Abstract
Hepatitis A virus is an hepatotrophic human picornavirus which demonstrates little antigenic variability. To topologically map immunogenic sites on hepatitis A virus which elicit neutralizing antibodies, eight neutralizing monoclonal antibodies were evaluated in competition immunoassays employing radiolabeled monoclonal antibodies and HM-175 virus. Whereas two antibodies (K3-4C8 and K3-2F2) bound to intimately overlapping epitopes, the epitope bound by a third antibody (B5-B3) was distinctly different as evidenced by a lack of competition between antibodies for binding to the virus. The other five antibodies variably blocked the binding of both K3-4C8-K3-2F2 and B5-B3, suggesting that these epitopes are closely spaced and perhaps part of a single neutralization immunogenic site. Several combinations of monoclonal antibodies blocked the binding of polyclonal human convalescent antibody by greater than 96%, indicating that the neutralization epitopes bound by these antibodies are immunodominant in humans. Spontaneously arising HM-175 mutants were selected for resistance to monoclonal antibody-mediated neutralization. Fourteen clonally isolated mutants demonstrated substantial resistance to multiple monoclonal antibodies, including K3-4C8-K3-2F2 and B5-B3. In addition, 13 mutants demonstrated a 10-fold or greater reduction in neutraliztion mediated by polyclonal human antibody. Neutralization resistance was associated with reduced antibody binding. These results suggest that hepatitis A virus may differ from poliovirus in possessing a single, dominant neutralization immunogenic site and therefore may be a better candidate for synthetic peptide or antiidiotype vaccine development.
Full text
PDF

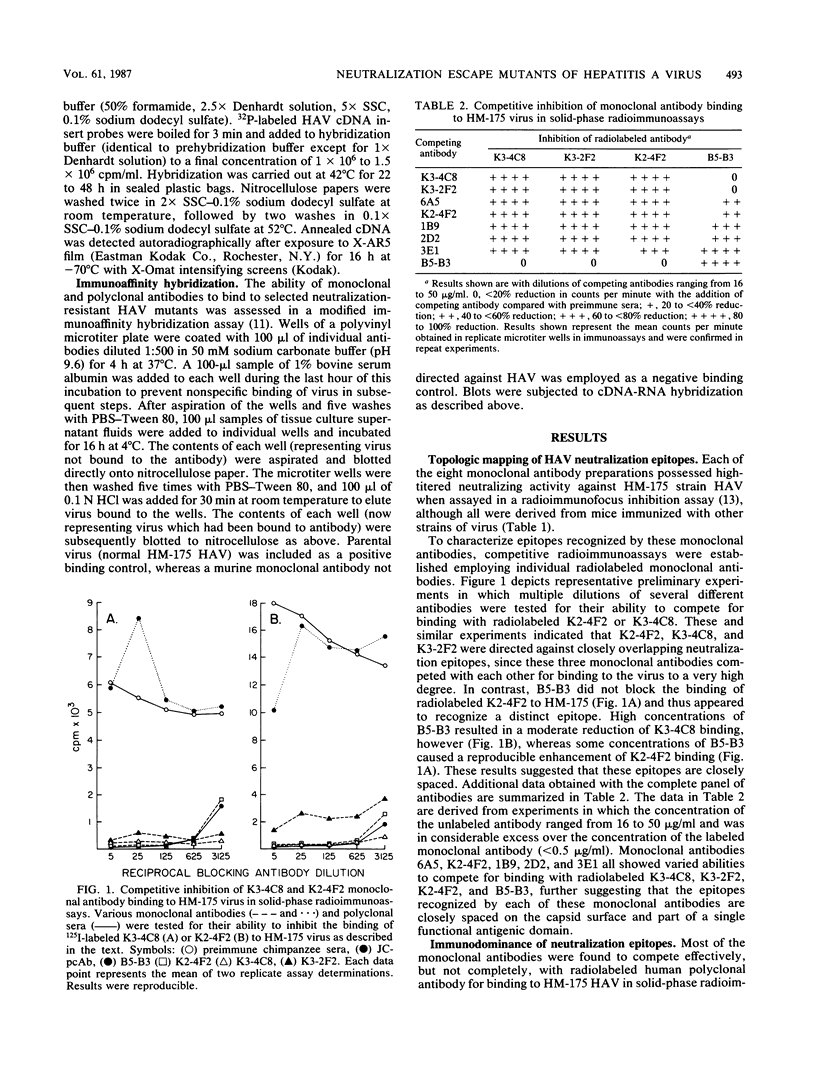
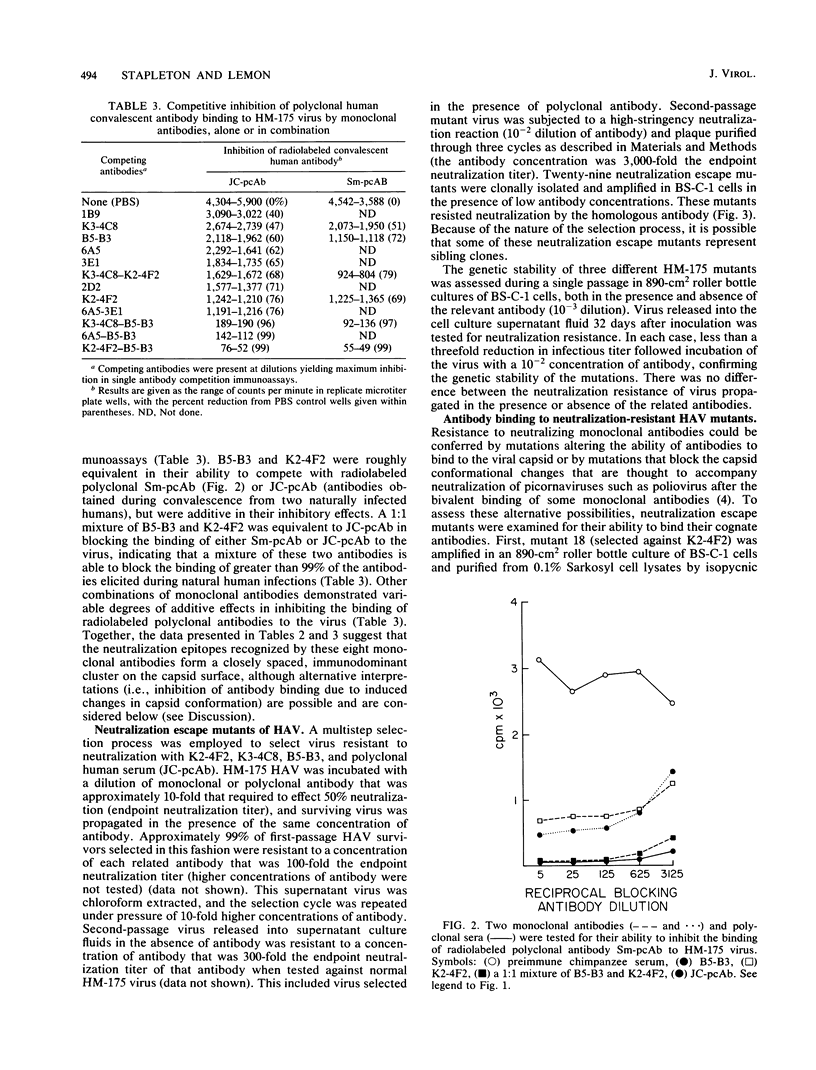



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binn L. N., Lemon S. M., Marchwicki R. H., Redfield R. R., Gates N. L., Bancroft W. H. Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J Clin Microbiol. 1984 Jul;20(1):28–33. doi: 10.1128/jcm.20.1.28-33.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulepis A. G., Locarnini S. A., Westaway E. G., Tannock G. A., Gust I. D. Biophysical and biochemical characterization of hepatitis A virus. Intervirology. 1982;18(3):107–127. doi: 10.1159/000149314. [DOI] [PubMed] [Google Scholar]
- Diamond D. C., Jameson B. A., Bonin J., Kohara M., Abe S., Itoh H., Komatsu T., Arita M., Kuge S., Nomoto A. Antigenic variation and resistance to neutralization in poliovirus type 1. Science. 1985 Sep 13;229(4718):1090–1093. doi: 10.1126/science.2412292. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. P., Hadler S. C., Prendergast T. J., Peterson E., Ginsberg M. M., Lookabaugh C., Holmes J. R., Maynard J. E. Occurrence of hepatitis A, B, and non-A/non-B in the United States. CDC sentinel county hepatitis study I. Am J Med. 1984 Jan;76(1):69–74. doi: 10.1016/0002-9343(84)90752-6. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Frösner G. G. Topology and immunoreactivity of capsid proteins in hepatitis A virus. Med Microbiol Immunol. 1983;172(2):101–106. doi: 10.1007/BF02124510. [DOI] [PubMed] [Google Scholar]
- Gust I. D., Coulepis A. G., Feinstone S. M., Locarnini S. A., Moritsugu Y., Najera R., Siegl G. Taxonomic classification of hepatitis A virus. Intervirology. 1983;20(1):1–7. doi: 10.1159/000149367. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hughes J. V., Stanton L. W., Tomassini J. E., Long W. J., Scolnick E. M. Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol. 1984 Nov;52(2):465–473. doi: 10.1128/jvi.52.2.465-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Combined immunoaffinity cDNA-RNA hybridization assay for detection of hepatitis A virus in clinical specimens. J Clin Microbiol. 1985 Dec;22(6):984–989. doi: 10.1128/jcm.22.6.984-989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knossow M., Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Three-dimensional structure of an antigenic mutant of the influenza virus haemagglutinin. Nature. 1984 Oct 18;311(5987):678–680. doi: 10.1038/311678a0. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N. Antigenic relatedness of two strains of hepatitis A virus determined by cross-neutralization. Infect Immun. 1983 Oct;42(1):418–420. doi: 10.1128/iai.42.1.418-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis. 1983 Dec;148(6):1033–1039. doi: 10.1093/infdis/148.6.1033. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Brown C. D., Brooks D. S., Simms T. E., Bancroft W. H. Specific immunoglobulin M response to hepatitis A virus determined by solid-phase radioimmunoassay. Infect Immun. 1980 Jun;28(3):927–936. doi: 10.1128/iai.28.3.927-936.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Jansen R. W. A simple method for clonal selection of hepatitis A virus based on recovery of virus from radioimmunofocus overlays. J Virol Methods. 1985 Jun;11(2):171–176. doi: 10.1016/0166-0934(85)90040-0. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Jansen R. W., Newbold J. E. Infectious hepatitis A virus particles produced in cell culture consist of three distinct types with different buoyant densities in CsCl. J Virol. 1985 Apr;54(1):78–85. doi: 10.1128/jvi.54.1.78-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A., Kornitschuk M., Hurrell J. G., Lehmann N. I., Coulepis A. G., Locarnini S. A., Gust I. D. Monoclonal antibodies against hepatitis A virus. J Clin Microbiol. 1983 Nov;18(5):1237–1243. doi: 10.1128/jcm.18.5.1237-1243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Evans D. M., Ferguson M., Schild G. C., Westrop G., Almond J. W. Principal and subsidiary antigenic sites of VP1 involved in the neutralization of poliovirus type 3. J Gen Virol. 1985 May;66(Pt 5):1159–1165. doi: 10.1099/0022-1317-66-5-1159. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Bootman J., Evans D. M., Ferguson M., Reeve P., Spitz M., Stanway G., Cann A. J., Hauptmann R. Location and primary structure of a major antigenic site for poliovirus neutralization. Nature. 1983 Feb 24;301(5902):674–679. doi: 10.1038/301674a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Sherry B., Mosser A. G., Colonno R. J., Rueckert R. R. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton J. T., Jansen R., Lemon S. M. Neutralizing antibody to hepatitis A virus in immune serum globulin and in the sera of human recipients of immune serum globulin. Gastroenterology. 1985 Sep;89(3):637–642. doi: 10.1016/0016-5085(85)90462-7. [DOI] [PubMed] [Google Scholar]
- Ticehurst J. R., Racaniello V. R., Baroudy B. M., Baltimore D., Purcell R. H., Feinstone S. M. Molecular cloning and characterization of hepatitis A virus cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5885–5889. doi: 10.1073/pnas.80.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]