antibody or 125I-labeled polyomavirus demonstrated several reactive proteins. Attachment of polyomavirus to mouse kidney cells, followed by extraction of the virus-receptor complex, identified polyomavirus-binding proteins similar to those observed in in vitro binding. Proteins with molecular weights of approximately 95,000, 50,000 and 25,000 to 30,000 were consistently observed in all receptor assays. The relationship between these proteins and their possible involvement as the cell receptor for polyomavirus are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Anders D. G., Trempy J., Consigli R. A. Differences in the subpopulations of the structural proteins of polyoma virions and capsids: biological functions of the multiple VP1 species. J Virol. 1981 Jan;37(1):80–91. doi: 10.1128/jvi.37.1.80-91.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Separation of neutralizing and hemagglutination-inhibiting antibody activities and specificity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1980 Apr;34(1):119–129. doi: 10.1128/jvi.34.1.119-129.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Fried H., Cahan L. D., Paulson J. C. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology. 1981 Feb;109(1):188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Decapsidation of polyoma virus: identification of subviral species. Virology. 1975 Nov;68(1):245–255. doi: 10.1016/0042-6822(75)90165-8. [DOI] [PubMed] [Google Scholar]
- Griffith G. R., Consigli R. A. Cross-linking of a polyomavirus attachment protein to its mouse kidney cell receptor. J Virol. 1986 Jun;58(3):773–781. doi: 10.1128/jvi.58.3.773-781.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G. R., Consigli R. A. Isolation and characterization of monopinocytotic vesicles containing polyomavirus from the cytoplasm of infected mouse kidney cells. J Virol. 1984 Apr;50(1):77–85. doi: 10.1128/jvi.50.1.77-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeGrue S. J., Macek C. M., Kahan B. D. Noncytolytic extraction of murine tumor-specific transplantation antigens with the nonionic detergent octyl-beta-D-glucopyranoside. J Natl Cancer Inst. 1982 Jul;69(1):131–136. [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Consigli R. A. Production and characterization of monoclonal antibodies to polyomavirus major capsid protein VP1. J Virol. 1985 Nov;56(2):365–372. doi: 10.1128/jvi.56.2.365-372.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. Lactoperoxidase-catalyzed iodination as a tool for investigation of proteins. Methods Enzymol. 1980;70(A):214–220. doi: 10.1016/s0076-6879(80)70051-4. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston V. D., Bolen J. B., Consigli R. A. Isolation and characterization of polyoma uncoating intermediates from the nuclei of infected mouse cells. J Virol. 1980 Mar;33(3):1173–1181. doi: 10.1128/jvi.33.3.1173-1181.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]