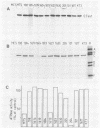
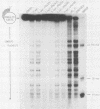
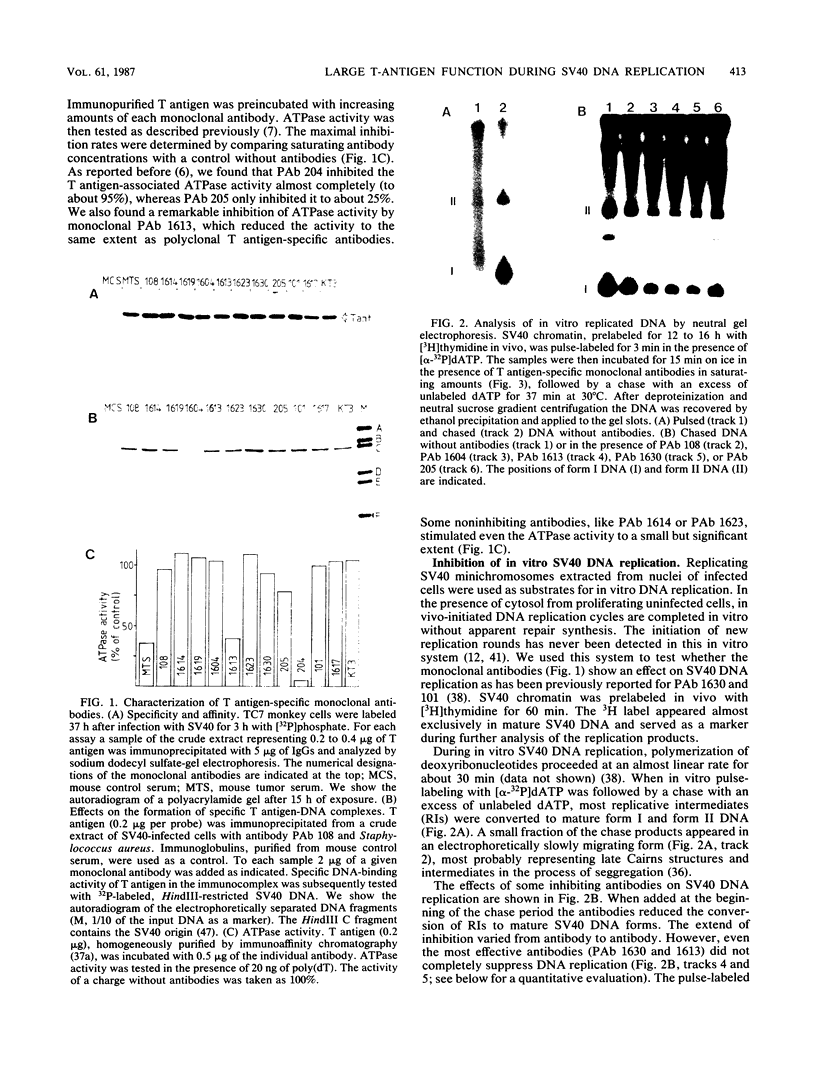
Abstract
Various monoclonal antibodies specific for simian virus 40 large tumor antigen (T antigen) inhibit the elongation process of viral DNA replication in an in vitro system. The results provide strong evidence for a function intrinsic to T antigen during ongoing replicative-chain elongation. The antibody inhibition studies were further used to establish a correlation between the known biochemical activities of T antigen and its function during the elongation phase. The data demonstrate that, in addition to DNA binding and ATPase, a third function of T antigen is required for replicative chain elongation. This function is most probably related to the recently described DNA helicase activity of T antigen. This conclusion is based on the following results: aphidicolin treatment of actively replicating simian virus 40 minichromosomes causes a partial uncoupling of parental DNA strand separation and DNA synthesis; the strand separation reaction is blocked by the same monoclonal antibodies which strongly inhibit the elongation process. DNA helicase activity of isolated T antigen is equally well inhibited by the same set of monoclonal antibodies that affect minichromosome replication in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Sugano S. Initiation of simian virus 40 DNA replication in vitro. J Virol. 1983 Nov;48(2):481–491. doi: 10.1128/jvi.48.2.481-491.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Balabanova H., Fridlender B. R., Anderer F. A. Stimulation of DNA polymerase alpha by a nuclear DNA/protein complex. J Supramol Struct Cell Biochem. 1981;16(1):1–13. doi: 10.1002/jsscb.1981.380160102. [DOI] [PubMed] [Google Scholar]
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann E. A. DNA-binding properties of phosphorylated and dephosphorylated D2-T antigen, a simian-virus-40 T-antigen-related protein. Eur J Biochem. 1985 Mar 15;147(3):495–501. doi: 10.1111/j.0014-2956.1985.00495.x. [DOI] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. L., Wright P. J., DeLucia A. L., Lewton B. A., Anderson M. E., Tegtmeyer P. Critical spatial requirement within the origin of simian virus 40 DNA replication. J Virol. 1984 Jul;51(1):91–96. doi: 10.1128/jvi.51.1.91-96.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Tornow J., Clark R., Tjian R. Properties of the simian virus 40 (SV40) large T antigens encoded by SV40 mutants with deletions in gene A. J Virol. 1986 Feb;57(2):539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Beard P., Berg P. Synthesis of Superhelical Simian Virus 40 Deoxyribonucleic Acid in Cell Lysates*. J Biol Chem. 1975 Jun 10;250(11):4340–4347. [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Uncoupling of SV40 tsA replicon activation from DNA chain elongation by temperature shifts and aphidicolin arrest. Nucleic Acids Res. 1982 Jan 22;10(2):763–773. doi: 10.1093/nar/10.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Brauer D., Otto B., Fanning E., Knippers R. Subclasses of simian-virus-40 large tumor antigen. Partial purification and DNA-binding properties of two subclasses of tumor antigen from productively infected cells. Eur J Biochem. 1982 Nov;128(1):53–62. [PubMed] [Google Scholar]
- Dröge P., Sogo J. M., Stahl H. Inhibition of DNA synthesis by aphidicolin induces supercoiling in simian virus 40 replicative intermediates. EMBO J. 1985 Dec 1;4(12):3241–3246. doi: 10.1002/j.1460-2075.1985.tb04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzpeter M., Fanning E., Deppert W. A new sensitive target-bound DNA binding assay for SV40 large T antigen. Virology. 1986 Jan 15;148(1):159–167. doi: 10.1016/0042-6822(86)90411-3. [DOI] [PubMed] [Google Scholar]
- Jones C., Su R. T. DNA polymerase alpha from the nuclear matrix of cells infected with simian virus 40. Nucleic Acids Res. 1982 Sep 25;10(18):5517–5532. doi: 10.1093/nar/10.18.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur H., Walter G. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J Virol. 1984 Nov;52(2):483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Mole S. E., Lane D. P. Use of simian virus 40 large T-beta-galactosidase fusion proteins in an immunochemical analysis of simian virus 40 large T antigen. J Virol. 1985 Jun;54(3):703–710. doi: 10.1128/jvi.54.3.703-710.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J., Renart J., Crawford L. V., Stark G. R. Specific association of simian virus 40 tumor antigen with simian virus 40 chromatin. J Virol. 1980 Jan;33(1):78–87. doi: 10.1128/jvi.33.1.78-87.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Scheu R., Otto B. Replication of simian virus 40 chromatin in vitro depends on the amount of DNA polymerase alpha associated with replicating chromatin. Eur J Biochem. 1980 Aug;109(1):67–73. doi: 10.1111/j.1432-1033.1980.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M., Sugano S., Yamaguchi N. Association of simian virus 40 T antigen with replicating nucleoprotein complexes of simian virus 40. J Virol. 1980 Aug;35(2):320–330. doi: 10.1128/jvi.35.2.320-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Simmons D. T. DNA-binding region of the simian virus 40 tumor antigen. J Virol. 1986 Mar;57(3):776–785. doi: 10.1128/jvi.57.3.776-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stahl H., Bauer M., Knippers R. The simian-virus-40 large-tumor antigen in replicating viral chromatin. A salt-resistant protein-DNA interaction. Eur J Biochem. 1983 Jul 15;134(1):55–61. doi: 10.1111/j.1432-1033.1983.tb07530.x. [DOI] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Zentgraf H., Knippers R. A large-tumor-antigen-specific monoclonal antibody inhibits DNA replication of simian virus 40 minichromosomes in an in vitro elongation system. J Virol. 1985 May;54(2):473–482. doi: 10.1128/jvi.54.2.473-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Knippers R. Simian virus 40 large tumor antigen on replicating viral chromatin: tight binding and localization on the viral genome. J Virol. 1983 Jul;47(1):65–76. doi: 10.1128/jvi.47.1.65-76.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., DePamphilis M. L. Analysis of simian virus 40 chromosome-T-antigen complexes: T-antigen is preferentially associated with early replicating DNA intermediates. J Virol. 1983 Oct;48(1):281–295. doi: 10.1128/jvi.48.1.281-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Free and viral chromosome-bound simian virus 40 T antigen: changes in reactivity of specific antigenic determinants during lytic infection. J Virol. 1986 May;58(2):635–646. doi: 10.1128/jvi.58.2.635-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Taylor T. S., Haines L. L., Bradley M. K., Martin R. G., Livingston D. M. Binding of simian virus 40 large T antigen from virus-infected monkey cells to wild-type and mutant viral replication origins. J Mol Biol. 1983 Aug 25;168(4):791–808. doi: 10.1016/s0022-2836(83)80075-8. [DOI] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Evans M. J., Burke J. F., Tsubota Y., Plummer M. J., Huberman J. A. In vitro DNA synthesis by an alpha-like DNA polymerase bound to replicating simian virus 40 chromosomes. J Virol. 1983 Oct;48(1):304–308. doi: 10.1128/jvi.48.1.304-308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. G., Tevethia M. J., Lewton B. A., Tegtmeyer P. DNA binding properties of simian virus 40 temperature-sensitive A proteins. J Virol. 1982 Nov;44(2):458–466. doi: 10.1128/jvi.44.2.458-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]