Abstract
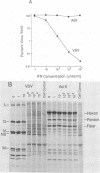
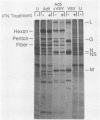
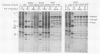
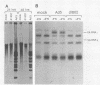
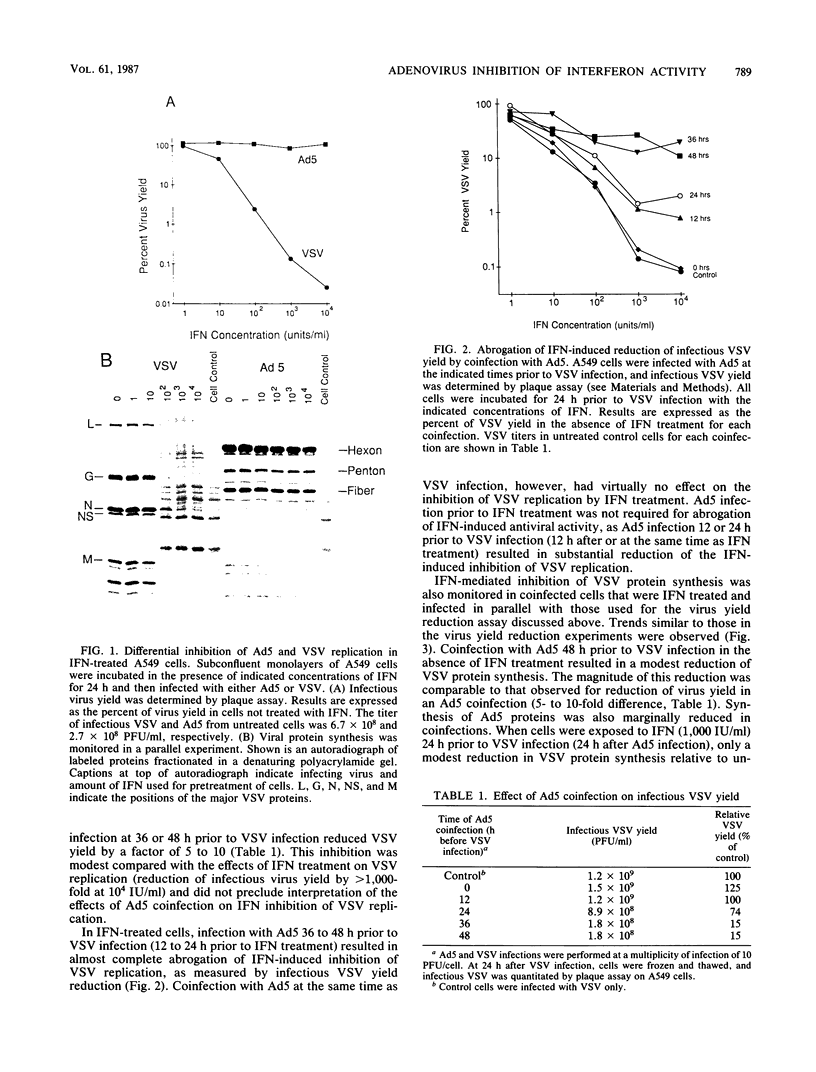
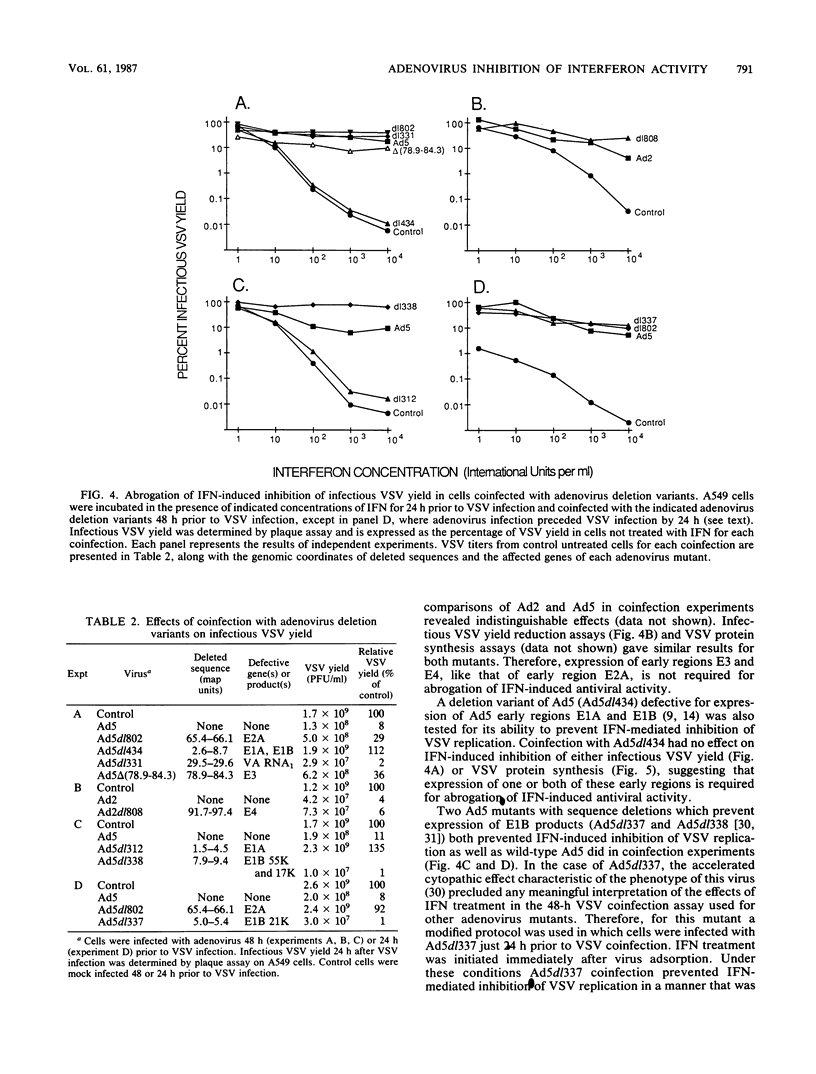
Human adenovirus type 5 (Ad5) is a DNA virus which replicates as efficiently in human A549 cells treated with human interferon-alpha 2 (IFN) as in untreated cells. Vesicular stomatitis virus (VSV), on the other hand, is a negative-strand RNA virus which is very sensitive to the effects of IFN treatment in A549 cells. The IFN-mediated inhibition of VSV replication was not observed in cells coinfected with Ad5. Abrogation of IFN-mediated antiviral activity was maximal when Ad5 infection preceded VSV infection by at least 36 h, but did not require adenovirus DNA synthesis for manifestation. Coinfection experiments with VSV and deletion variants of adenovirus demonstrated that neither virus-associated RNA synthesis nor expression of adenovirus early regions E1B, E2A, E3, or E4 are required for abrogation of IFN-mediated inhibition of VSV replication. However, expression of early region E1A was essential, suggesting that E1A products can modulate, either directly or indirectly, IFN activity in adenovirus-infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Folk W. R. Differential activation of RNA polymerase III-transcribed genes by the polyomavirus enhancer and the adenovirus E1A gene products. Nucleic Acids Res. 1985 Feb 25;13(4):1413–1428. doi: 10.1093/nar/13.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Lewis J. B., Broker T. R. RNA transcription and splicing at early and intermediate times after adenovirus-2 infection. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):401–414. doi: 10.1101/sqb.1980.044.01.044. [DOI] [PubMed] [Google Scholar]
- Czarniecki C. W., Hamilton E. B., Fennie C. W., Wolf R. L. In vitro biological activities of Escherichia coli-derived bovine interferons-alpha, -beta, and -gamma. J Interferon Res. 1986 Feb;6(1):29–37. doi: 10.1089/jir.1986.6.29. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Feldman L. T., Berk A. J. Transcription of class III genes activated by viral immediate early proteins. Science. 1985 Oct 25;230(4724):447–450. doi: 10.1126/science.2996135. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Klessig D. F. Expression of unselected adenovirus genes in human cells co-transformed with the HSV-1 tk gene and adenovirus 2 DNA. Cell. 1980 Sep;21(2):453–463. doi: 10.1016/0092-8674(80)90482-1. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Quinlan M. P., Grodzicker T. Proteins containing only half of the coding information of early region 1b of adenovirus are functional in human cells transformed with the herpes simplex virus type 1 thymidine kinase gene and adenovirus type 2 DNA. J Virol. 1982 Feb;41(2):423–434. doi: 10.1128/jvi.41.2.423-434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Symington J. S., Green M. Biosynthesis and properties of the adenovirus 2 L1-encoded 52,000- and 55,000-Mr proteins. J Virol. 1986 Mar;57(3):839–847. doi: 10.1128/jvi.57.3.839-847.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R. K., Demsey A. E., Mohanty S. B., Friedman R. M. Interferon-treated cells release vesicular stomatitis virus particles lacking glycoprotein spikes: correlation with biochemical data. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2284–2287. doi: 10.1073/pnas.77.4.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders E. K., Tilles J. G., Huang A. S. Interferon-mediated inhibition of virion-directed transcription. Virology. 1972 Aug;49(2):573–581. doi: 10.1016/0042-6822(72)90508-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Interferon action III. The rate of primary transcription of vesicular stomatitis virus is inhibited by interferon action. J Gen Virol. 1978 Mar;38(3):391–408. doi: 10.1099/0022-1317-38-3-391. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Samuel C. E. Mechanism of interferon action: inhibition of vesicular stomatitis virus replication in human amnion U cells by cloned human leukocyte interferon. I. Effect on early and late stages of the viral multiplication cycle. J Biol Chem. 1983 Oct 10;258(19):12019–12025. [PubMed] [Google Scholar]
- Masters P. S., Samuel C. E. Mechanism of interferon action: inhibition of vesicular stomatitis virus replication in human amnion U cells by cloned human leukocyte interferon. II. Effect on viral macromolecular synthesis. J Biol Chem. 1983 Oct 10;258(19):12026–12033. [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- O'Malley R. P., Mariano T. M., Siekierka J., Mathews M. B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986 Feb 14;44(3):391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Resistance of vaccinia virus to interferon is related to an interference phenomenon between the virus and the interferon system. Virology. 1984 Apr 15;134(1):12–28. doi: 10.1016/0042-6822(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pilder S., Logan J., Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984 Nov;52(2):664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986 Feb;6(2):470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Forget B. G., Weissman S. M. RNA of low molecular weight in KB cells infected with adenovirus type 2. J Mol Biol. 1966 Jun;17(2):428–439. doi: 10.1016/s0022-2836(66)80153-5. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985 Dec;56(3):767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Knutson G. S. Mechanism of interferon action: human leukocyte and immune interferons regulate the expression of different genes and induce different antiviral states in human amnion U cells. Virology. 1983 Oct 30;130(2):474–484. doi: 10.1016/0042-6822(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C. Biochemical pathways in interferon-action. Pharmacol Ther. 1984;24(2):235–257. doi: 10.1016/0163-7258(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Symington J. S., Lucher L. A., Brackmann K. H., Virtanen A., Pettersson U., Green M. Biosynthesis of adenovirus type 2 i-leader protein. J Virol. 1986 Mar;57(3):848–856. doi: 10.1128/jvi.57.3.848-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Rescue of vesicular stomatitis virus from interferon-induced resistance by superinfection with vaccinia virus. I. Rescue in cell cultures from different species. Virology. 1973 Dec;56(2):505–511. doi: 10.1016/0042-6822(73)90053-6. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Rescue of vesicular stomatitis virus from interferon-induced resistance by superinfection with vaccinia virus. II. Effect of UV-inactivated vaccinia and metabolic inhibitors. Virology. 1973 Dec;56(2):512–522. doi: 10.1016/0042-6822(73)90054-8. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R., Perry L. J., Estell D. A., Lin N., Levine H. L., Slinker B., Fields F., Ross M. J., Shively J. Properties of a human alpha-interferon purified from E. coli extracts. J Interferon Res. 1981;1(3):381–390. doi: 10.1089/jir.1981.1.381. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Characterization of a specific kinase inhibitory factor produced by vaccinia virus which inhibits the interferon-induced protein kinase. Virology. 1984 Aug;137(1):171–181. doi: 10.1016/0042-6822(84)90020-5. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Vaccinia rescue of VSV from interferon-induced resistance: reversal of translation block and inhibition of protein kinase activity. Virology. 1983 Nov;131(1):128–136. doi: 10.1016/0042-6822(83)90539-1. [DOI] [PubMed] [Google Scholar]