INTRODUCTION
The organization and function of the Golgi complex has intrigued cell biologists for many years (Farquhar and Palade, 1998; Mazzarello and Bentaivoglio, 1998). This is due both to the Golgi’s central role in secretory membrane traffic and sorting, and to its unique structure and dynamics. The Golgi complex receives newly synthesized proteins and lipid from the ER, covalently modifies them, and then repackages them for delivery to plasma membrane, lysosomes, or secretory granules. It also recycles membrane components back to the ER for continued use. These diverse functions depend on small membrane-bound transport intermediates that shuttle protein into and out of the Golgi’s stack of flattened cisternae and associated tubules and vesicles. Such transport intermediates regulate protein and membrane flux into and out of the Golgi complex and thereby control the steady-state size and location of this organelle. In so doing, they ensure the proper functioning of the Golgi complex, enabling the cell to tailor its biosynthetic and secretory products for specific cellular needs.
The emergence of green fluorescent protein (GFP) technology has enabled proteins to be visualized in the unperturbed environment of the living cell (Chalfie et al., 1994). This allows direct observation of intracellular structures, whose life history and pathways previously have only been deduced. In the movies shown below, membrane proteins fused to GFP were used to visualize the dynamics of the Golgi complex and the transport intermediates that shuttle membrane proteins into and out of this organelle. Included are time-lapse sequences that show the formation, translocation, and fate of Golgi-targeted/ derived transport intermediates. Also shown are movies revealing how the distribution/morphology of the Golgi complex is affected when such processes are perturbed. In addition, results of photobleaching experiments characterizing the lateral mobility of proteins residing within the Golgi complex are presented. These movies demonstrate the dynamic, steady-state nature of the Golgi complex and provide insight into the tubule-vesicular carriers that mediate membrane flow into and out of this organelle.
VIDEO MOVIES
Movie 1: Formation and Life History of ER-to-Golgi Transport Intermediates
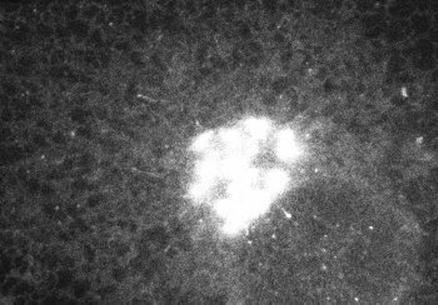
The ts045 vesicular stomatis viral G (VSVG) protein misfolds and is retained in the ER at 40°C and upon shift to 32°C moves as a synchronous population to the Golgi complex before being transported to the plasma membrane (Bergmann, 1989). These properties of VSVG are preserved upon addition of GFP to the cytoplasmic tail of the protein, allowing detailed analysis of the morphological characteristics of ER-to-Golgi transport to be visualized in living cells (Presley et al., 1997; Scales et al., 1997). Movie 1 (from Presley et al., 1997) (Figure 1) shows confocal images acquired at 8.6-s time intervals after shift from 40 to 32°C in COS cells expressing VSVG-GFP. Note that within seconds of temperature shift, ER-localized VSVG-GFP became concentrated within bright, fluorescent pre-Golgi intermediates that were widely distributed at ER sites scattered throughout the cytoplasm. Pre-Golgi structures never appeared to arise at the same site as previous ones, which suggested they formed de novo from dynamically generated nonfixed ER sites. Once formed, the pre-Golgi structures had only a finite lifetime and quickly translocated as units through the cytoplasm to the Golgi complex with no loss of fluorescent material. During transport, the pre-Golgi intermediates often stretched into long tubular elements as they moved along curvilinear tracks to the Golgi complex. This indicated that they were not small vesicles but larger, pleiomorphic structures. Because pre-Golgi intermediates and their associated cargo translocate as discrete entities into the central Golgi region, they may function both in membrane traffic and Golgi biogenesis. Additional studies are required to understand how pre-Golgi structures arise from the ER and the extent they engage in membrane sorting and recycling processes.
Figure 1.
Movie 1 depicts formation and life history of ER-to-Golgi transport intermediates.
Movie 2: Translocation of Pre-Golgi Intermediates
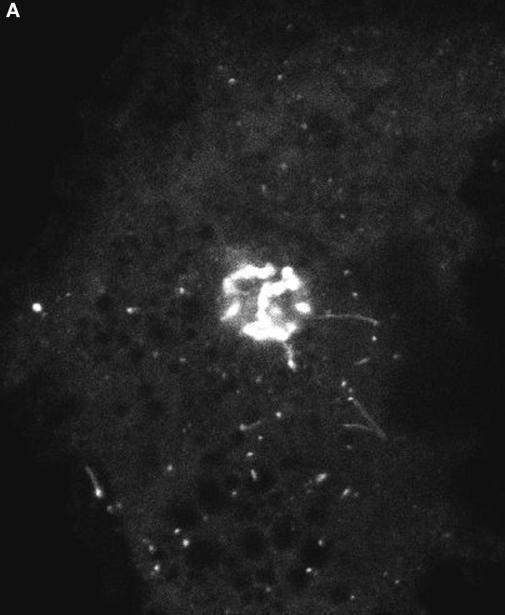
This movie shows a confocal time-lapse sequence of VSVG-GFP transport in COS cells incubated at 15°C for several hours before temperature shift to 32°C (from Presley et al., 1997) (Figure 2). At 15°C, pre-Golgi structures fail to translocate efficiently through the cytoplasm and remain closely associated with ER exit sites where they can continue to receive cargo (Saraste and Kuismanen, 1992). This results in the formation of larger pre-Golgi intermediates (up to 1.5 μm in diameter) which are more easily visualized within cells. As shown in the movie (images collected at 3.6-s time intervals on a confocal microscope), enlarged pre-Golgi intermediates quickly detached from ER exit sites and translocated into the Golgi region upon warmup from 15 to 32°C. They moved in an identical fashion as the smaller pre-Golgi intermediates observed in cells shifted directly from 40 to 32°C. In both cases, the pre-Golgi intermediates translocated as discrete entities in a stop-and-go fashion through the cytoplasm. Movement was unidirectional along curvilinear tracks toward the cell center at rates of up to 1.4 μm/s. Long tubules extending in the direction of motion of the pre-Golgi intermediates were often seen, suggesting a motor on the tip of the tubule was pulling the pre-Golgi structures inward toward the Golgi complex. These results indicate pre-Golgi structures can be variable in size and still translocate efficiently through the cytoplasm to the Golgi complex.
Figure 2.
Movie 2 shows translocation of pre-Golgi intermediates.
Movie 3: Role of Microtubules in ER-to-Golgi Traffic
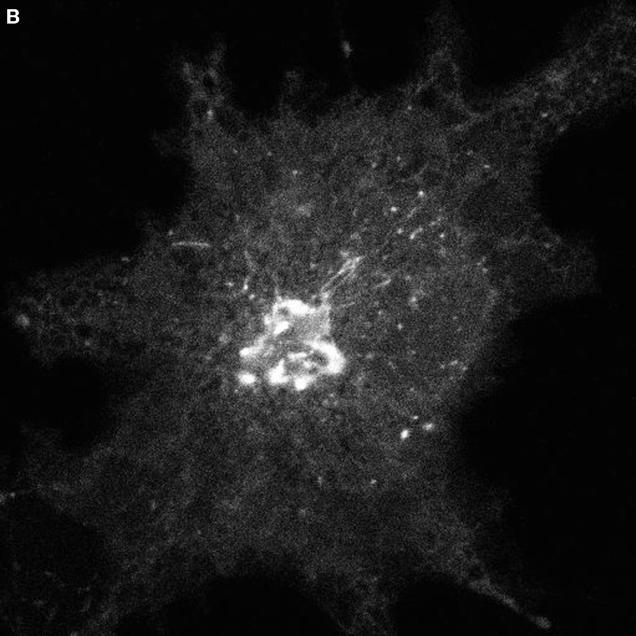
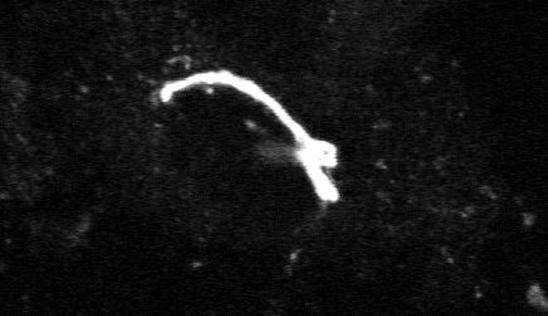
Microtubules have long been thought to play a role in ER-to-Golgi traffic (Ho et al., 1989; Saraste and Svensson, 1991). These cytoplasmic filaments emanate from the centrosomal Golgi region in many cells and form a polarized radial array—microtubule minus ends converging at the cell center and plus ends scattered in the cell periphery. This organization is ideal for directing peripheral ER-derived membrane into the Golgi region. The requirement of microtubules for translocation of pre-Golgi structures toward the central Golgi complex is shown in Movie 3 (Figure 3). (Images were acquired at 4-min intervals using a confocal microscope.) Here, VSVG-GFP–expressing COS cells were treated with nocodazole to depolymerize microtubules prior to temperature shift from 40 to 32°C. Note that soon after temperature shift, VSVG-GFP was efficiently exported out of the ER into peripheral pre-Golgi elements, but these structures remained at peripheral sites. This effect of microtubule depolymerization was reversible, with pre-Golgi structures quickly reattaching to newly formed microtubules and translocating microtubule minus end-directed into the Golgi region upon removal of nocodazole (Presley et al., 1997). The microtubule motor complex of dynein/dynactin was shown to power this transport to the Golgi complex (Burkhardt et al., 1997; Presley et al., 1997). How dynein/dynactin associates with pre-Golgi intermediates and how its interaction with microtubules is regulated are important questions for future work.
Figure 3.
Movie 3 shows role of microtubules in ER-to-Golgi traffic.
Movie 4, A and B: Fate of Pre-Golgi Intermediates upon Reaching the Golgi Complex
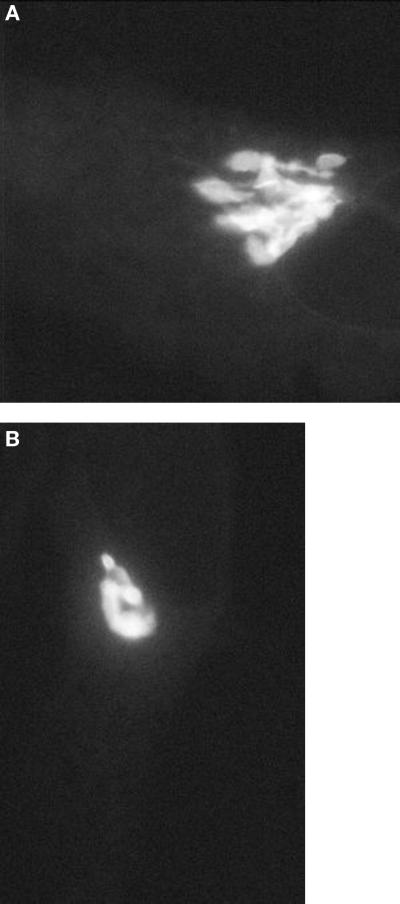
Targeting and fusion of pre-Golgi structures with Golgi membranes was visualized in VSVG-GFP expressing COS cells whose Golgi-associated fluorescence was photobleached with high-intensity laser light at the time cells were warmed from 15 to 32°C. After photobleaching, time-lapse images of the entire cell were collected with low-power laser light to follow movement of pre-Golgi structures into the Golgi region. As shown in the movie sequence 4A (from Presley et al., 1997) (Figure 4A), pre-Golgi structures translocated inward to the Golgi complex, docked, and then delivered their contents of VSVG-GFP into the Golgi system. These results indicate that pre-Golgi structures are the major source of delivery of VSVG-GFP to the Golgi complex. The fact that the entire profile of the Golgi complex refilled with fluorescence within a short period after photobleaching (see Movie 4B [Figure 4B]) further suggested that VSVG-GFP is rapidly transported throughout the Golgi complex after its delivery by pre-Golgi intermediates.
Figure 4.
Movie 4 shows fate of pre-Golgi intermediates upon reaching the Golgi complex.
Movie 5: Intra-Golgi Traffic Revealed by Photobleaching Recovery
The detailed path followed by cargo and Golgi resident proteins within the Golgi complex cannot easily be resolved at the light microscopic level, because Golgi stacks are often <488 nm in depth (e.g., the wavelength of blue light). Nevertheless, insight into the lateral mobility of proteins within Golgi membranes can be obtained using fluorescence recovery after photobleaching (FRAP). This technique can help distinguish between retention and retrieval mechanisms for Golgi protein localization (Cole et al., 1996b). In FRAP, a small region of the cell is photobleached, and recovery of fluorescence into the bleached region is monitored over time. The kinetics of fluorescence recovery allow calculation of the diffusion coefficient, D, which is a measure of the speed of protein diffusion in the membrane, and the immobile fraction, which is the fraction of proteins that do not recover during the time course of the experiment. Photobleaching permanently inactivates the fluorophore, so return of fluorescence is due only to exchange of nonfluorescent with fluorescent proteins by lateral diffusion within the membrane.
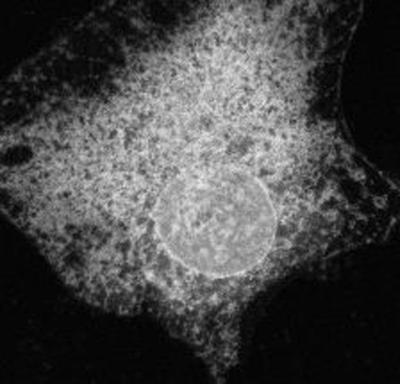
Movie 5 shows recovery after photobleaching of the Golgi enzyme galactosyltransferase tagged with GFP in HeLa cells (from Cole et al., 1996b) (Figure 5). In this experiment, the region of the Golgi complex above the dotted line was photobleached with high-energy illumination from a confocal microscope. Recovery of fluorescence into the photobleached region was then followed by imaging the entire Golgi body at low-energy light at 4-s time intervals. Note that within 1 min complete recovery of fluorescence into the bleached box occurred. This indicates that Golgi resident proteins such as GalTase diffuse rapidly, without constraints, through the Golgi complex. The diffusion coefficient calculated from FRAP experiments was 5 × 10−9 cm2/s with >90% of the proteins mobile (Cole et al., 1996b).
Figure 5.
Movie 5 shows intra-Golgi traffic revealed by photobleaching recovery.
Movie 6: Protein Trafficking between Golgi Stacks
A variation of FRAP, called fluorescence loss in photobleaching (FLIP), can be used to monitor movement of fluorescently tagged molecules between two different regions of a cell or organelle (Cole et al., 1996b) (Figure 6). In FLIP, fluorescence in one region of the cell is photobleached with high-intensity illumination while another region is imaged with illumination sufficiently low to avoid significant photobleaching. If photobleaching one region reduces the fluorescence in the other region as well, diffusional exchange of molecules between the two regions has occurred. As shown in Movie 6 (from Cole et al., 1996b), repetitive photobleaching in a small area of the Golgi complex led to loss of GFP fluorescence from the entire Golgi complex with only outlying Golgi elements retaining fluorescence. This result implies that the numerous compact stacks of cisternae that constitute the Golgi complex are extensively interconnected, allowing highly mobile proteins such as GalTase to move quickly from one part of the Golgi to another. Movement is likely to occur by lateral diffusion of these proteins across thin membrane tubules that in ultrastructural studies have been shown to interconnect Golgi stacks (Rambourg and Clermont, 1990). Interestingly, ATP depletion of cells, which blocks vesicle transport, showed little or no effects in FLIP experiments of GalTase-GFP–expressing cells (Cole et al., 1996b). This result indicates that the extensive movement of Golgi enzymes observed in FLIP experiments is not due to vesicle budding and fusion processes.
Figure 6.
Movie 6 shows traffic between Golgi stacks.
Movie 7: Golgi Tubules: Potential Vehicles for Golgi-to-ER Retrograde Traffic
Golgi membrane tubules are known from ultrastructural studies to be a major characteristic of Golgi morphology. Both the cis and trans face of the Golgi stack are composed of extensive tubule networks of membrane, and tubule processes frequently interconnect Golgi stacks (Rambourg et al., 1979; Tanaka et al., 1986; Ladinsky et al., 1994). As shown in Movie 7 (Figure 7), these structures may also play a role in Golgi to ER retrograde traffic. KDEL-R, which is known to constitutively cycle between the ER and Golgi complex (Pelham, 1991), was imaged with a confocal microscope in HeLa cells expressing a GFP tagged version of this molecule (KDEL-R-GFP). Note that in addition to labeling Golgi membranes, KDEL-R-GFP was found in membrane tubules that extended out from the Golgi complex. After forming, the tubules often broke off from the Golgi and moved at rates of 0.6 μm/s along curvilinear tracks to the cell periphery. Microtubules were required for the peripheral movement of Golgi tubules, suggesting that a plus end–directed motor was associated with their surface (Sciaky et al., 1997). After detaching from the Golgi complex and moving to the cell periphery, Golgi tubules containing KDEL-R-GFP often disappeared from view (possibly fusing with the ER, which extends throughout the cytoplasm). Consistent with this possibility, KDEL-R-GFP was never found on the plasma membrane but only in Golgi, ER, and tubule structures. Golgi tubules may therefore represent retrograde transport intermediates, whose role is to recycle membrane back to the peripheral ER system. The molecular basis and regulation of Golgi tubule budding and scission remains to be addressed.
Figure 7.
Movie 7 depicts Golgi tubule traffic: potential vehicles for Golgi-to-ER retrograde traffic.
Movie 8: Effects of Brefeldin A (BFA) on Golgi Morphology and Dynamics
Golgi tubules are known to mediate retrograde membrane traffic in cells treated with BFA, a drug that prevents assembly of cytosolic coat proteins onto Golgi membranes. As shown in Movie 8A (Figure 8A), Golgi tubules resembling those of untreated cells are observed in BFA-treated cells, but they formed at a more rapid rate. Moreover, the tubules failed to detach from Golgi structures. This led to the formation of a dynamic Golgi tubule network within 5–8 min of adding the drug. When one or more of the Golgi tubules fused with the ER, Golgi membranes redistributed rapidly into the ER, leaving no Golgi structure behind. The fact that Golgi membranes redistributed unidirectionally into the ER (and not ER membrane into the Golgi) during BFA treatment suggests that the ER provides a lower free energy environment for membrane proteins and lipids than the Golgi system.
Figure 8.
Movie 8 shows the effects of BFA on Golgi morphology and dynamics.
The sequences in Movie 8A are of GalTase-GFP in HeLa cells. They were acquired at 3-s time intervals on a cooled charge-coupled device microscope. Note that Golgi protein redistribution into the ER occurred within 30 s after fusion of Golgi with ER. The speed of this redistribution has been shown by Sciaky et al. (1997) to be too fast to be explained by simple protein diffusion. Instead, these authors suggest it is driven by a membrane tension-driven membrane flow (Bloom et al., 1991) that arises from chemical potential or free energy differences between ER and Golgi membranes. This leads to unidirectional protein transport when ER and Golgi membranes fuse during BFA treatment.
A dramatic illustration of unidirectional membrane flow from Golgi into the ER in BFA-treated cells is shown in Movie 8B (Figure 8B). In this experiment cells were treated with nocodazole before addition of BFA. Note that upon addition of BFA to the cells no Golgi tubules formed, since microtubules are required for peripheral extension and movement of tubules. Nevertheless, the time for Golgi redistribution into the ER was extremely rapid once Golgi and ER membranes fused. This suggests that extension of Golgi tubules along microtubules serves only to increase the probability of fusion between Golgi and ER membranes. Once fusion between these two organelles takes place (which can occur in the absence of Golgi tubule extension, because ER membranes are distributed through the cell), the Golgi rapidly and completely empties into the ER.
The chemical basis for the free energy difference between ER and Golgi membranes that underlies unidirectional absorption of Golgi into the ER during BFA treatment is unknown. An interesting possibility is that the work required to “pump” Golgi-destined components up in energy is supplied by the peripheral “coat” system, which concentrates and packages proteins exported from the ER (Balch et al., 1994; Schekman and Orci, 1996). The fact that an immediate target of BFA is ADP ribosylation factor, which in physical terms is an energy-driven assembly transport system for supplying COPI to membranes (Donaldson et al., 1992; Rothman and Wieland, 1996), is consistent with this idea. When nucleotide exchange onto ADP ribosylation factor is inhibited by BFA, COP I proteins are quickly depleted from Golgi membranes. As shown in Movie 8A, Golgi tubules proliferate and become longer in response. They also do not so readily detach from the Golgi complex. Ultimately, fusion with the ER occurs and Golgi membrane quickly empties into the ER. These findings suggest that one function of COP I is to maintain the integrity of the Golgi complex, possibly by severing potential links to other organelles. Future work needs to address the mechanisms that regulate Golgi tubule formation and detachment from the Golgi complex and how free energy differences between the ER and Golgi complex arise.
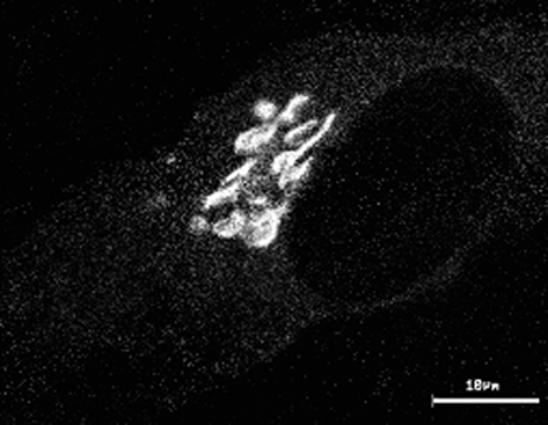
Movie 9: Remodeling of the Golgi Complex in Response to Microtubule Depolymerization
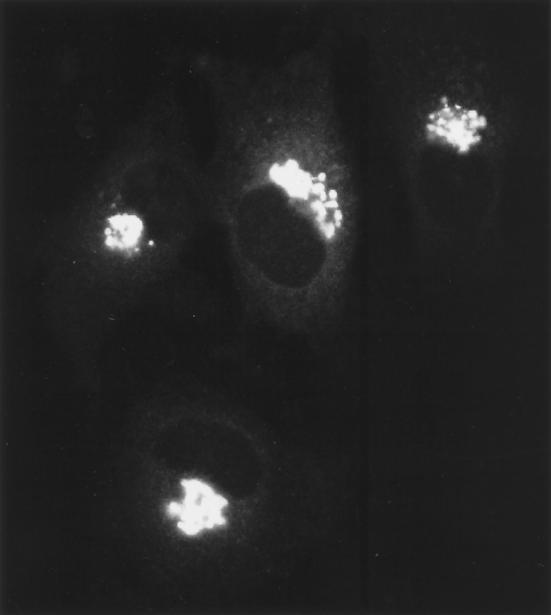
The normal distribution of Golgi membranes within cells appears to represent a dynamic balance of forward and recycling pathways connecting with the ER. Thus, inhibition of forward traffic into the Golgi complex without a corresponding effect on recycling typically leads to redistribution of Golgi material to the site of perturbation. Fragmentation of the Golgi complex in nocodazole-treated cells is a good example of this phenomenon. Microtubule disruption by nocodazole is known to block the inward translocation of pre-Golgi intermediates along microtubules (see Movie 3) without significant effects on Golgi to ER traffic (Saraste and Svensson, 1991; Cole et al., 1996a). Because Golgi enzymes constitutively cycle between the ER and Golgi complex via these forward and reverse pathways (Cole et al., 1998), they accumulate at ER exit sites when forward trafficking along microtubules is inhibited (Cole et al., 1996a; Yang and Storrie, 1998). This leads to rebuilding of Golgi stacks at ER exit sites and results in the formation of hundreds of Golgi fragments distributed through the cell (Cole et al., 1996; Burkhardt, 1998; Storrie and Yang, 1998). Movie 9 (Figure 9) (images collected at 1-min intervals on a confocal microscope) shows this process in HeLa cells expressing GalTase-GFP. Note that small Golgi fragments are generated at significant distances from the central Golgi region within 15 min after nocodazole treatment. These fragments do not move in any directed fashion after their formation and resemble the fragments containing VSVG-GFP in nocodazole-treated cells shifted from 40 to 32°C. The exact mechanism for redistribution of Golgi enzymes to fragment sites is still under debate (e.g., recycling through the ER or vesicle transport through the cytoplasm) (Cole et al., 1996a; Burkhardt, 1998; Sima et al., 1998; Storrie and Yang, 1998). Nevertheless, these observations indicate the Golgi complex is capable of de novo regeneration at ER exit sites and imply a fundamental relationship between pre-Golgi structures and the Golgi complex.
Figure 9.
Movie 9 depicts remodeling of the Golgi complex in response to microtubule depolymerization.
Supplementary Material
Footnotes
REFERENCES
- Balch WE, McCafferey JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bergmann JE. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol. 1989;32:85–110. doi: 10.1016/s0091-679x(08)61168-1. [DOI] [PubMed] [Google Scholar]
- Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Burkhardt, J.K. (1998). The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim. Biophys. Acta (in press). [DOI] [PubMed]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirschen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140:1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996a;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996b;272:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein bCOP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. In: Mobility and Proximity in Biological Membranes. Damjanovich S, Edidin M, Szollosi J, Tron L, editors. Boca Raton: CRC Press; 1994. pp. 109–135. [Google Scholar]
- Farquhar M, Palade G. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WC, Allan VJ, Van Meer G, Berger EG, Kreis TE. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- Ladinsky MS, Kremer JR, Furcinitti PS, McIntosh JR, Howell KE. HVEM tomography of the trans-Golgi network: structural insights and identification of a lace-like vesicle coat. J Cell Biol. 1994;127:29–38. doi: 10.1083/jcb.127.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Presley J, Zaal K, Hirschberg K, Miller C, Ellenberg J. GFP Biofluorescence: Imaging, Gene Expression, and Protein Dynamics in Living Cells. K. Sullivan and S. Kay, New York: Academic Press; 1998. Monitoring the dynamics and mobility of membrane proteins tagged with green fluorescent protein. (in press). [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Smith CL. Insights into secretory and endocytic membrane traffic using green fluorescent protein chimeras. Curr Opin Neurobiol. 1997;7:631–639. doi: 10.1016/s0959-4388(97)80082-7. [DOI] [PubMed] [Google Scholar]
- Mazzarello P, Bentaivoglio M. The Centenarian Golgi apparatus. Nature. 1998;392:543–544. doi: 10.1038/33266. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991;3:585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol. 1990;51:189–200. [PubMed] [Google Scholar]
- Rambourg A, Clermont Y, Hermo L. Three-dimensional architecture of the Golgi apparatus in Sertoli cells of the rat. Am J Anat. 1979;154:455–476. doi: 10.1002/aja.1001540402. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the golgi complex. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action of COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley J, Smith C, Zaal KJM, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D, Cabrera-Poch N, Pepperkok R, Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie, B., and Yang, W. (1998). Dynamics of the interphase mammalian Golgi complex as revealed through drugs producing reversible Golgi disassembly. Biochim. Biophys. Acta (in press). [DOI] [PubMed]
- Tanaka K, Mitsushima A, Fukudome H, Kashima Y. Three-dimensional architecture of the Golgi complex observed by high resolution scanning electron microscopy. J Submicrosc Cytol. 1986;18:1–9. [PubMed] [Google Scholar]
- Yang W, Storrie B. Scattered Golgi elements during microtubule disruption are initially enriched in trans Golgi proteins. Mol Biol Cell. 1998;9:191–207. doi: 10.1091/mbc.9.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.