Abstract
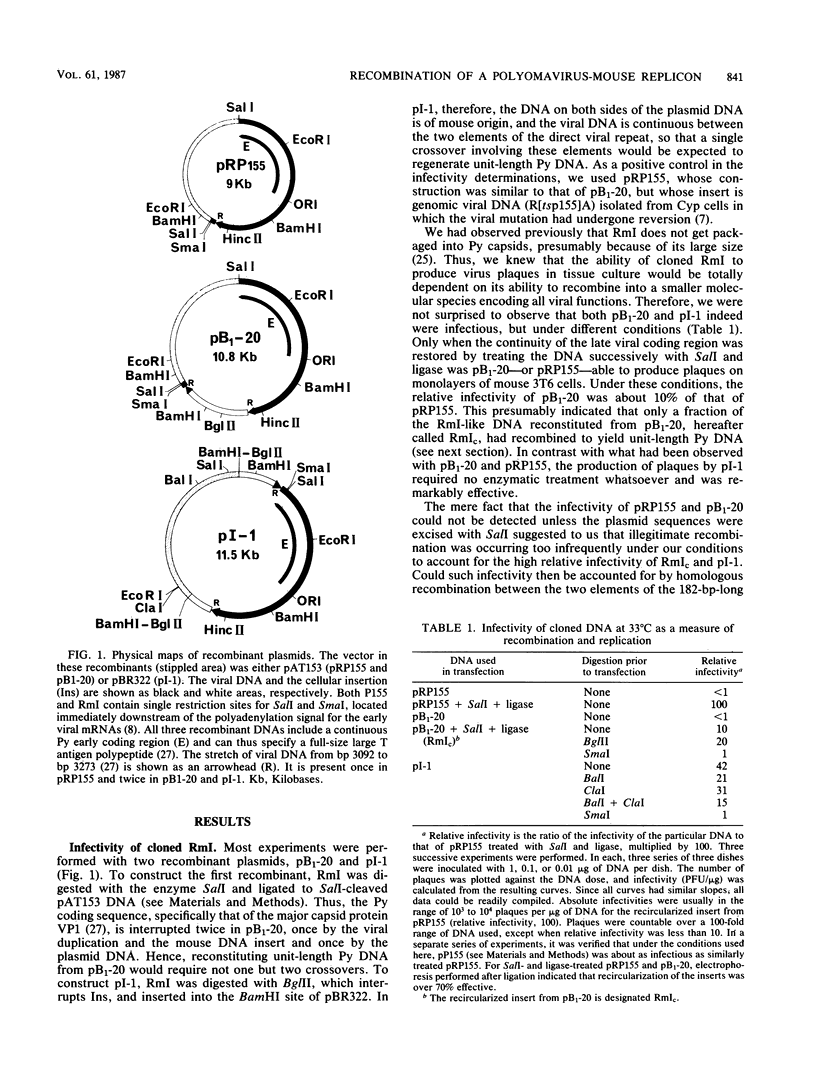
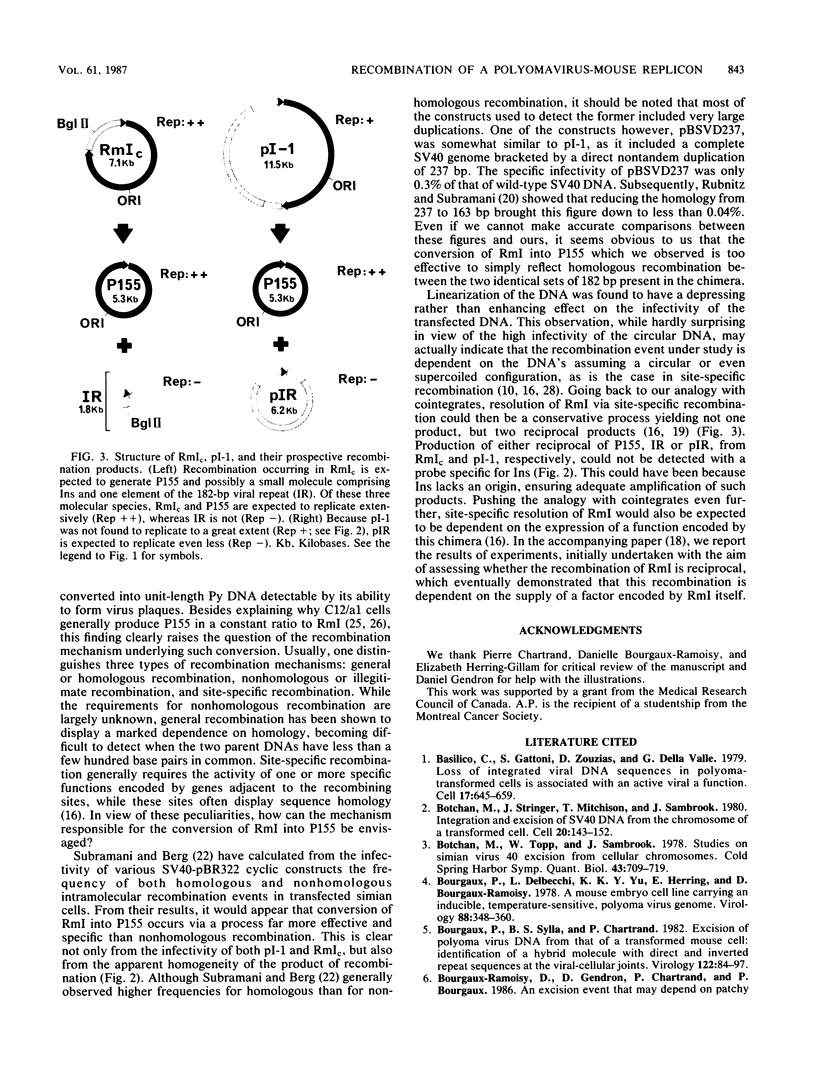
RmI is a circular chimera containing 1.03 copies of polyomavirus DNA and 1,628 base pairs of mouse DNA, joined through direct and inverted repeat sequences. It is excised from the chromosome of a transformed cell via a site-specific recombination event that is dependent on the activation of the viral gene coding for large T antigen. RmI is shown here to be highly infectious for normal mouse cells. This infectivity reflects the ability of RmI to effectively yield unit-length viral DNA via intramolecular recombination. The effectiveness with which infectious viral DNA is produced from RmI is consistent with the idea that the underlying recombination event is site specific, rather than homologous or illegitimate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Gattoni S., Zouzias D., Valle G. D. Loss of integrated viral DNA sequences in polyomatransformed cells is associated with an active viral A function. Cell. 1979 Jul;17(3):645–659. doi: 10.1016/0092-8674(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Bourgaux-Ramoisy D., Gendron D., Chartrand P., Bourgaux P. An excision event that may depend on patchy homology for site specificity. Mol Cell Biol. 1986 Jul;6(7):2727–2730. doi: 10.1128/mcb.6.7.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Delbecchi L., Yu K. K., Herring E., Bourgaux-Ramoisy D. A mouse embryo cell line carrying an inducible, temperature-sensitive, polyoma virus genome. Virology. 1978 Jul 15;88(2):348–360. doi: 10.1016/0042-6822(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Sylla B. S., Chartrand P. Excision of polyoma virus DNA from that of a transformed mouse cell: identification of a hybrid molecule with direct and inverted repeat sequences at the viral-cellular joints. Virology. 1982 Oct 15;122(1):84–97. doi: 10.1016/0042-6822(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Delbecchi L., Gendron D., Bourgaux P. Inducible permissive cells transformed by a temperature-sensitive polyoma virus: superinfection does not allow excision of the resident viral genome. J Virol. 1981 Jul;39(1):196–206. doi: 10.1128/jvi.39.1.196-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galup C., Herring-Gillam E., Sylla B. S., Bourgaux P. The temperature-sensitive defect in polyoma virus P155 mutant. Virus Res. 1984 Sep;1(6):469–475. doi: 10.1016/0168-1702(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Gattoni S., Colantuoni V., Basilico C. Relationship between integrated and nonintegrated viral DNA in rat cells transformed by polyoma virus. J Virol. 1980 Jun;34(3):615–626. doi: 10.1128/jvi.34.3.615-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D., Nash H. A. Genetic rearrangement of DNA induces knots with a unique topology: implications for the mechanism of synapsis and crossing-over. Proc Natl Acad Sci U S A. 1985 May;82(10):3124–3128. doi: 10.1073/pnas.82.10.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huberdeau D., Sylla B. S., Herring-Gillam E., Bourgaux-Ramoisy D., Bourgaux P. Alternative excision products originating from a single integration of polyomavirus DNA. Mol Cell Biol. 1985 Oct;5(10):2608–2612. doi: 10.1128/mcb.5.10.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania L., Boast S., Fried M. Excision of polyoma virus genomes from chromosomal DNA by homologous recombination. Nature. 1982 Jan 28;295(5847):349–350. doi: 10.1038/295349a0. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: viral function required for recombination. J Virol. 1987 Mar;61(3):845–850. doi: 10.1128/jvi.61.3.845-850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. R. Resolution of cointegrates between transposons gamma delta and Tn3 defines the recombination site. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3428–3432. doi: 10.1073/pnas.78.6.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Subramani S., Berg P. Homologous and nonhomologous recombination in monkey cells. Mol Cell Biol. 1983 Jun;3(6):1040–1052. doi: 10.1128/mcb.3.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla B. S., Allard D., Roy G., Bourgaux-Ramoisy D., Bourgaux P. A mouse DNA sequence that mediates integration and excision of polyoma virus DNA. Gene. 1984 Sep;29(3):343–350. doi: 10.1016/0378-1119(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Bourgaux-Ramoisy D., Bourgaux P. Induction of viral DNA synthesis in clonal derivatives of a permissive cell line transformed by a temperature-sensitive polyoma virus. Virology. 1980 Jan 30;100(2):357–369. doi: 10.1016/0042-6822(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Huberdeau D., Bourgaux-Ramoisy D., Bourgaux P. Site-specific excision of integrated polyoma DNA. Cell. 1984 Jun;37(2):661–667. doi: 10.1016/0092-8674(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Wasserman S. A., Dungan J. M., Cozzarelli N. R. Discovery of a predicted DNA knot substantiates a model for site-specific recombination. Science. 1985 Jul 12;229(4709):171–174. doi: 10.1126/science.2990045. [DOI] [PubMed] [Google Scholar]