Abstract
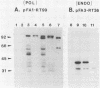
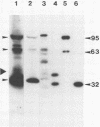
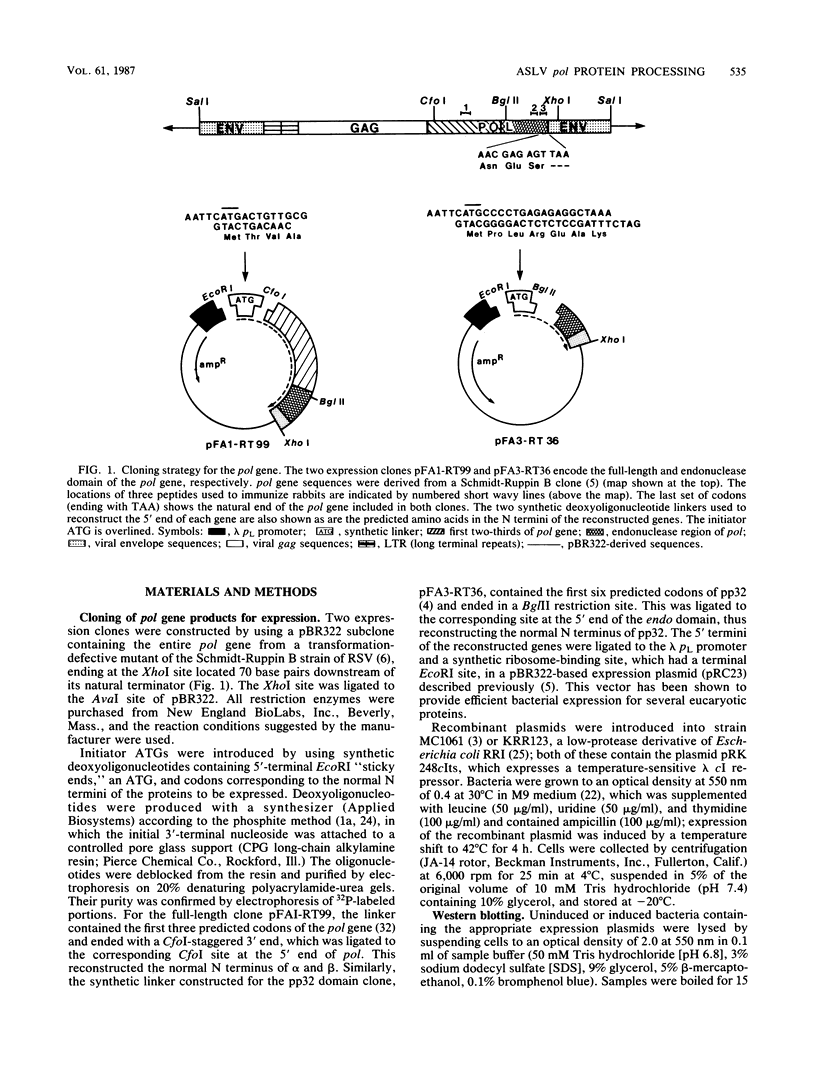
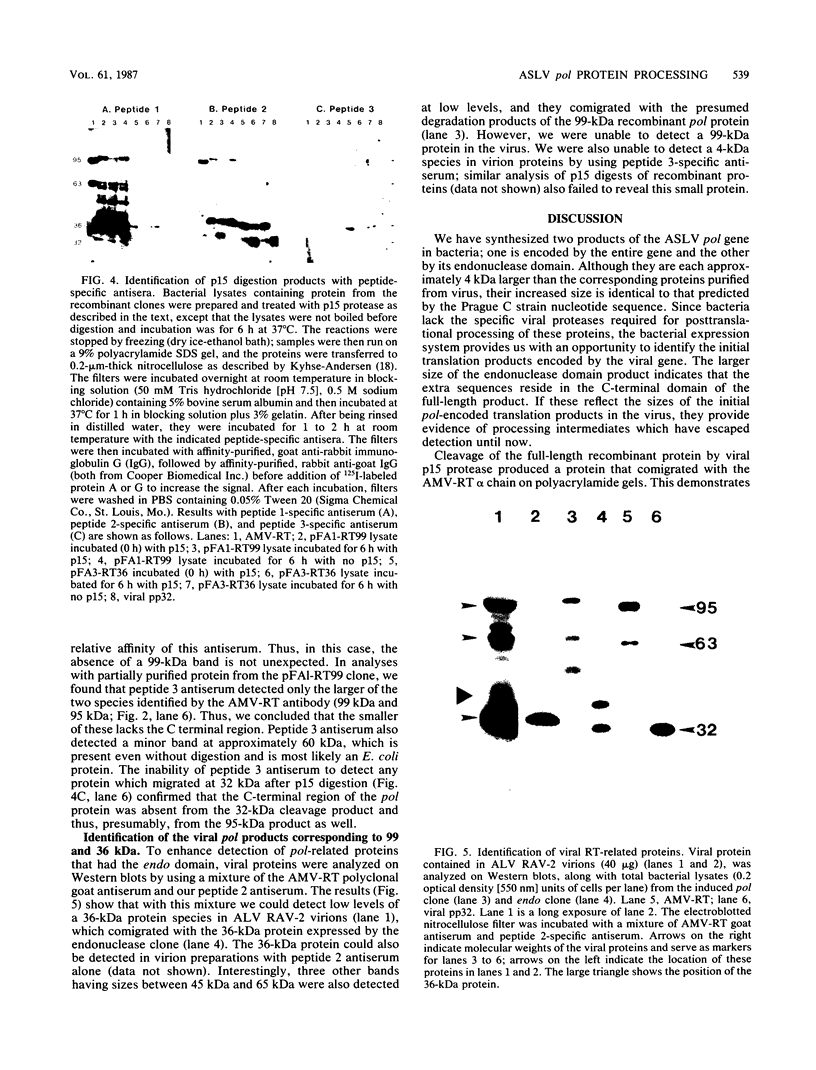
Three pol gene products have been identified in avian retroviral particles: the full-length 95-kilodalton (kDa) beta chain of reverse transcriptase and two proteolytic cleavage products of beta, a 63-kDa reverse transcriptase alpha chain derived from the amino terminus of beta and a 32-kDa (pp32) endonuclease from its carboxy terminus. By using molecularly cloned retroviral DNA and synthetic oligonucleotides to introduce initiator ATGs and codons corresponding to the authentic N termini, we constructed two bacterial-expression clones; one clone contains the entire pol gene, and the other contains the region encoding the pp32 domain. A 99-kDa protein was synthesized in Escherichia coli by the full-length clone, and a 36-kDa protein was synthesized by the endonuclease domain clone. The recombinant proteins exceeded the size of both the mature viral beta chain and the pp32, respectively, by approximately 4 kDa. These larger sizes, however, are consistent with predictions from the DNA sequence of the pol gene. Processing of the recombinant pol proteins was examined by using p15 protease purified from virus particles and antisera directed against synthetic peptides corresponding to three domains in pol. Proteolytic digestion of the 99-kDa product with p15 produced a 63-kDa protein that comigrated on polyacrylamide gels with the alpha chain of reverse transciptase and a 36-kDa fragment that comigrated with the endonuclease domain product. Further digestion of the 36-kDa protein yielded a 32-kDa protein that comigrated with viral pp32 endonuclease. Thus, we concluded that two p15-sensitive sites exist in pol. Cleavage at the previously identified site produces alpha, and cleavage at the newly discovered site removes approximately 4 kDa from the C terminus of the primary protein product. Since the 36-kDa protein was also detected in protein isolated from virus particles, it seems probable that processing at the C-terminal site is a normal step in the production of mature beta and pp32 endonuclease products.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Butnick N. Z., Miyamoto C., Chizzonite R., Cullen B. R., Ju G., Skalka A. M. Regulation of the human c-myc gene: 5' noncoding sequences do not affect translation. Mol Cell Biol. 1985 Nov;5(11):3009–3016. doi: 10.1128/mcb.5.11.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Grandgenett D. P., Oroszlan S. Amino acid sequence analysis of reverse transcriptase subunits from avian myeloblastosis virus. J Virol. 1980 Oct;36(1):115–119. doi: 10.1128/jvi.36.1.115-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Kopchick J. J., Stacey D. W. Effect of intron size on splicing efficiency in retroviral transcripts. Nucleic Acids Res. 1982 Oct 11;10(19):6177–6190. doi: 10.1093/nar/10.19.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979 Mar 10;254(5):1606–1613. [PubMed] [Google Scholar]
- Grandgenett D. P., Knaus R. J., Hippenmeyer P. J. Antibodies against a synthetic peptide of the avian retrovirus pp32 protein and the beta DNA polymerase subunit. Virology. 1983 Oct 15;130(1):257–262. doi: 10.1016/0042-6822(83)90137-x. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Schiff R. D. A 32,000-dalton nucleic acid-binding protein from avian retravirus cores possesses DNA endonuclease activity. Virology. 1978 Aug;89(1):119–132. doi: 10.1016/0042-6822(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Grandgenett D., Quinn T., Hippenmeyer P. J., Oroszlan S. Structural characterization of the avian retrovirus reverse transcriptase and endonuclease domains. J Biol Chem. 1985 Jul 15;260(14):8243–8249. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leis J., Duyk G., Johnson S., Longiaru M., Skalka A. Mechanism of action of the endonuclease associated with the alpha beta and beta beta forms of avian RNA tumor virus reverse transcriptase. J Virol. 1983 Feb;45(2):727–739. doi: 10.1128/jvi.45.2.727-739.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J., Johnson S., Collins L. S., Traugh J. A. Effects of phosphorylation of avian retrovirus nucleocapsid protein pp12 on binding of viral RNA. J Biol Chem. 1984 Jun 25;259(12):7726–7732. [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C., Chizzonite R., Crowl R., Rupprecht K., Kramer R., Schaber M., Kumar G., Poonian M., Ju G. Molecular cloning and regulated expression of the human c-myc gene in Escherichia coli and Saccharomyces cerevisiae: comparison of the protein products. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7232–7236. doi: 10.1073/pnas.82.21.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. Circles with two tandem LTRs are precursors to integrated retrovirus DNA. Cell. 1984 Mar;36(3):673–679. doi: 10.1016/0092-8674(84)90347-7. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Karess R. E., Anderson S. M., Hanafusa H. Tryptic peptide analysis of avian oncovirus gag and pol gene products. J Virol. 1979 Oct;32(1):102–113. doi: 10.1128/jvi.32.1.102-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H. M., Grandgenett D. P., Green M. Sequence relatedness between the subunits of avian myeloblastosis virus reverse transcriptase. J Biol Chem. 1975 Jul 10;250(13):5278–5280. [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Nebes S., Leis J. Production of large amounts of 35S RNA and complementary DNA from avian RNA tumor viruses. Anal Biochem. 1977 Jan;77(1):226–234. doi: 10.1016/0003-2697(77)90308-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Pepinsky R. B., Southard L. E. Primary structure of p19 species of avian sarcoma and leukemia viruses. J Virol. 1985 Oct;56(1):31–39. doi: 10.1128/jvi.56.1.31-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]