Abstract
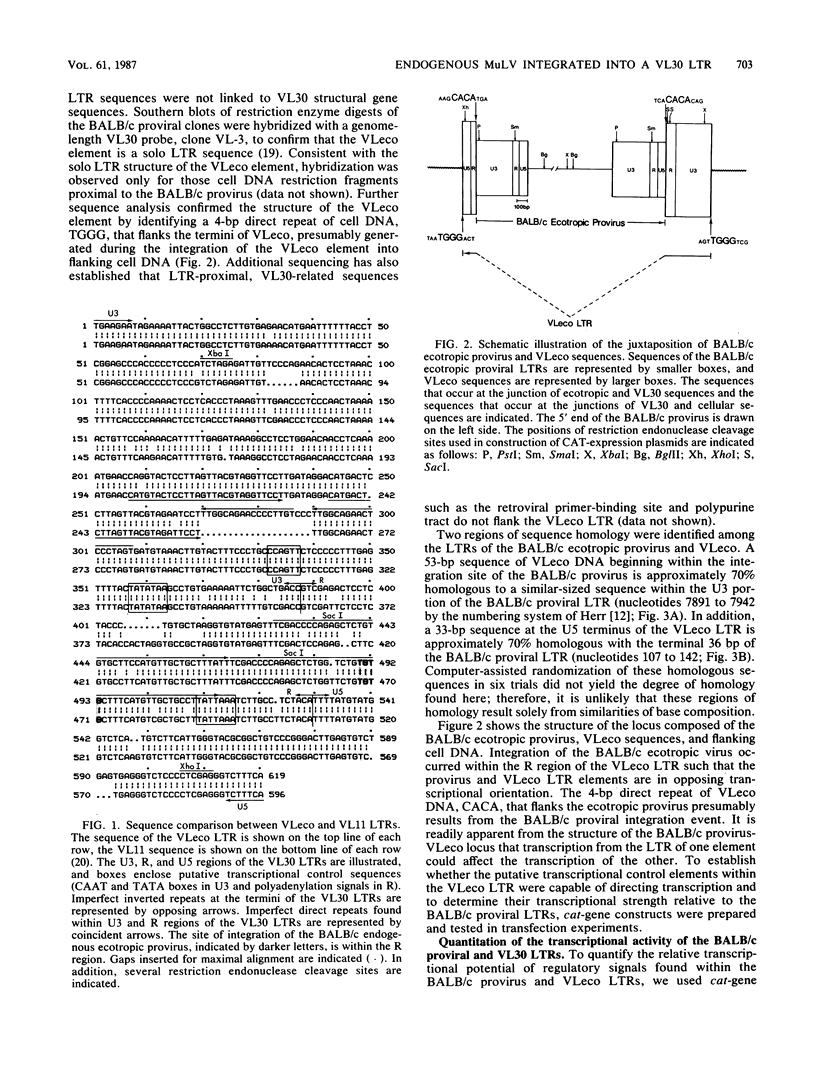
Nucleotide sequence analysis of the cellular sequences flanking the integrated ecotropic (mouse-infectious) murine leukemia provirus of BALB/c mice indicated that the murine leukemia provirus is integrated in opposing transcriptional orientation within a solo long terminal repeat (LTR) of the VL30 family of endogenous retrovirus-related sequences. To quantify the effect of this integration event on the ability of the ecotropic provirus to be expressed, we constructed recombinant molecules that carried the chloramphenicol acetyltransferase (cat) gene and various viral LTRs and determined the CAT activity induced by these constructs after transfection of NIH 3T3 cells. Our results indicate that the BALB/c ecotropic LTR is about 10-fold more active than the VL30 LTR. The presence of the VL30 LTR did not affect the transcriptional activity of the ecotropic LTR in the context of the integration event. Our results also indicate that the LTRs of the BALB/c provirus are less transcriptionally active than are the proviral LTRs of AKR murine leukemia virus and the Harvey murine sarcoma virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedigian H. G., Copeland N. G., Jenkins N. A., Salvatore K., Rodick S. Emv-13 (Akv-3): a noninducible endogenous ecotropic provirus of AKR/J mice. J Virol. 1983 May;46(2):490–497. doi: 10.1128/jvi.46.2.490-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Nath U., Jähner D., Jaenisch R. DNase I sensitivity of endogenous and exogenous proviral genome copies in M-MuLV-induced tumors of Mov-3 Mice. Virology. 1982 May;119(1):204–208. doi: 10.1016/0042-6822(82)90078-2. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Bedigian H. G., Thomas C. Y., Jenkins N. A. DNAs of two molecularly cloned endogenous ecotropic proviruses are poorly infectious in DNA transfection assays. J Virol. 1984 Feb;49(2):437–444. doi: 10.1128/jvi.49.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Skalka A. M., Ju G. Endogenous avian retroviruses contain deficient promoter and leader sequences. Proc Natl Acad Sci U S A. 1983 May;80(10):2946–2950. doi: 10.1073/pnas.80.10.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson C. P., Elder P. K., Ono T., Foster D. N., Getz M. J. Structure and expression of mouse VL30 genes. Mol Cell Biol. 1983 Dec;3(12):2221–2231. doi: 10.1128/mcb.3.12.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. W., Steffen D., Gusella J., Tabin C., Bird S., Cowing D., Weinberg R. A. DNA methylation affecting the expression of murine leukemia proviruses. J Virol. 1982 Oct;44(1):144–157. doi: 10.1128/jvi.44.1.144-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Besmer P., DeLeo A. B., Law L. W. High-frequency cotransfer of the transformed phenotype and a tumor-specific transplantation antigen by DNA from the 3-methylcholanthrene-induced Meth A sarcoma of BALB/c mice. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7555–7559. doi: 10.1073/pnas.78.12.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. A locus that enhances the induction of endogenous ecotropic murine leukemia viruses is distinct from genome-length ecotropic proviruses. J Virol. 1982 Dec;44(3):950–957. doi: 10.1128/jvi.44.3.950-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. Molecular and biological characterization of the endogenous ecotropic provirus of BALB/c mice. J Virol. 1985 Dec;56(3):798–806. doi: 10.1128/jvi.56.3.798-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Apparent recombinants between virus-liKE (VL30) and murine leukemia virus-related sequences in mouse DNA. J Virol. 1983 Jul;47(1):178–184. doi: 10.1128/jvi.47.1.178-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Horowitz J. M., Risser R. Structure and expression of endogenous ecotropic murine leukemia viruses in RF/J mice. J Exp Med. 1982 Nov 1;156(5):1461–1474. doi: 10.1084/jem.156.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Allelism and linkage studies of murine leukemia virus activation genes in low leukemic strains of mice. J Exp Med. 1982 Apr 1;155(4):1233–1238. doi: 10.1084/jem.155.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in induction of endogenous murine leukemia virus from low leukemic mice. Cell. 1982 Apr;28(4):881–888. doi: 10.1016/0092-8674(82)90067-8. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in the spontaneous production of endogenous murine leukemia virus in low leukemic mouse strains. J Exp Med. 1982 Aug 1;156(2):337–349. doi: 10.1084/jem.156.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Connor J., Avery R. J. Unusual long terminal repeat sequence of a retrovirus transmissible mouse (VL 30) genetic element: identification of functional domains. Nucleic Acids Res. 1984 Apr 25;12(8):3445–3460. doi: 10.1093/nar/12.8.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. L., Hartley J. W., Spahn G. J., Rabstein L. S., Whitmire C. E., Turner H. C., Huebner R. J. Prevalence of the group-specific (gs) antigen and infectious virus expressions of the murine C-type RNA viruses during the life span of BALB-cCr mice. Int J Cancer. 1972 Sep 15;10(2):283–289. doi: 10.1002/ijc.2910100208. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Rabstein L. S., Spahn G. J., Madison R. M., Huebner R. J. Incidence of spontaneous neoplasms in breeding and retired breeder BALB-cCr mice throughout the natural life span. Int J Cancer. 1972 Sep 15;10(2):273–282. doi: 10.1002/ijc.2910100207. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. 'Solo' large terminal repeats (LTR) of an endogenous retrovirus-like gene family (VL30) in the mouse genome. Nucleic Acids Res. 1984 Mar 12;12(5):2273–2282. doi: 10.1093/nar/12.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. No apparent nucleotide sequence specificity in cellular DNA juxtaposed to retrovirus proviruses. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7357–7361. doi: 10.1073/pnas.77.12.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]



