Abstract
Specificity of vesicular transport is determined by pair-wise interaction between receptors (SNAP receptors or SNAREs) associated with a transport vesicle and its target membrane. Two additional factors, N-ethylmaleimide-sensitive fusion protein (NSF) and soluble NSF attachment protein (SNAP) are ubiquitous components of vesicular transport pathways. However, the precise role they play is not known. On the basis that NSF and SNAP can be recruited to preformed SNARE complexes, it has been proposed that NSF- and SNAP-containing complexes are formed after SNARE-dependent docking of transport vesicles. This would enable ATPase-dependent complex disassembly to be coupled directly to membrane fusion. Alternatively, binding and release of NSF/SNAP may occur before vesicle docking, and perhaps be involved in the activation of SNAREs. To gain more information about the point at which so-called 20S complexes form during the transport vesicle cycle, we have examined NSF/SNAP/SNARE complex turnover on clathrin-coated vesicle–derived membranes in situ. This has been achieved under conditions in which the extent of membrane docking can be precisely monitored. We demonstrate by UV-dependent cross-linking experiments, coupled to laser light-scattering analysis of membranes, that complexes containing NSF, SNAP, and SNAREs will form and dissociate on the surface of undocked transport vesicles.
INTRODUCTION
Transport of proteins between intracellular compartments is mediated by vesicles. Although the molecular basis for the docking and fusion of transport vesicles with their target membrane is still poorly understood, biochemical and genetic studies have enabled the identification of several proteins required for vesicle transport (Rothman, 1994).
Recent progress has demonstrated the central role in vesicle docking/fusion played by the SNARE (SNAP receptor) family of proteins (Rothman and Warren, 1994). These proteins are associated with the membranes of both the transport vesicle and target compartment, with the majority of the protein exposed to the cytoplasm. SNAREs are characterized by the presence of one or more amphipathic helices. These regions have a propensity to form coiled coils with neighboring helices and hence are likely to form the basis of intermolecular interactions. Although all SNARE family members share a common motif (Weimbs et al., 1997), they can be classified into three distinct subfamilies. Syntaxins are type I integral membrane proteins of ∼35–45 kDa (Weimbs et al., 1997). Syntaxin I was originally identified as a brain-specific protein that localized to the presynaptic membrane and was essential for synaptic transmission (Bennett et al., 1992). Several mammalian and yeast isoforms of syntaxin have now been isolated (Bennett et al., 1992, 1993; Aalto et al., 1993; Bock et al., 1996), and their restricted cellular locations imply a role for syntaxins in determining the specificity of membrane transport. Likewise, isoforms of SNAP25 (synaptosomal protein of molecular mass 25 kDa) have been identified (Oyler et al., 1989; Hess et al., 1992; Brennwald et al., 1994). SNAP25 is associated with the cytoplasmic face of membranes via multiple palmitoylation sites (Hess et al., 1992; Veit et al., 1996) and is able to form a binary complex with syntaxin (Pevsner et al., 1994). Since syntaxin and SNAP25 predominate on target membranes, they have been termed target membrane or t-SNAREs (Söllner et al., 1993b). The third subfamily of SNARE proteins is related to synaptobrevin, a small type II integral membrane protein associated with synaptic vesicles (Trimble et al., 1988), and are termed vesicle-associated (v)-SNAREs (Söllner et al., 1993b).
Current models for the function of SNAREs during membrane targeting are based on the SNARE hypothesis formulated by Rothman and colleagues (Söllner et al., 1993b). Here, distinct cellular locations are defined by a specific repertoire of t-SNAREs. These interact with specific v-SNARE(s) on transport vesicles to form a docking complex, the turnover of which is linked to membrane fusion. There is considerable evidence that SNARE-SNARE interactions contribute toward the specificity of targeting. First, specific cleavage of SNAREs underlies the potent inhibition of synaptic transmission brought about by clostridial neurotoxins (Schiavo et al., 1992). Second, 7S SDS-resistant complexes between v- and t-SNAREs can be formed in vitro (Hayashi et al., 1994) and isolated from cell extracts (Söllner et al., 1993a). Third, the specificity of binding between v- and t-SNAREs observed in vitro mirrors the pairing of v- and t-SNAREs within the cell (Calakos et al., 1994; Pevsner et al., 1994). Fourth, fusion between yeast vacuoles in vitro is dependent on appropriate SNAREs being present on both participating membranes (Nichols et al., 1997).
Two soluble proteins have been identified that interact with SNAREs and play essential roles in a number of intracellular transport steps. N-ethylmaleimide-sensitive fusion protein (NSF) is a 76 kDa homo-oligomeric ATPase (Whiteheart et al., 1994), related to several other ATPases of diverse function (Confalonieri and Duguet, 1995). Interaction between NSF and SNAREs is mediated by the soluble NSF attachment proteins (SNAPs), of which α-SNAP is the best characterized (Clary et al., 1990; Whiteheart and Kubalek, 1995). α-SNAP will bind with low affinity directly to either syntaxin or SNAP25, but binds most effectively to SDS-resistant 7S SNARE complexes (Hayashi et al., 1995; McMahon and Südhof, 1995). SNAP-dependent NSF recruitment generates a larger complex of 20S (Söllner et al., 1993a). Subsequently, NSF-dependent ATP hydrolysis induces alterations in syntaxin folding (Hanson et al., 1995) and, as a consequence, drives 20S complex disassembly (Söllner et al., 1993a). Although several partial reactions in this pathway have been reconstituted, the precise functions of NSF and SNAP during membrane fusion are a matter of much debate (see Bock and Scheller, 1997). According to the SNARE hypothesis, SNAP and NSF recruitment is contingent upon SNARE-dependent vesicle docking, and disassembly of the complex is coupled directly to membrane fusion (Rothman and Warren, 1994). In contrast, other models propose that NSF and SNAP fulfil their functions before membrane fusion (Morgan and Burgoyne, 1995). Here, it is proposed that the formation and turnover of the 20S complex is coupled to the activation of SNAREs, which drives docking between vesicle and target membrane.
Although much recent evidence is consistent with a predocking role for NSF and α-SNAP, the precise point at which they participate in membrane transport reactions is not known. Toward this goal, we have examined 20S complex formation on clathrin-coated vesicle–derived membranes. These are the earliest transport vesicle intermediates that can be purified. 20S Complex formation was followed in situ using a cross-linking approach, and the extent of vesicle docking was measured in parallel by a sensitive and precise light-scattering method. Our results demonstrate that 20S complex turnover occurs on coated vesicle-derived membranes and is not dependent upon vesicle docking.
MATERIALS AND METHODS
Antibodies
The following antibodies were used in this study: monoclonal anti-polyhistidine (His-1; Sigma Chemical, St. Louis, MO), monoclonal anti-NSF (clone 2E5; [Tagaya et al., 1993]), monoclonal anti-synaptobrevin (SP10 and SP11; Serotech, Oxford, England), monoclonal anti-syntaxin (HPC-1; Sigma), monoclonal anti-SNAP25 (SP12; Serotech), and monoclonal anti-myc (9E10 [Evan et al., 1985]).
Recombinant Proteins
His6-α-SNAP was prepared as described (Whiteheart et al., 1993) to a purity of greater than 95%. His6-NSF-myc was prepared by the same method, and final purity was ∼90%. Where used, NSF-myc was prepared as described (Wilson and Rothman, 1992). GST-tagged SNAREs were prepared as described (McMahon and Südhof, 1995). To obtain GST-α-SNAP, the coding sequence for bovine α-SNAP was excised from Qiagen (Chatsworth, CA) vector pQE-9 (Whiteheart et al., 1993) using BamHI and HindIII. The α-SNAP DNA fragment was blunt-ended using T4 DNA polymerase and inserted into the SmaI site of pGEX-4T-3 (Pharmacia, Piscataway, NJ). GST-α-SNAP was prepared by affinity purification following the manufacturer’s instructions. Radiolabeled α-SNAP was made by incubating a plasmid containing the α-SNAP cDNA (Whiteheart et al., 1992) in a combined transcription/translation kit (Promega, Madison, WI). The translated protein was enriched according to published procedures (Whiteheart et al., 1992). Translations typically contained 1 μg DNA and 60 μCi [35S]methionine. Specific incorporation of radiolabel into α-SNAP was confirmed by SDS-PAGE and autoradiography; virtually all the radiolabeled product comigrated with α-SNAP, although some incomplete translation products were also present (our unpublished observations). For generation of 4-(trifluoromethyldiazirino) benzoyl-N-hydroxysuccinic acid (TDBA)-lysyl [35S] α-SNAP, TDBA-modified lysyl tRNA was prepared as described (Görlich et al., 1991) and used to supplement in vitro translation reactions at the level of 40 pmol per 25 μl translation mix.
Formation and Recovery of SNARE Complexes
To follow formation of 20S complexes, sodium carbonate-extracted crude brain membranes or Tris-HCl–extracted clathrin-coated vesicles were incubated on ice for 6 min with in vitro-translated [35S]α-SNAP or TDBA-lysyl [35S]α-SNAP (an estimated 2.5 ng) and His6-NSF-myc (2 μg) in either HNE buffer (20 mM HEPES-NaOH, pH 7.4, 100 mM NaCl, 2 mM EDTA) (brain membranes) or TE buffer (20 mM Tris-HCl, pH 9.0, 2 mM EDTA) (coated vesicles) containing 1 mM ATP and 1 mM DTT. Triton X-100 was added to a final concentration of 1% (wt/vol), and the incubation continued for a further 20 min with intermittent mixing. Samples were cleared by centrifugation (5 min, 12,000 × g), and the resulting supernatants were diluted twofold in the appropriate buffer and incubated for a further 2 h in the presence of monoclonal anti-polyhistidine antibody (1 μl) or 9E10 anti-myc (10 μg) as indicated. The incubation was then supplemented with 10 μl Protein A- or G-agarose beads (Calbiochem, San Diego, CA) that had been preincubated for 1 h at 4°C in HNE buffer containing 1 mg/ml BSA. After incubation overnight at 4°C on a rotator, the agarose beads were recovered by centrifugation and washed three times with wash buffer (Balch et al., 1984). The final pellets were resuspended in scintillation cocktail and counted for radioactivity.
To follow 7S SNARE complexes in coated vesicle membranes, Tris-washed vesicles were resuspended in SDS-PAGE sample buffer and incubated for 5 min at either 95°C or 37°C, before running on a 10% acrylamide gel. SNARE complexes were analyzed by Western blotting with a mixture of anti-syntaxin, anti-SNAP25, and anti-synaptobrevin antibodies followed by an HRP-labeled secondary antibody (Hayashi et al., 1994). To test the ability of recombinant SNAREs to form SDS-resistant complexes, GST-syntaxin and GST-SNAP25 (3 μg each) were mixed with or without GST-cellubrevin (3 μg) and incubated for 2 h at 4°C in HNE buffer including 1% (wt/vol) Triton X-100. Incubation products were diluted in sample buffer either at 37°C or 95°C, then analyzed by Western blotting.
Cross-linking
Samples containing membranes were incubated with modified TDBA-lysyl [35S]α-SNAP and recombinant NSF as described above, and at the time indicated were irradiated for 5 min on ice using a Spectroline B-100F UV lamp from Ultra Violet Products (Cambridge, United Kingdom) (100 W mercury bulb, 365-nm filter). Unless described specifically, samples were treated as for 20S complex formation. The protein A-agarose beads were washed twice in wash buffer, once in HNE buffer, and then boiled in sample buffer. Samples were analyzed by SDS-PAGE followed by phosphorimaging or autoradiography. For coated vesicle-derived membranes, cross-linking was performed in TE buffer. These conditions provide the optimal pH for NSF-dependent ATPase activity. The efficiency of 20S complex assembly and turnover was identical in this buffer compared with HNE buffer (our unpublished observations).
In some experiments, samples were incubated with the homobifunctional cross-linking reagent succinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (SMCC). SMCC (1 mM) was added to samples and incubated on ice for 10 min. Cross-linker was quenched with 100 mM glycine for a further 10 min on ice, after which samples were treated as above.
For secondary immunoprecipitations, protein A-agarose beads containing bound 20S complexes were incubated with 30 μl denaturing buffer (100 mM Tris-HCl, pH 7.6, 140 mM NaCl, 1% [wt/vol] SDS) for 5 min at 95°C unless indicated. After addition of 170 μl immunoprecipitation buffer (10 mM Tris-HCl, pH 7.6, 140 mM NaCl, 1% Triton X-100), samples were incubated on ice with mixing for 30 min and then centrifuged, and the resulting supernatants were incubated with a second antibody for 2 h at 4°C. Protein G- or protein A-agarose beads were added as appropriate and samples treated as above.
Membrane Preparations
For preparation of crude brain membranes, porcine brains were homogenized in a Waring blender with 2 volumes of homogenization buffer (140 mM sucrose, 20 mM HEPES, 70 mM potassium acetate, pH 7.2) containing 1 mM DTT and 40 μg/ml PMSF. Homogenates were centrifuged for 30 min at 5000 rpm in a Beckman JA10 rotor (Beckman, Fullerton, CA) and the supernatant was centrifuged for 3 h at 30,000 × gav. Pellets were resuspended in homogenization buffer, and crude membranes were prepared by overlaying gradients of 40% (wt/vol) sucrose (5 ml) and 20% (wt/vol) sucrose (15 ml) containing 10 mM Tris-HCl, pH 7.5. Gradients were centrifuged for 3 h at 27,000 rpm in a Beckman SW28 rotor, and membranes were isolated from the interface. For preparation of carbonate-extracted membranes, crude membranes were diluted with 4 volumes of 125 mM sodium carbonate, pH 11.5, and incubated for 30 min at 4°C. Membranes were recovered by centrifugation and resuspended in HNE buffer.
Coated vesicles were purified from porcine brain essentially as described (Steel et al., 1996). To maximize the degree of purity, vesicle-containing fractions were recovered from a first D2O/Ficoll gradient, and then loaded onto a second, identical gradient. Purity of preparations was assessed by negative staining as described previously. Invariably, preparations were essentially devoid of contaminating membranes. To generate uncoated vesicles, samples were incubated for 30 min on ice with TE buffer, and then centrifuged for 10 min at 100,000 rpm in a Beckman TLA100.3 rotor. Uncoated vesicles were resuspended in a small volume of TE buffer and clarified to remove any possible aggregates by centrifuging at 28,000 rpm for 20 min in a TLA100.3 rotor.
Determination of Vesicle Size
Light-scattering experiments were performed on a Malvern 4700C spectrometer (Malvern Instruments, Worcester, England) and 128-channel digital autocorrelator equipped with a 100 mW argon-ion laser. The 488-nm line was employed for all experiments. All experiments used clarified vesicles in a final volume of 200 μl and were performed at an angle of 90° at 25°C. The homogeneity and cleanliness of the samples were checked continuously by monitoring the absolute light-scattering intensity. Quasi-elastic scattering from the vesicles was analyzed using the standard Malvern PCS software in two ways. In the first method (monomodal analysis), the optimum correlation time is obtained after an automated search for the “far point,” i.e., the time after which there is no detectable correlation in the scattered light. Correlation is then performed a number of times (generally 10) over the selected time and the correlograms are statistically assessed for noise due to extraneous material. The correlation function is fitted by the method of cumulants to obtain an average diffusion coefficient, which by Stoke’s equation is related to an average particle size. A similar average particle size may arise from a variety of mixtures of different sized particles. Thus, in general, the problem is said to be “ill conditioned.” The close agreement between the average size obtained by electron microscopy and light scattering suggests that this specific problem is well conditioned. To confirm the uniformity of the sample, the data were subjected to a second method of analysis (multimodal analysis). In this method, the correlator is reconfigured as four separate correlators of 32 channels each, which are optimized in correlation times to span the particle size distribution of interest. The data are subsequently analyzed using an exponential sampling method. In this method, a fit to the correlogram is sought employing different size categories of particles spanning the size range of interest. This is a sensitive test for heterogeneity in population size as opposed to polydispersity in size. As a result, this configuration is well suited to allow the detection of “monomeric” and “multimeric” species of vesicles.
Electron Microscopy
Samples were adsorbed onto formvar/carbon-coated grids for 1 min and stained with saturated (5%) uranyl acetate.
RESULTS
Detection and Characterization of α-SNAP Cross-linking Partners
Since NSF and α-SNAP interact with each other only when they are part of a 20S complex, formation and disassembly of these complexes in solution can be followed by immunoprecipitation of [35S]α-SNAP with antibodies to NSF (Wilson et al., 1992). This protocol was modified, by cross-linking [35S]α-SNAP before immunoprecipitation, so that complex formation occurring on membranes in situ could be detected. The reagent of choice for producing α-SNAP-protein adducts was TDBA-modified lysine residues incorporated into the radiolabeled, in vitro-translated, protein (Krieg et al., 1986; Görlich et al., 1991; Brunner, 1996). Upon UV irradiation, the TDBA group is converted to a highly reactive carbene species that will covalently modify neighboring molecules. The reactivity of this intermediate limits the efficiency of cross-linking, since it can be readily quenched by water. However, this property ensures that cross-linking is specific, since only groups tightly apposed to the TDBA-lysine will be modified. Moreover, cross-linking is not dependent upon the presence of specific side chains within the acceptor protein. This reagent has been used successfully to investigate a number of intracellular processes, and in particular to identify specific protein-protein interactions during the translocation and insertion of newly synthesized proteins at the ER membrane (Görlich et al., 1992; High et al., 1993; Martoglio and Dobberstein, 1996).
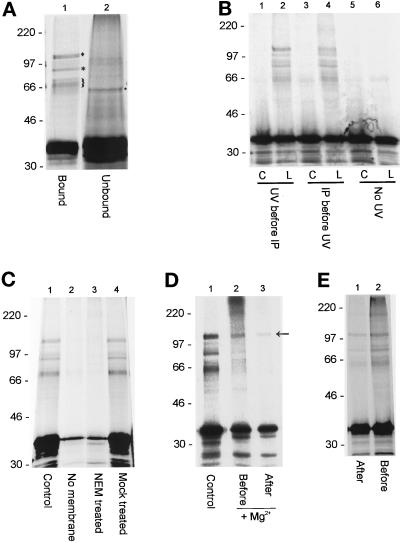
To optimize conditions for detection of cross-links on membranes, experiments were first performed using detergent extracts of crude brain-derived membranes, since these are rich in SNAREs (Söllner et al., 1993b). Radiolabeled TDBA lysyl α-SNAP was incubated with detergent-solubilized, sodium carbonate-extracted membranes and His6-NSF-myc. The reaction conditions allowed NSF to bind but not hydrolyze ATP (i.e., in the presence of ATP and absence of magnesium ions). UV irradiation of samples generated several α-SNAP cross-linking products (Figure 1A). Cross-linking was specific, since the pattern of cross-linking differed between fractions either incorporated into 20S complexes or excluded (Figure 1A, cf. lanes 1 and 2). A prominent radiolabeled species of about 62 kDa was observed in the supernatant of immunoprecipitations, consistent with interaction of α-SNAP with a 27-kDa protein (Figure 1A, lane 2, marked with dot). In contrast, cross-linking products of a 110-kDa doublet (diamond), 80 kDa (star), and 62–68 kDa (brackets) were found routinely in NSF immunoprecipitates (Figure 1A, lane 1, marked). Other products (100, 85, and 50 kDa) were observed in some experiments but were not seen routinely (see, for example, Figure 1B, lanes 2 and 4). High molecular mass adducts were seen in some experiments (see Figure 1D, lane 2, for example), but such products were not a general feature of these studies. The efficiency of cross-linking was similar whether samples were irradiated before or after recovery of NSF-containing complexes on protein G-agarose (Figure 1B). Cross-linking was dependent on UV irradiation and the presence of TDBA-lysine in the translated α-SNAP (Figure 1B). Cross-linking products required the addition of membranes, excluding the possibility that some were a consequence of interactions between α-SNAP and soluble factors (Figure 1C). As expected, cross-linking was detected only in the presence of functional NSF (Figure 1C, cf. lanes 3 and 4). Furthermore, the induction of ATP hydrolysis by addition of magnesium ions led to significant reduction in formation of the 110-kDa cross-linking product and almost complete disappearance of other products. This was true whether magnesium was added before or after binding [35S]α-SNAP and NSF to membranes (Figure 1D, cf. lane 1 and lanes 2 and 3). We therefore conclude that each cross-link resulted from a specific interaction between α-SNAP and another protein component of the 20S “fusion” complex. Maximal efficiency of cross-linking was observed when samples were irradiated before membrane solubilization, suggesting that 20S complex formation occurred more readily with intact membranes (Figure 1E).
Figure 1.
Characterization of α-SNAP cross-links. (A) Samples containing carbonate-washed brain membranes, [35S]TDBA-α-SNAP, and His6-NSF-myc were solubilized, irradiated, and then immunoprecipitated with anti-polyHis antibody on protein G-agarose beads. Fractions of the pellet (lane 1) or supernatant (lane 2) were analyzed by SDS PAGE. Cross-linking products that were seen in all experiments are marked. (B) Samples containing TDBA-lysyl [35S]α-SNAP (L) or control [35S]-α-SNAP (C), were irradiated before (lanes 1 and 2) or after (lanes 3 and 4) immunoprecipitation of 20S complexes on protein G-agarose beads, as indicated. Alternatively, samples were not irradiated (lanes 5 and 6). (C) Cross-linking was performed using complete reactions (control; lane 1), reactions lacking brain-derived membranes (no membranes; lane 2), reactions containing NSF previously treated for 15 min at 4°C with 3 mM NEM and then quenched with 3 mM DTT (NEM treated; lane 3), or reactions containing NSF pretreated with 3 mM NEM and 3 mM DTT together (mock-treated; lane 4). (D) Samples were treated as above (control; lane 1), or 10 mM MgCl2 was added before (lane 2) or after (lane 3) binding of [35S]TDBA-α-SNAP and His6-NSF-myc. The 110-kDa band, corresponding to an α-SNAP-NSF adduct, is arrowed. (E) Complete samples were irradiated before (lane 2) or after (lane 1) addition of detergent, before recovery of 20S complexes.
To identify the specific cross-linking products described above, the immunoprecipitated samples were eluted under denaturing conditions and reprecipitated with specific antibodies recognizing known components of the 20S complex (Figure 2A). This allowed the unambiguous identification of the 110-kDa doublet as α-SNAP-NSF cross-linking products (Figure 2A, lane 2). The 62- to 68-kDa band corresponded to an α-SNAP-SNAP25 cross-link (Figure 2A, lane 3). Antibodies to syntaxin or synaptobrevin did not precipitate any labeled products (our unpublished observations), and the prominent band at 80 kDa could not be ascribed to interactions with a known protein. Nevertheless, these data confirmed that UV cross-linking is an effective means of following both 20S complex formation and turnover.
Figure 2.
Identification of α-SNAP cross-links. (A) Complete incubations containing carbonate-washed brain membranes were recovered on protein G-agarose and analyzed by SDS PAGE (control). Alternatively, samples were subjected to secondary immunoprecipitations using monoclonal anti-NSF or anti-SNAP25 antibodies. For secondary precipitations, twice the amount of sample was used, to compensate for loss of material during the second precipitation step. (B) Samples containing membranes, His6-NSF-myc, and unmodified [35S]-α-SNAP were incubated with SMCC. After quenching with glycine, 20S complexes were recovered and analyzed by SDS-PAGE (lane 1). Alternatively, a secondary immunoprecipitation step was performed with anti-syntaxin antibody before analysis (lane 2).
Although α-SNAP-syntaxin/synaptobrevin adducts could not be detected by UV cross-linking, this may simply reflect the absence of modified lysine residues in appropriate regions of α-SNAP. To address this possibility, an alternative cross-linking approach was used. Samples containing [35S]α-SNAP with unmodified lysine residues were incubated with the bifunctional cross-linking reagent SMCC, before immunoprecipitation of 20S complexes and secondary immunoprecipitation with specific antibodies. As expected, the pattern of cross-linking products observed using the bifunctional cross-linking reagent was distinct from that obtained by UV-dependent cross-linking, reflecting the different properties of the respective cross-linking reagents. In particular, a specific α-SNAP-syntaxin cross-linking product was detected using SMCC (Figure 2B, lane 2). Efficient cross-linking between α-SNAP and SNAP25 also occurred (our unpublished observations). However, no evidence for an interaction between α-SNAP and synaptobrevin was observed using SMCC or several other bifunctional cross-linking reagents (our unpublished observations).
Characterization of Coated Vesicles
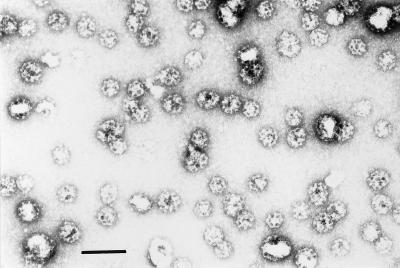
Previous research has established that clathrin-coated vesicles purified from brain contain both v- and t-SNAREs (Walch-Solimena et al., 1995). Moreover, coated vesicle-derived membranes from brain or other tissue sources form 20S complexes in detergent extracts (Steel et al., 1996). Given the purity and homogeneity of coated vesicle preparations, they proved an ideal source of membranes for this study. The purity of the brain-derived coated vesicle preparations used was confirmed by electron microscopy (Figure 3). Furthermore, little evidence of vesicle aggregation, which might affect the interpretation of results, was apparent.
Figure 3.
Negative staining of clathrin-coated vesicles. Coated vesicles were purified from porcine brain as described. Bar, 0.2 μm.
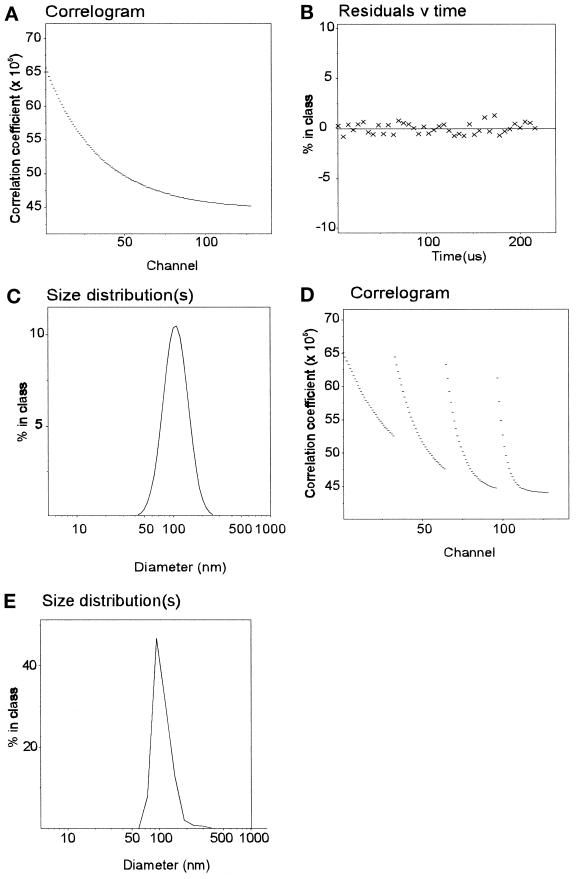
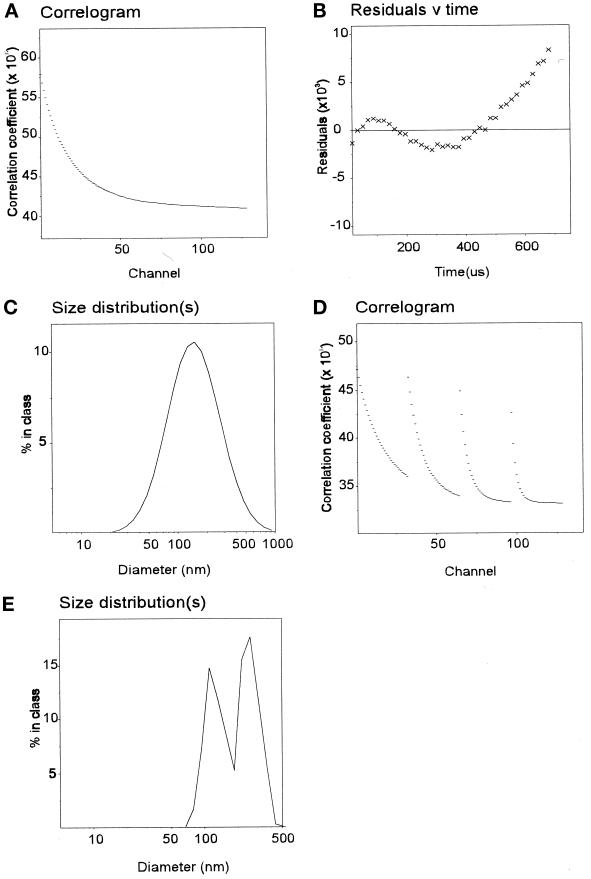
As a more sensitive and precise means of assessing vesicle size in solution, preparations were analyzed by photon correlation spectroscopy using laser light scattering. During correlation experiments, the absolute light- scattering intensity was continuously monitored and indicated that the solutions employed were very clean and homogeneous, there being no extraneous scattering detectable. The autocorrelation function extracted from the quasi elastic scattering (Figure 4A) was fitted by the method of cumulants analysis. The residuals obtained for this fit were very low and randomly distributed around the fitted function (Figure 4B), consistent with a homogeneous preparation. By monomodal analysis, preparations of coated vesicles were shown to contain a uniform distribution of particles with an average diameter of 98.6 nm, consistent with the size of particles observed by negative staining (Figure 4C and Table 1). The quality of the data and the approach were confirmed by applying the more demanding multimodal analysis (Figure 4D; see MATERIALS AND METHODS), which yielded essentially the same average particle diameter (Figure 4E). Vesicle size was consistent between preparations (see Table 1).
Figure 4.
Size analysis of clathrin-coated extracted vesicles. The correlogram for clathrin-coated vesicles (A) was obtained over a 5.4- to 7.8-μs collection time as described in MATERIALS AND METHODS. When analyzed by a third-order cumulants analysis, the residuals indicated a good fit (B) by particles of a median diameter of 99 nm (C). Multimodal analysis was performed after setting the particle size limits between 30 and 300 nm (see MATERIALS AND METHODS). The parallel 4 × 32 channel correlator data (D) were analyzed (see MATERIALS AND METHODS) and confirmed the presence of a homogeneous distribution of particles (E) of median size 99 nm.
Table 1.
Summary of sizing data
| Vesicle preparation | Mean diameter (monomodal) | Mean diameter (multimodal) | n | Fit error |
|---|---|---|---|---|
| Coated vesicles | 98.7 ± 5.4 nm | 98.6 ± 7.6 nm | 3 | 9 × 10−3 |
| Tris-extracted vesicles | 89.9 ± 2.0 nm | 90.9 ± 2.2 nm | 3 | 14 × 10−3 |
| Tris-extracted vesicles + NSF, [35S]α-SNAP | 87.9 ± 2.0 nm | 88.4 ± 2.2 nm | 3 | 14 × 10−3 |
Data regarding the sizing of vesicle preparations are summarized. The errors (SD) refer to those found between vesicle preparations, rather than those obtained between analyses within the same experiment.
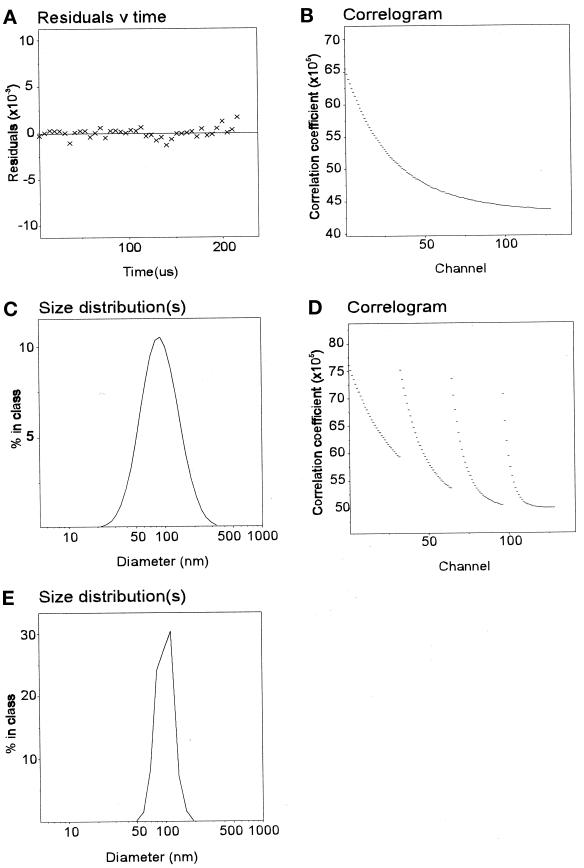
To make sure that exogenously added proteins could interact with the coated vesicle membrane, the clathrin coat was removed by extraction in Tris-HCl. Conditions that resulted in almost complete extraction of coat proteins (95% of clathrin and adaptors were removed; our unpublished observations) led to a rapid reduction in the total intensity of light scattering and a substantial increase in the range of particle sizes by monomodal analysis. This was confirmed by multimodal analysis that showed two species, most likely clathrin triskelia and stripped vesicles (our unpublished observations). To confirm this interpretation of the light-scattering data and simplify subsequent analysis, the stripped vesicles were separated from coat proteins by centrifugation and thereafter washed and resuspended in Tris-HCl buffer. Monomodal analysis (Figure 5, A–C) and multimodal analysis (Figure 5, D and E) confirmed the presence of a homogeneous distribution of particles of ∼90 nm. The reduction in particle size resulting from Tris-HCl extraction is consistent with removal of the clathrin coat.
Figure 5.
Size analysis of Tris-HCl–extracted vesicles. Monomodal (A–C) and multimodal (D and E) analysis of vesicles extracted in Tris-HCl gave a median size of 89 nm. For methods see Figure 4.
Analysis of vesicle size provided no evidence that coated vesicle-derived membranes were docked. However, it was important to know how effectively changes in vesicle size resulting from docking/aggregation could be detected by this method. Previous studies have shown that when vesicles stripped of clathrin but retaining significant amounts of adaptors are exposed to neutral pH, extensive adaptor-mediated vesicle docking/aggregation occurs (Beck et al., 1992). Sodium carbonate extraction of coated vesicles led to efficient removal of clathrin and partial loss of adaptors (our unpublished observations). Multimodal analysis indicated the presence of particles of 90–100 nm in diameter, as expected (our unpublished observations). When these vesicles were resuspended in buffer at neutral pH, a pronounced increase in average particle size was observed by monomodal analysis (Figure 6, A–C). Moreover, the size distribution was much greater, and the residuals for the cumulants analysis systematically deviated from the fit (Figure 6B). The presence of more than one particle species was confirmed by multimodal analysis, which showed size peaks at ∼100 and 300 nm (Figure 6E). A similar, although less dramatic, size shift was detected when Tris-HCl–extracted vesicles (which contained fewer adaptors than carbonate-extracted vesicles) were resuspended at neutral pH (our unpublished observations). Thus, the quality of the primary light-scattering data and its response to changes in particle behavior established that the technique would monitor vesicle docking with a high degree of confidence.
Figure 6.
Size analysis of aggregated vesicles. Carbonate-extracted vesicles were diluted tenfold into HNE buffer. The correlogram of these vesicles (A), when fitted by a third-order cumulants analysis, showed a significant and systematic deviation of the residuals (B) from the mean. Size analysis yielded a broad distribution spanning 30–700 nm diameter with a median of 220 nm (C). Multimodal analysis was performed setting particle sizes between 50 and 500 nm (see MATERIALS AND METHODS) and the parallel 4 × 32 channel correlograms (D) were analyzed by the Pike-Ostrowsky method. The analysis indicates the presence of single vesicles (∼100 nm) and a polydisperse distribution of multimers of mean size 350 nm.
Uncoated Vesicles Support 20S Complex Formation
Preparations of uncoated vesicles were assayed for their ability to act as substrates for 20S complex assembly. Vesicles were incubated with recombinant NSF and TDBA-lysyl [35S]α-SNAP at the ratio already established to maximize the detection of cross-linking products and then UV irradiated. Neither TDBA-lysyl [35S]α-SNAP nor NSF caused significant light scattering either alone or in combination (our unpublished observations). No change in vesicle size was seen after addition of NSF and α-SNAP (Table 1). Hence, vesicle docking could not be detected at any time during the course of the reaction.
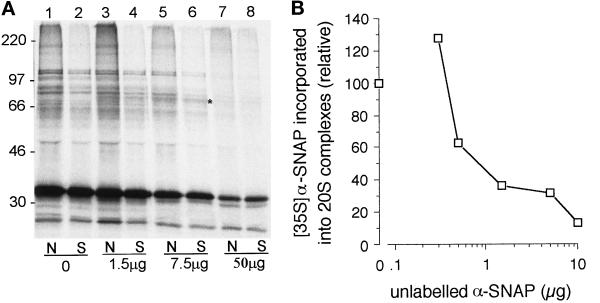
After irradiation, vesicle membranes were solubilized and 20S complexes isolated. The pattern of cross-linking products obtained using coated vesicle-derived membranes was very similar to that obtained using carbonate-extracted brain membranes (Figure 7A, cf. lanes 1 and 2). In particular, cross-links to NSF (diamond) and SNAP25 (brackets) were clearly distinguishable and were confirmed by secondary immunoprecipitation (Figure 7B, lanes 2 and 3). The prominent cross-link at 85 kDa was also present (Figure 7A, lane 2, star). Products at 50 kDa (dot) and 60 kDa (Figure 7A, lane 2, square) were observed more readily than when crude membranes were used, suggesting that the balance of cross-linked products may vary slightly according to the source of membrane. However, the data are fully consistent with the formation of functional 20S complexes. Addition of magnesium ions before UV irradiation and detergent solubilization caused some reduction in α-SNAP cross-linking to NSF (Figure 7C, lane 2) and a more substantial loss of cross-linking to other components of the complex (Figure 7C, cf. lanes 1 and 2). This demonstrated that 20S complexes were turned over, as well as formed, on undocked membranes. The partial resistance of SNAP-NSF cross-linking to ATP hydrolysis, in both crude detergent extracts (see Figure 1D) and purified membrane preparations, suggests that at least a portion of these molecules remain associated with each other after release from their receptor.
Figure 7.
α-SNAP cross-linking with coated vesicle-derived membranes. (A) Carbonate-washed brain membranes (lane 1) or coated vesicle-derived membranes (lane 2) were incubated with similar amounts of [35S]TDBA-α-SNAP and His6-NSF-myc, and then analyzed as described. Specific cross-linking products are indicated and referred to in the text. (B) Complete samples containing coated vesicle-derived membranes were irradiated, and then 20S complexes were immunoprecipitated and analyzed as standard (lane 1). Alternatively, samples were subjected to secondary immunoprecipitations using anti-NSF (lane 2), anti-SNAP25 (lane 3), or control (anti-clathrin heavy chain; lane 4) antibodies, as indicated. (C) Complete samples containing coated vesicle-derived membranes were incubated without (control, lane 1) or with (lane 2) 10 mM MgCl2 for 15 min, and then irradiated before recovery of 20S complexes.
Despite the enrichment of synaptobrevin in clathrin-coated vesicles (Maycox et al., 1992), it was formally possible that we had detected low affinity binding of α-SNAP to t-SNARE monomers or syntaxin/SNAP25 complexes, rather than high-affinity binding to components of 7S SNARE complexes. This seemed unlikely given the dependence of this cross-linking approach on high affinity interactions, and the low affinity with which α-SNAP binds to monomeric t-SNAREs or syntaxin/SNAP25 complexes (Hayashi et al., 1995; McMahon and Südhof, 1995). To completely exclude this possibility, we examined cross-linking products from experiments in which SDS had been added before recovery of NSF-containing complexes. Since trimeric 7S SNARE complexes are distinguished from other SNARE-containing complexes by their resistance to SDS treatment (Hayashi et al., 1994), demonstration of SDS resistance should provide proof for the existence of such complexes. We found, first, that cross-linking to many components was preserved when SDS was added at a point before recovery of 20S complexes (cf. Figure 8A/B and Figure 7). In fact, similar cross-linking patterns were obtained whether UV irradiation was performed before or after SDS solubilization of vesicle membranes (cf. Figure 8, A and B, lane 1). Moreover, when reaction products were incubated at 37°C rather than 95°C before SDS-PAGE (Figure 8, A and B, lane 2), the adduct(s) apparent at 62–68 kDa were largely removed and were replaced by higher molecular mass adducts. Specifically, inclusion of all α-SNAP-SNAP25 adducts within SDS-resistant SNARE complexes was confirmed by secondary immunoprecipitation; all of the 62- to 68-kDa SNAP25 adduct remained part of much higher molecular mass species when samples were incubated at 37°C rather than 95°C (Figure 8C, lane 4, diamond and bracket) (it was also noted that the α-SNAP-NSF adduct, which reprecipitates with residual anti-NSF during secondary precipitations, changed mobility slightly between samples treated at 37°C or 95°C [Figure 8, A–C, asterisk]). Control experiments based on incorporation of α-SNAP into 20S complexes using membrane-derived SNAREs, or the in vitro generation of 7S complexes from recombinant SNAREs, showed that high molecular mass SDS-resistant SNARE complexes could not be formed from monomeric SNAREs that had been exposed to SDS (our unpublished observations). To confirm that only complexes containing all three SNAREs were resistant to SDS, recombinant SNAREs were combined under conditions that favor spontaneous SNARE-SNARE binding, and the incubation products were analyzed by Western blotting (Figure 8D). Only those incubations containing all three SNAREs gave rise to SDS-resistant complexes, consistent with recently published data that SDS resistance is an exclusive property of the trimeric complex (Fasshauer et al., 1997). Further evidence that 7S SNARE complexes were present in coated vesicle-derived membranes was obtained by examining endogenous SNAREs by Western blotting (Figure 8E). In addition to SNARE monomers, several species between ∼70 kDa and 200 kDa were observed, consistent with the presence of SDS-resistant SNARE complexes (Hayashi et al., 1994) in vesicle membranes.
Figure 8.
α-SNAP cross-links to SNAP25 within SNARE complexes. (A) Coated vesicles were solubilized in 0.5% SDS and incubated for 10 min at 4°C before addition of Triton X-100 to a final concentration of 2%. Cross-linking with [35S]TDBA-α-SNAP and recovery of 20S complexes were then performed as standard. Samples were incubated at 95°C (lane 1) or 37°C (lane 2) before SDS-PAGE. (B) Cross-linking was performed in situ on vesicle membranes as standard, and then vesicles were solubilized in SDS. After addition of Triton X-100, 20S complexes were reisolated. Samples were incubated at 95°C (lane 1) or 37°C (lane 2) before SDS-PAGE. (C) Samples were treated as in panel B, and 20S complexes were isolated. Complexes were analyzed by SDS-PAGE after preparation in sample buffer at 95°C (lane 1) or 37°C (lane 2). Alternatively, immunoprecipitates were eluted from beads with SDS at room temperature and immunoprecipitated with anti-SNAP25. These were analyzed by SDS-PAGE after treatment at 37°C (lane 3) or 95°C (lane 4). (D) GST-syntaxin and GST-SNAP25 were incubated with or without GST-cellubrevin as indicated. Samples were prepared for SDS-PAGE at 37°C or 95°C, and then analyzed by Western blotting for the formation of SDS-resistant SNARE complexes. (E) Coated vesicles were solubilized in SDS-PAGE sample buffer at 95°C (lane 1) or 37°C (lane 2), and then analyzed by Western blotting for SNAREs.
It was important to establish that the formation of functional 20S complexes observed above was not confined to the very small proportion of docked/aggregated vesicles that might be present in the vesicle preparation but which may not be detected by light-scattering analysis. Therefore, the efficiency of cross-linking when samples were irradiated before membrane solubilization was compared with that obtained when samples were irradiated after addition of detergent. In these latter samples, one would expect maximal formation of 20S complexes, since the orientation of SNAREs with respect to each other would not be restricted by the presence of membranes. In fact, more efficient cross-linking was routinely observed using intact, compared with solubilized, membranes (Figure 9A, cf. lanes 1 and 2). Addition of moderate amounts of unlabeled His6α-SNAP led to the appearance of a cross-linking product of ∼75 kDa, presumably corresponding to an α-SNAP-α-SNAP dimer, whether or not samples were solubilized before cross-linking (Figure 9A, asterisked, lanes 3–6). Further addition of unlabeled His6α-SNAP to a level that prevented inclusion of radiolabeled α-SNAP in 20S complexes (Figure 9B) led to a parallel reduction in cross-linking both on intact membranes and in solution (Figure 9A, lanes 7 and 8). Thus, the inclusion of increasing amounts of unlabeled α-SNAP confirmed that the number of 20S complexes formed on intact membranes was at least as great as that formed in solution. Light-scattering analysis of samples confirmed that no significant change in particle size occurred at any concentration of unlabeled α-SNAP that was used (88.1 nm with no α-SNAP; 86.0 nm with 50 μg α-SNAP).
Figure 9.
20S complex formation on membranes is more efficient than in solution. (A) Coated vesicle-derived membranes were incubated as standard without (lanes 1, 3, 5, and 7) or with (lanes 2, 4, 6, and 8) detergent and then UV-irradiated. Increasing amounts of unlabeled α-SNAP were present, as indicated. The appearance of a 75-kDa, α-SNAP-dependent cross-link (lanes 3–6) is indicated by an asterisk. (B) Coated vesicle-derived membranes (one fifth of that used in panel A) were incubated with radiolabeled α-SNAP and increasing amounts of unlabeled α-SNAP. Formation of 20S complexes containing radiolabeled α-SNAP was assayed as described.
It has been suggested that NSF-dependent disassembly of the 20S complex could drive conformational changes in SNARE(s) that are required for vesicle docking (Nichols et al., 1997). To examine whether this reaction might be sufficient by itself to promote vesicle docking, the sizing of vesicles was repeated in the presence of magnesium ions, to allow NSF-dependent ATP hydrolysis. Greater than 70% of 20S complexes disassembled under these conditions (our unpublished observations). A small increase in the total intensity of light scattering was observed, but essentially no change in the mean particle size was detected (89.0 ± 0.6 [n = 5] in the absence of magnesium, 87.3 ± 2.0 [n = 10] with magnesium added). Hence, it appears that these conditions are not sufficient to give rise to efficient vesicle docking. Although this implies that additional factors are required, it may be that the concentration of vesicles that we have achieved is insufficient to allow docking to occur; we have estimated that the effective concentration of syntaxin in these vesicle preparations is in the micromolar range, while the affinity constant for SNARE interactions is ∼5 × 10−6 M (Calakos et al., 1994).
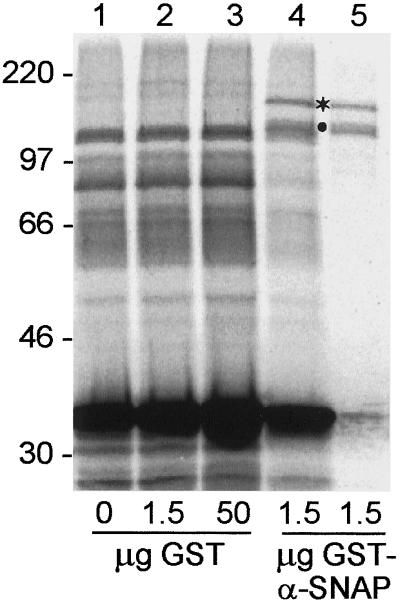
The novel 75-kDa cross-linking product formed in the presence of recombinant His6-α-SNAP seemed likely to be an α-SNAP-α-SNAP dimer. To confirm this interaction, a similar experiment was performed using the higher molecular mass GST-α-SNAP fusion protein, and the migration of exogenous α-SNAP-dependent cross-linking products was analyzed by SDS-PAGE. As shown in Figure 10 (cf. lanes 1 and 4), inclusion of 1.5 μg GST-α-SNAP diminished the intensity of α-SNAP-NSF/SNARE cross-linking products (parallel experiments showed that GST-α-SNAP competed somewhat more effectively than His6-α-SNAP for inclusion in 20S complexes [our unpublished observations]). However, it also led to the appearance of novel cross-linking products of ∼120 and 150 kDa (Figure 10, lane 4; dot and asterisk). Both of these high molecular mass adducts were immunoprecipitated with an antibody to GST (Figure 10, lane 5). As a control, addition of increasing amounts of recombinant GST neither diminished the intensity of α-SNAP-NSF/SNARE cross-linking products nor led to the formation of high molecular mass cross-linking species (Figure 10, lanes 1–3).
Figure 10.
Evidence for α-SNAP–α-SNAP interactions within 20S complexes. Standard cross-linking reactions were performed using coated vesicle-derived membranes without additions (lane 1), or with 1.5 μg (lane 2) or 50 μg (lane 3) GST, or 1.5 μg (lanes 4 and 5) GST-α-SNAP. Pellets from 20S complexes (lanes 1–4) or a secondary immunoprecipitation with anti-GST (lane 5) were analyzed for cross-linking products.
DISCUSSION
The soluble proteins NSF and α-SNAP are critical components of membrane transport steps, although their precise function is not known. Both proteins are recruited to a complex of membrane-associated receptors (SNAREs) and the resultant 20S complex is turned over by NSF-dependent ATPase activity (Söllner et al., 1993a, 1993b; Hayashi et al., 1995). It has been proposed by Rothman and colleagues that recruitment of NSF and SNAP to SNARE complexes occurs after SNARE-mediated vesicle docking (Rothman, 1994; Rothman and Warren, 1994). In contrast, others have suggested that NSF and SNAP perform a function before docking that may be related to SNARE activation (Morgan and Burgoyne, 1995; Nichols et al., 1997). A variety of evidence supports the latter view, without being definitive (see (Woodman, 1997) for review). In particular, fusion of docked synaptic vesicles with the presynaptic membrane is rapid, reversible, and independent of added ATP (Bruns and Jahn, 1995). These characteristics are not consistent with an NSF-dependent reaction. Additionally, NSF has been located on undocked synaptic vesicles (Hong et al., 1994) and clathrin-coated vesicles (Steel et al., 1996), implying a predocking role for the protein. Evidence derived from homotypic fusion of endosomes (Rodriguez et al., 1994; Colombo et al., 1996) or yeast vacuoles (Mayer et al., 1996; Mayer and Wickner, 1997) also suggests that NSF and SNAP act before membrane docking.
These studies do not examine the precise role these proteins play in vesicle docking/fusion. Toward this end, we have investigated whether NSF-dependent 20S complex formation and disassembly can occur on clathrin-coated vesicle–derived membranes. This is the earliest transport intermediate that it is possible to purify. We have also examined whether complex formation is contingent upon membrane docking. Previous in vitro studies of 20S complex formation have utilized detergent extracts and immunoprecipitation (Wilson et al., 1992; Söllner et al., 1993a). Under such conditions, it is unclear whether complexes contain SNAREs derived from opposing membranes (consistent with a postdocking role) or the same membrane (consistent with a predocking role). We have used a cross-linking approach to stabilize SNAP-protein interactions before membrane solubilization, and hence follow the formation and turnover of NSF- and SNAP-containing complexes on the surface of intact membranes.
Using the UV-activatible cross-linker TDBA-lysine, we observed cross-linking of α-SNAP to NSF and to the t-SNARE SNAP25. The efficiency of cross-linking was comparable to that achieved with this reagent in other studies (Martoglio and Dobberstein, 1996), suggesting high-affinity interactions were occurring. No cross-linking of α-SNAP to syntaxin or synaptobrevin, the other characterized components of 20S complexes, was observed using TDBA-modified lysine residues, although an α-SNAP-syntaxin adduct was detected when the bifunctional reagent SMCC was used. Our inability to observe α-SNAP-synaptobrevin adducts with any reagent tested does not necessarily imply that this interaction is absent in our system. However, our results are consistent with previous studies (Hayashi et al., 1995; McMahon and Südhof, 1995) which have been unable to demonstrate the direct binding of α-SNAP to synaptobrevin. To demonstrate unequivocally that 7S complexes, rather than monomeric SNAREs or syntaxin/SNAP25 dimers, were substrates for α-SNAP binding in isolated vesicles, we exploited the finding that 7S complexes are resistant to SDS. Essentially all of the cross-linking was to such high molecular mass, SDS-resistant, complexes.
Several discrete UV-dependent cross-linking products were observed that could not be ascribed to proteins known to interact with α-SNAP. For example, a 62-kDa product that did not assemble into 20S complexes indicated that α-SNAP may bind to a membrane-associated protein distinct from SNAREs. Unidentified cross-linking products were also observed within 20S complexes, in particular one of 85 kDa. Interestingly, we also observed α-SNAP–α-SNAP cross-linking within the 20S complex. This provides the first evidence that oligomerization of α-SNAP might play a role in membrane fusion.
We have additionally provided substantive evidence that 20S complex formation occurs independently of vesicle docking. By employing precise light- scattering analysis we have shown that, under all conditions, vesicle preparations are homogeneous with a mean size of 90 nm. If docking between vesicles were to occur, fusion would not proceed in the absence of Mg-ATP and cytosol. The species generated would thus have an apparent diameter of at least 180 nm, and probably much greater due to the increased diffusion coefficient of a particle with such a shape. Such particles are virtually absent from our preparations. We cannot completely exclude the possibility that complex formation occurred between SNAREs present on a very small fraction of vesicles that might be docked. This appears very unlikely, however, given that the efficiency of complex formation was at least as efficient on intact vesicles as solubilized ones. Solubilization should allow reorientation of SNAREs to maximize the extent of their interaction with each other. Since our data suggest that the same proportion of SNAREs can form complexes on intact membranes as in solution, the majority of, if not all, complex formation must be occurring between SNAREs within the same membrane.
Our findings extend previous observations regarding the formation of SNARE complexes on transport vesicles. SDS- and botulinum toxin-resistant SNARE complexes have been found on purified chromaffin granule membranes (Hohne-Zell and Gratzl, 1996). During the preparation of this manuscript, Jahn and colleagues reported that similar high molecular mass complexes containing SNAREs are present on synaptic vesicles and are partially sensitive to ATP hydrolysis (Otto et al., 1997). These studies focused on arrested vesicular intermediates formed after an NSF-dependent priming event (Chamberlain et al., 1995), where fusion with the cell surface can occur rapidly after a stimulus is provided. We have now demonstrated that SNARE complexes are formed even earlier in the transport vesicle cycle, on the coated vesicle membrane. Similar results are obtained using coated vesicles derived from placenta (our unpublished observations) and together with the results presented here imply that preassembly of SNARE complexes on undocked membranes may be a more general property of transport vesicle function. The exact role that this partial reaction plays in fusion is not clear. Nevertheless, our data are fully consistent with a priming event that reorganizes SNAREs and allows them to mediate vesicle docking.
ACKNOWLEDGMENTS
TDBA was a generous gift from Josef Brunner, Department of Biochemistry, ETHZ, Zürich, Switzerland. We are also grateful to Harvey McMahon (LMB, Cambridge, United Kingdom) for his gift of recombinant SNAREs. P.W. is an Medical Research Council Senior Research Fellow (grant G117/153). This work is also supported by Medical Research Council grant G9533795 MA (P.W.) and funding from the Biotechnology and Biological Sciences Research Council (S.H.).
REFERENCES
- Aalto MK, Ronne H, Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Beck KA, Chang M, Brodsky FM, Keen JH. Clathrin assembly protein AP-2 induces aggregation of membrane vesicles: a possible role for AP-2 in endosome formation. J Cell Biol. 1992;119:787–796. doi: 10.1083/jcb.119.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular-transport vesicles. J Biol Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. A fusion of new ideas. Nature. 1997;387:133–135. doi: 10.1038/387133a0. [DOI] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. SEC9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of SEC4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brunner J. Use of photocrosslinkers in cell biology. Trends Cell Biol. 1996;6:154–157. doi: 10.1016/0962-8924(96)40001-0. [DOI] [PubMed] [Google Scholar]
- Bruns D, Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Roth D, Morgan A, Burgoyne RD. Distinct effects of α-SNAP, 14–3-3-proteins and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Colombo MI, Taddese M, Whiteheart SW, Stahl PD. A possible predocking attachment site for N-ethylmaleimide-sensitive fusion protein. J Biol Chem. 1996;271:18810–18816. doi: 10.1074/jbc.271.31.18810. [DOI] [PubMed] [Google Scholar]
- Confalonieri F, Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995;17:639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop MJ. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Otto H, Eliason WK, Jahn R, Brünger AT. Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J Biol Chem. 1997;272:28036–28041. doi: 10.1074/jbc.272.44.28036. [DOI] [PubMed] [Google Scholar]
- Görlich D, Kurzchalia TV, Wiedmann M, Rapoport TA. Probing the molecular environment of translocating polypeptide chains by cross-linking. Methods Cell Biol. 1991;34:241–262. doi: 10.1016/s0091-679x(08)61684-2. [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Hartmann E, Kalies K-U, Rapoport TA. A mammalian homologue of Sec61p and SecYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992;71:489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Otto H, Barton N, Jahn R. The N-ethylmaleimide-sensitive fusion protein and α-SNAP induce a conformational change in syntaxin. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DT, Slater TM, Wilson MC, Skene JHP. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12:4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S, Andersen SS, Gorlich D, Hartmann E, Prehn S, Rapoport TA, Dobberstein B. Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J Cell Biol. 1993;121:743–750. doi: 10.1083/jcb.121.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohne-Zell B, Gratzl M. Adrenal chromaffin cells contain functionally different SNAP-25 monomers and SNAP-25/syntaxin heterodimers. FEBS Lett. 1996;394:109–116. doi: 10.1016/0014-5793(96)00931-3. [DOI] [PubMed] [Google Scholar]
- Hong R-M, Mori H, Fukui T, Moriyama Y, Futai M, Yamamoto A, Tashiro Y, Tagaya M. Association of N-ethylmaleimide-sensitive factor with synaptic vesicles. FEBS Lett. 1994;350:253–257. doi: 10.1016/0014-5793(94)00778-0. [DOI] [PubMed] [Google Scholar]
- Krieg UC, Walter P, Johnson AE. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci USA. 1986;83:8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martoglio B, Dobberstein B. Snapshots of membrane-translocating proteins. Trends Cell Biol. 1996;6:142–147. doi: 10.1016/0962-8924(96)10001-5. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Link E, Reetz A, Morris SA, Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalysed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. SEC18p (NSF)-driven release of SEC17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin and SNAP-25 forms high-affinity α-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Morgan A, Burgoyne RD. Is NSF a fusion protein? Trends Cell Biol. 1995;5:335–339. doi: 10.1016/s0962-8924(00)89059-5. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner W, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6120. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JEA, Calakos N, Ting AE, Bennett MK. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Stirling CJ, Woodman PG. Multiple N-ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994;5:773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–223. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Lareto P, DasGupta BR, Montecucco C. Tetanus and Botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Steel GJ, Tagaya M, Woodman PG. Association of the fusion protein NSF with clathrin-coated vesicle membranes. EMBO J. 1996;15:745–752. [PMC free article] [PubMed] [Google Scholar]
- Tagaya M, Wilson DW, Brunner M, Arango N, Rothman JE. Domain structure of an N-ethylmaleimide-sensitive fusion protein involved in vesicular transport. J Biol Chem. 1993;268:2662–2666. [PubMed] [Google Scholar]
- Trimble WS, Cowan DM, Scheller RH. VAMP-1: A synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci USA. 1988;85:4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Söllner TH, Rothman JE. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 1996;385:119–123. doi: 10.1016/0014-5793(96)00362-6. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, Von Mollard GF, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc Natl Acad Sci USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart SW, Brunner M, Wilson DW, Wiedmann M, Rothman JE. Soluble N-ethylmaleimide-sensitive fusion attachment proteins (SNAPs) bind to a multi-SNAP receptor complex in Golgi membranes. J Biol Chem. 1992;267:12239–12243. [PubMed] [Google Scholar]
- Whiteheart SW, Griff IC, Brunner M, Clary DO, Mayer T, Buhrow SA, Rothman JE. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Kubalek EW. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 1995;5:64–68. doi: 10.1016/s0962-8924(00)88948-5. [DOI] [PubMed] [Google Scholar]
- Whiteheart, S.W., Rossnagel, K., Buhrow, S.A., Brunner, M., Jaenicke, R., and Rothman, J.E. (1994). N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 126. [DOI] [PMC free article] [PubMed]
- Wilson DW, Rothman JE. Expression and purification of recombinant NSF from Escherichia coli. Methods Enzymol. 1992;219:309–318. doi: 10.1016/0076-6879(92)19031-z. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman PG. The roles of NSF, SNAPs and SNAREs during membrane fusion. Biochim Biophys Acta. 1997;1357:155–172. doi: 10.1016/s0167-4889(97)00039-6. [DOI] [PubMed] [Google Scholar]