Abstract
A bacterially synthesized 28-kilodalton carboxyl-terminal fragment (28K-EBNA of Epstein-Barr virus nuclear antigen shows highly concentration dependent binding to monomer, dimer, and trimer copies of synthetic DNA-binding site 5' GATCTAGGATAGCATATGCTACCCCGGGG 3' 3' ATCCTATCGTATACGATGGGGCCCCCTAG 5' in bacterial plasmids. The rate of the binding reaction is independent of the number of sites, but dependent upon the length of the DNA containing the sites. These data are consistent with 28K-EBNA locating its binding sites by a process of facilitated transfer or sliding along the DNA. The highly concentration dependent binding suggests that multiple 28K-EBNA monomer polypeptides form a complex before or during binding. Binding occurs equally well at 24 and 37 degrees C, but not at 0 degrees C. A 28K-EBNA complex bound to a single site has unoccupied binding sites capable of interacting with additional DNA molecules. Such interaction is confirmed by agarose gel electrophoresis of protein-DNA complexes which indicate that a 28K-EBNA complex forms bridges between two DNA molecules. A bridge between the two binding regions in the Epstein-Barr virus origin of plasmid replication (oriP) would form a loop structure which could be an important feature for the regulatory function of authentic Epstein-Barr virus nuclear antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Lu C., Williams R. C., Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Wickner S., Auerbach J., Echols H. Role of the Xis protein of bacteriophage lambda in a specific reactive complex at the attR prophage attachment site. Cell. 1983 Jan;32(1):161–168. doi: 10.1016/0092-8674(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Echols H., Dodson M., Better M., Roberts J. D., McMacken R. The role of specialized nucleoprotein structures in site-specific recombination and initiation of DNA replication. Cold Spring Harb Symp Quant Biol. 1984;49:727–733. doi: 10.1101/sqb.1984.049.01.082. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Halprin J., Scott A. L., Jacobson L., Levine P. H., Ho J. H., Niederman J. C., Hayward S. D., Milman G. Enzyme-linked immunosorbent assay of antibodies to Epstein-Barr virus nuclear and early antigens in patients with infectious mononucleosis and nasopharyngeal carcinoma. Ann Intern Med. 1986 Mar;104(3):331–337. doi: 10.7326/0003-4819-104-3-331. [DOI] [PubMed] [Google Scholar]
- Hearing J. C., Levine A. J. The Epstein-Barr virus nuclear antigen (BamHI K antigen) is a single-stranded DNA binding phosphoprotein. Virology. 1985 Aug;145(1):105–116. doi: 10.1016/0042-6822(85)90205-3. [DOI] [PubMed] [Google Scholar]
- Hummel M., Arsenakis M., Marchini A., Lee L., Roizman B., Kieff E. Herpes simplex virus expressing Epstein-Barr virus nuclear antigen 1. Virology. 1986 Jan 30;148(2):337–348. doi: 10.1016/0042-6822(86)90330-2. [DOI] [PubMed] [Google Scholar]
- Keegan L., Gill G., Ptashne M. Separation of DNA binding from the transcription-activating function of a eukaryotic regulatory protein. Science. 1986 Feb 14;231(4739):699–704. doi: 10.1126/science.3080805. [DOI] [PubMed] [Google Scholar]
- Lotz B., Keith H. D. The crystal structures of poly(LAla-Gly-Gly-Gly)II and Poly(LAla-Gly-Gly)II. J Mol Biol. 1971 Oct 14;61(1):195–200. doi: 10.1016/0022-2836(71)90216-6. [DOI] [PubMed] [Google Scholar]
- Milman G., Scott A. L., Cho M. S., Hartman S. C., Ades D. K., Hayward G. S., Ki P. F., August J. T., Hayward S. D. Carboxyl-terminal domain of the Epstein-Barr virus nuclear antigen is highly immunogenic in man. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6300–6304. doi: 10.1073/pnas.82.18.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M. F., Shedd D., Weigel R. J., Fischer D. K., Miller G. Expression in COS-1 cells of Epstein-Barr virus nuclear antigen from a complete gene and a deleted gene. J Virol. 1984 Jun;50(3):822–831. doi: 10.1128/jvi.50.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculley T. B., Kreofsky T., Pearson G. R., Spelsberg T. C. Partial purification of the Epstein-Barr virus nuclear antigen(s). J Biol Chem. 1983 Mar 25;258(6):3974–3982. [PubMed] [Google Scholar]
- Sugden B. Epstein-Barr virus: a human pathogen inducing lymphoproliferation in vivo and in vitro. Rev Infect Dis. 1982 Sep-Oct;4(5):1048–1061. doi: 10.1093/clinids/4.5.1048. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. Polymers of tripeptides as collagen models. V. An x-ray study of poly(L-prolyl-glycyl-glycine). J Mol Biol. 1969 Aug 14;43(3):479–485. doi: 10.1016/0022-2836(69)90353-2. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
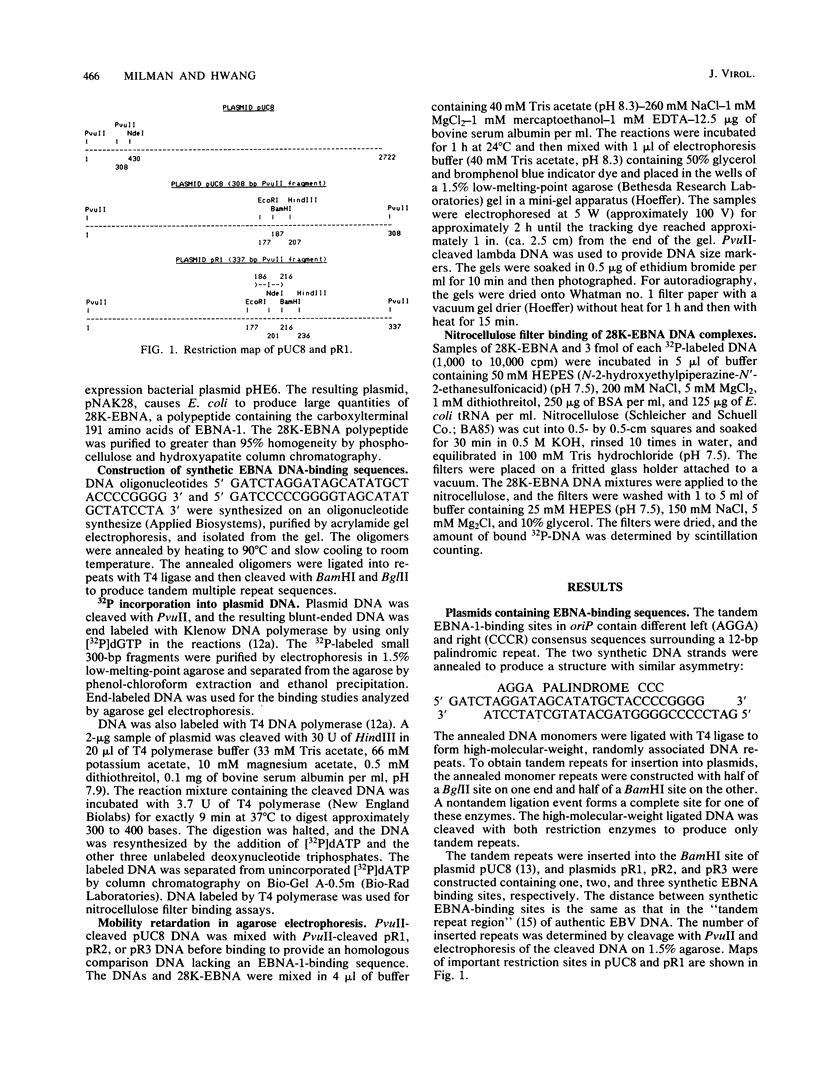
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]