Abstract
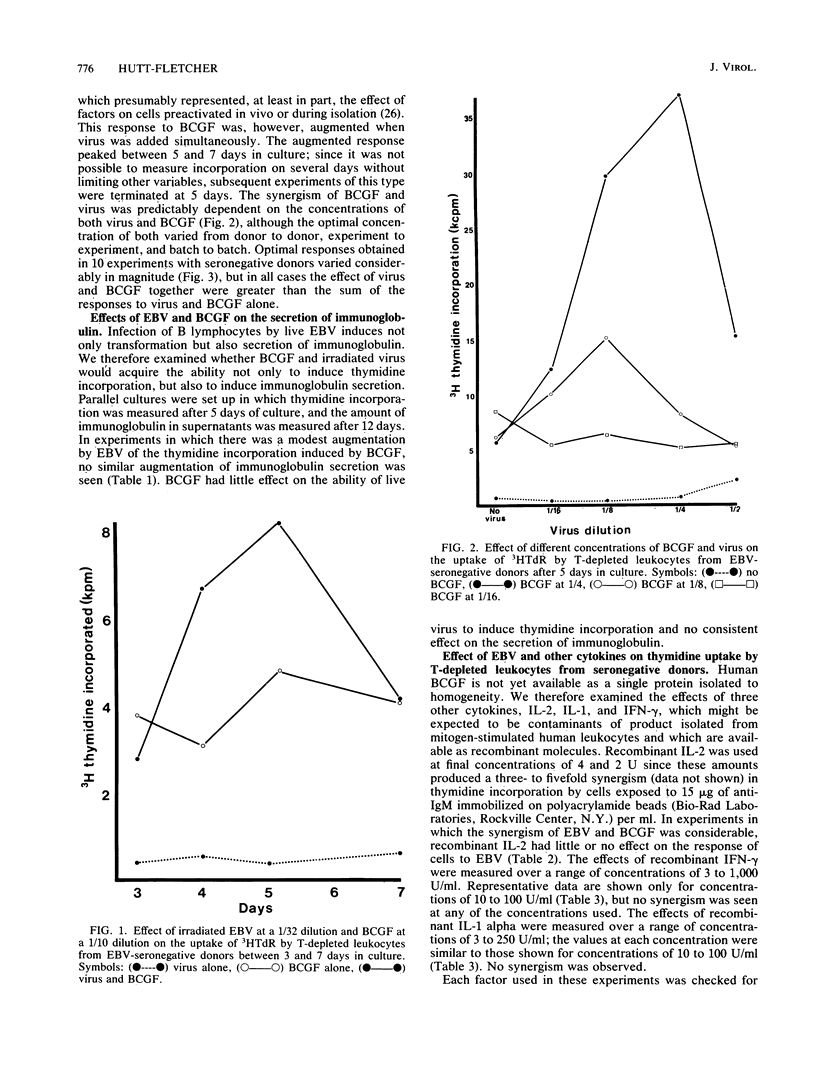
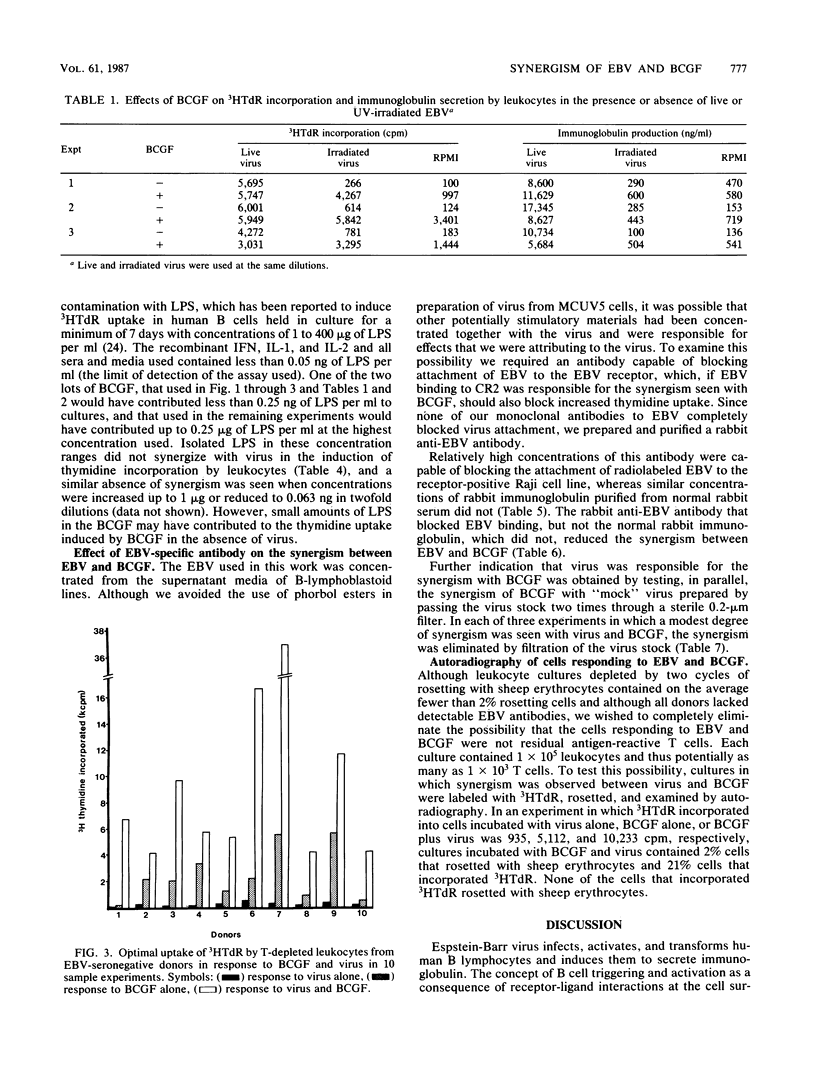
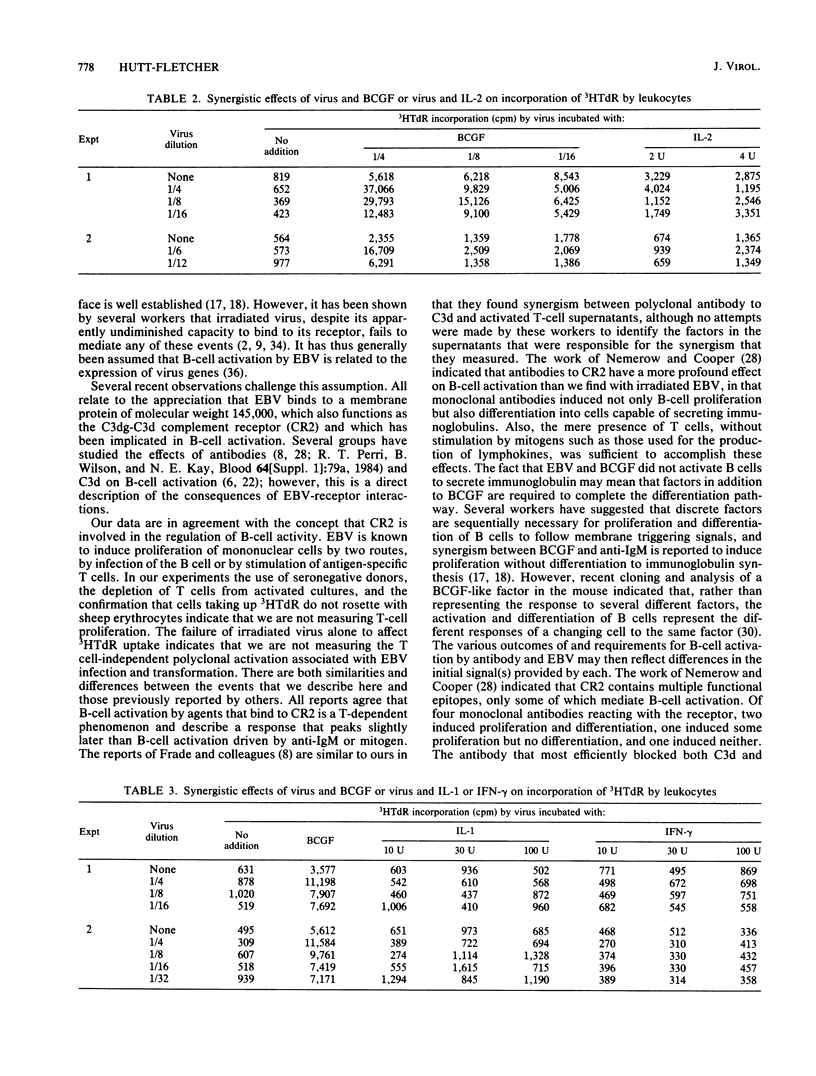
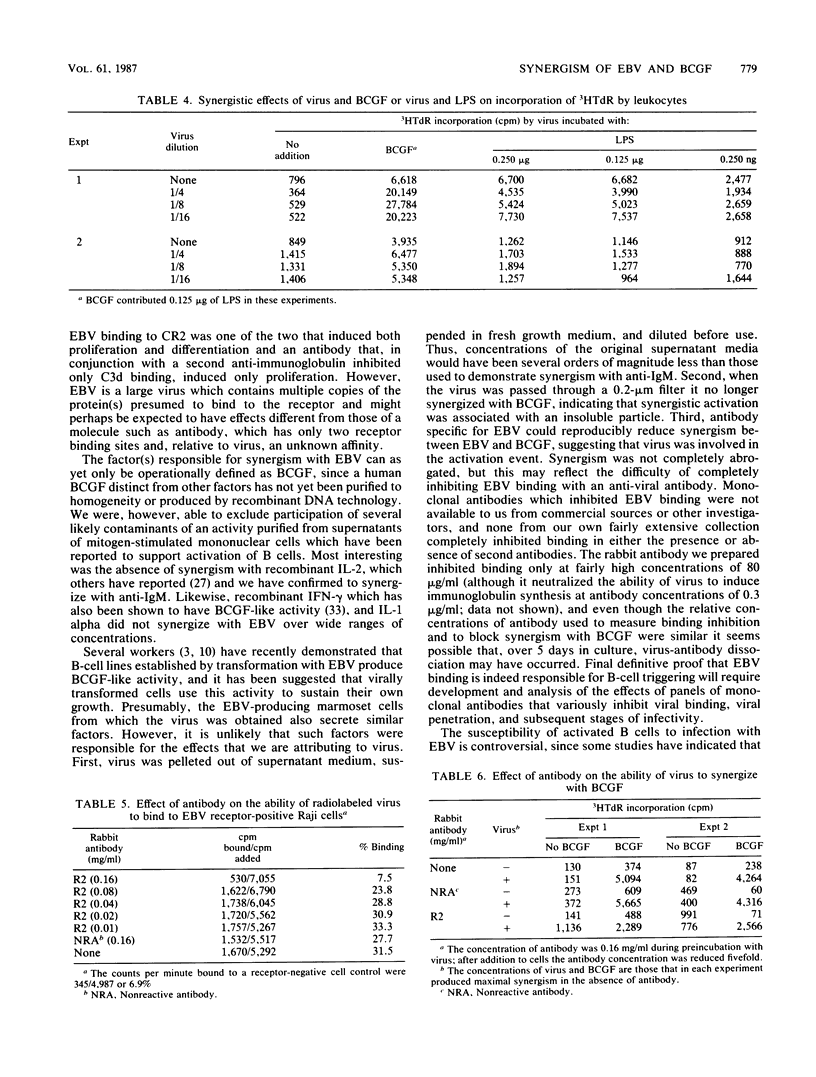
Infection with Epstein-Barr virus (EBV) is initiated by virus binding to the C3dg-C3d receptor CR2. Several workers have implicated this receptor in the control of B-cell activation by examining the effects of antibodies to CR2 and isolated C3d on B-cell proliferation and differentiation. We report here on the activating effects of irradiated EBV, which retains its capacity to bind to CR2 but loses its ability to function as a T-independent B-cell activator. EBV synergized with B-cell growth factor in the induction of uptake of tritiated thymidine by T cell-depleted leukocytes from seronegative donors but did not induce secretion of immunoglobulin. Synergism could be inhibited with an anti-viral antibody that inhibited binding of EBV to CR2. No similar synergism was found between EBV and recombinant interleukin 2, interleukin 1 alpha, or gamma interferon or with the lipid A fraction of bacterial lipopolysaccharide. EBV may thus initiate B-cell activation as it binds to CR2. Infectious virus may, under normal circumstances, induce the cell to make those growth factors necessary to support B-cell proliferation; the difficulty of transforming cells with transfected EBV DNA may in part reflect the absence of an activation event provided by intact virus as it attaches to CR2. The synergism of EBV and B-cell growth factor more clearly distinguishes the effects of B-cell growth factor from those of interleukin 1 and interleukin 2 in other models of B-cell activation. Thus, this may be a useful model for further delineation of unique effects of B-cell growth factor on B-cell function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Ehlin-Henriksson B., Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984 Jan 1;159(1):208–220. doi: 10.1084/jem.159.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A new approach to the study of human B lymphocyte function using an indirect plaque assay and a direct B cell activator. Immunol Rev. 1979;45:41–67. doi: 10.1111/j.1600-065x.1979.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Blazar B. A., Sutton L. M., Strome M. Self-stimulating growth factor production by B-cell lines derived from Burkitt's lymphomas and other lines transformed in vitro by Epstein-Barr virus. Cancer Res. 1983 Oct;43(10):4562–4568. [PubMed] [Google Scholar]
- Chan M. A., Stein L. D., Dosch H. M., Sigal N. H. Heterogeneity of EBV-transformable human B lymphocyte populations. J Immunol. 1986 Jan;136(1):106–112. [PubMed] [Google Scholar]
- Einhorn L., Klein E. EBV infection of mitogen-stimulated human B lymphocytes. Int J Cancer. 1981 Feb 15;27(2):181–183. doi: 10.1002/ijc.2910270209. [DOI] [PubMed] [Google Scholar]
- Erdei A., Melchers F., Schulz T., Dierich M. The action of human C3 in soluble or cross-linked form with resting and activated murine B lymphocytes. Eur J Immunol. 1985 Feb;15(2):184–188. doi: 10.1002/eji.1830150214. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Crevon M. C., Barel M., Vazquez A., Krikorian L., Charriaut C., Galanaud P. Enhancement of human B cell proliferation by an antibody to the C3d receptor, the gp 140 molecule. Eur J Immunol. 1985 Jan;15(1):73–76. doi: 10.1002/eji.1830150114. [DOI] [PubMed] [Google Scholar]
- Gerber P., Lucas S. J. In vitro stimulation of human lymphocytes by Epstein-Barr virus. Cell Immunol. 1972 Oct;5(2):318–324. doi: 10.1016/0008-8749(72)90057-3. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., LeBlanc P. A. Modification of Epstein-Barr virus replication by tunicamycin. J Virol. 1986 Jan;57(1):117–123. doi: 10.1128/jvi.57.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Fowler E., Lambris J. D., Feighny R. J., Simmons J. G., Ross G. D. Studies of the Epstein Barr virus receptor found on Raji cells. II. A comparison of lymphocyte binding sites for Epstein Barr virus and C3d. J Immunol. 1983 Mar;130(3):1309–1312. [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Gilbert C. J. Cytotoxic cells induced in vitro by Epstein-Barr Virus antigens. Cell Immunol. 1981 Sep 1;63(1):91–105. doi: 10.1016/0008-8749(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Butler J. L., Falkoff R. J., Fauci A. S. Human B cell activation, proliferation and differentiation. Immunol Rev. 1984 Apr;78:75–96. doi: 10.1111/j.1600-065x.1984.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Yoshizaki K., Kimoto M., Okada M., Kuritani T., Kikutani H., Shimizu K., Nakagawa T., Nakagawa N., Miki Y. B cell growth and differentiation factors and mechanism of B cell activation. Immunol Rev. 1984 Apr;78:97–118. doi: 10.1111/j.1600-065x.1984.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Miller G., Grogan E., Heston L., Robinson J., Smith D. Epstein-Barr viral DNA: infectivity for human placental cells. Science. 1981 Apr 24;212(4493):452–455. doi: 10.1126/science.6259735. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Gartner S., Kaplan H. S. Stimulation of mitogenic responses in human peripheral blood lymphocytes by lipopolysaccharide: serum and T helper cell requirements. J Immunol. 1978 Dec;121(6):2160–2164. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Hirano T., Nakagawa N., Yoshizaki K., Kishimoto T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J Immunol. 1985 Feb;134(2):959–966. [PubMed] [Google Scholar]
- Nemerow G. R., McNaughton M. E., Cooper N. R. Binding of monoclonal antibody to the Epstein Barr virus (EBV)/CR2 receptor induces activation and differentiation of human B lymphocytes. J Immunol. 1985 Nov;135(5):3068–3073. [PubMed] [Google Scholar]
- Nemerow G. R., Wolfert R., McNaughton M. E., Cooper N. R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J Virol. 1985 Aug;55(2):347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Mingari C., Maggi E., Liang C. M., Moretta L. B cell growth factor activity of interferon-gamma. Recombinant human interferon-gamma promotes proliferation of anti-mu-activated human B lymphocytes. J Immunol. 1986 May 15;136(10):3513–3516. [PubMed] [Google Scholar]
- Schooley R. T., Haynes B. F., Payling-Wright C. R., Grouse J. E., Dolin R., Fauci A. S. Mechanism of Epstein-Barr virus-induced human B-lymphocyte activation. Cell Immunol. 1980 Dec;56(2):518–525. doi: 10.1016/0008-8749(80)90126-4. [DOI] [PubMed] [Google Scholar]
- Simmons J. G., Hutt-Fletcher L. M., Fowler E., Feighny R. J. Studies of the Epstein-Barr virus receptor found on Raji cells. I. Extraction of receptor and preparation of anti-receptor antibody. J Immunol. 1983 Mar;130(3):1303–1308. [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Epstein-Barr virus infection and immunoregulation in man. Adv Immunol. 1985;37:99–149. doi: 10.1016/s0065-2776(08)60339-9. [DOI] [PubMed] [Google Scholar]
- Young L. S., Clark D., Sixbey J. W., Rickinson A. B. Epstein-Barr virus receptors on human pharyngeal epithelia. Lancet. 1986 Feb 1;1(8475):240–242. doi: 10.1016/s0140-6736(86)90776-2. [DOI] [PubMed] [Google Scholar]


