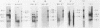Abstract
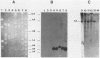
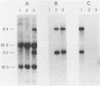
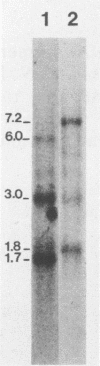
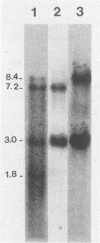
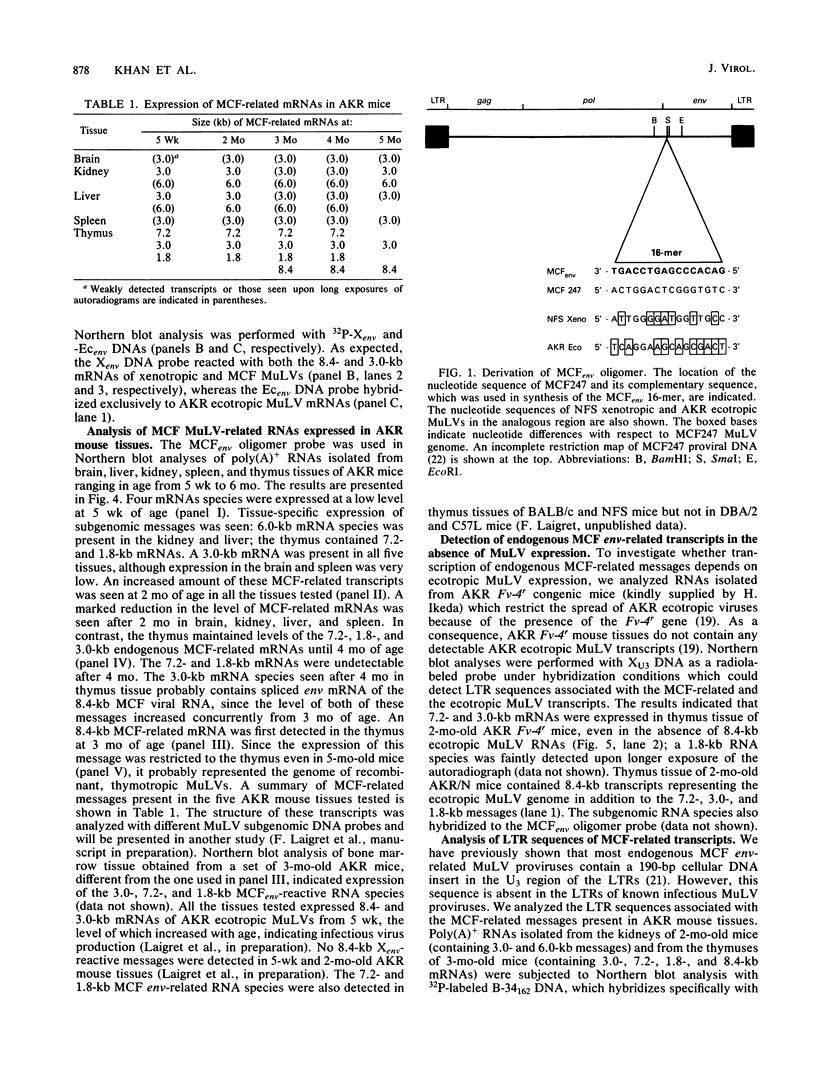
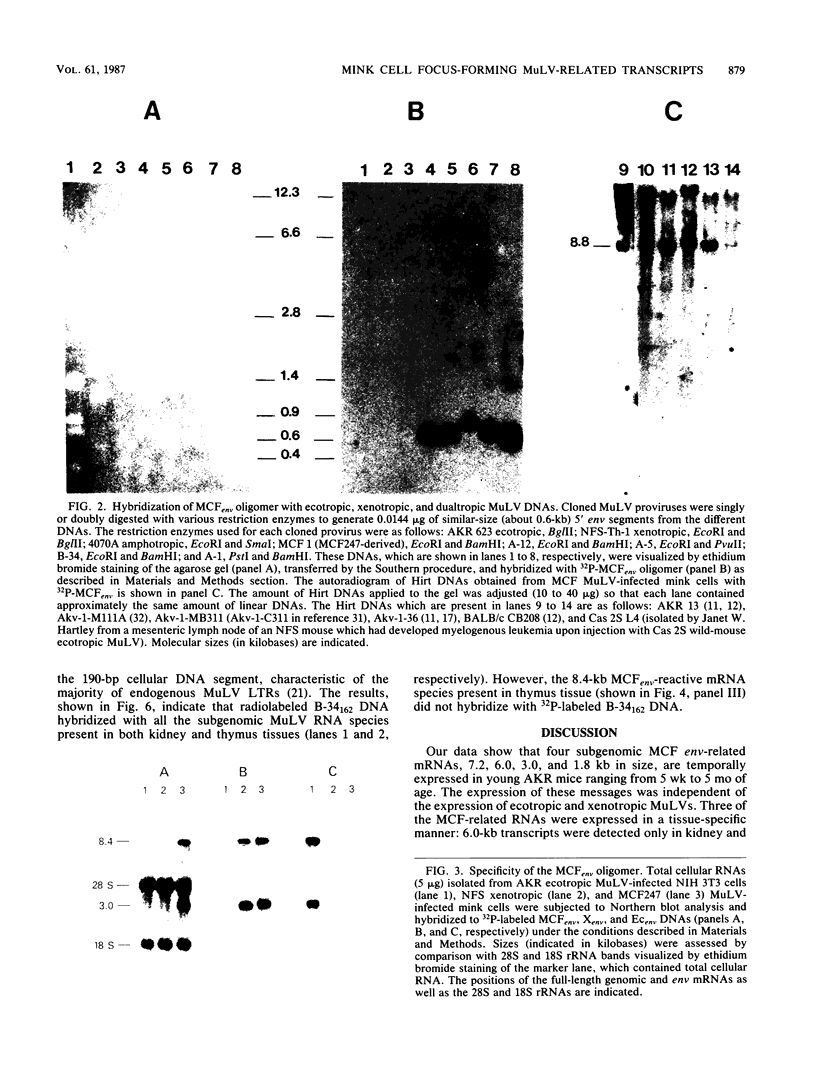
We used a synthetic 16-base-pair mink cell focus-forming (MCF) env-specific oligomer as radiolabeled probe to study MCF murine leukemia virus (MuLV)-related transcripts in brain, kidney, liver, spleen, and thymus tissues of AKR mice ranging from 5 weeks to 6 months (mo) of age. Tissue-specific expression of poly (A) + RNAs was seen: 6.0-kilobase (kb) transcripts were detected in the liver and kidney; 7.2- and 1.8-kb RNA species were present in the thymus. In addition, all the tissues tested contained 3.0-kb messages. The transcription of these MCF-related mRNAs was independent of the presence of ecotropic and xenotropic MuLVs. In general, expression of the MCF env-related transcripts appeared to peak at 2 mo of age; these messages were barely detectable in brain, kidney, liver, and spleen tissues after 2 mo and in thymus tissue after 4 mo of age. All of the subgenomic MCF env-related mRNAs (6.0, 7.2, 1.8, and 3.0 kb) appeared to contain the 190-base-pair cellular DNA insert, characteristic of the long terminal repeats associated with endogenous MCF env-related proviruses (A. S. Khan and M. A. Martin, Proc. Natl. Acad. Sci. USA 80:2699-2703, 1983). No genomic-size (8.4-kb) transcripts corresponding to endogenous MCF-related proviruses were detected. An 8.4-kb MCF env-related mRNA was first seen at 3 mo of age, exclusively in thymus tissue. This species most likely represents the first appearance of a recombinant MCF-related MuLV genome. The transcripts which were detected in thymus tissue might be involved in the generation of leukemogenic MCF viruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacheler L. T. Molecular clones of endogenous murine leukemia virus-related DNA sequences from Balb/c mice: characterization of integration sites. Virology. 1984 Oct 15;138(1):129–142. doi: 10.1016/0042-6822(84)90153-3. [DOI] [PubMed] [Google Scholar]
- Boccara M., Souyri M., Magarian C., Stavnezer E., Fleissner E. Evidence for a new form of retroviral env transcript in leukemic and normal mouse lymphoid cells. J Virol. 1983 Oct;48(1):102–109. doi: 10.1128/jvi.48.1.102-109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Hoggan M. D., Chan H. W., Sears J. F., Khan A. S., Moore J. L., Hartley J. W., Rowe W. P., Martin M. A. Cloning and characterization of an envelope-specific probe from xenotropic murine leukemia proviral DNA. J Virol. 1982 Jan;41(1):228–236. doi: 10.1128/jvi.41.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Chattopadhyay S. K. A new class of retrovirus present in many murine leukemia systems. Virology. 1986 May;151(1):31–40. doi: 10.1016/0042-6822(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Cell-surface antigens associated with recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1979 Mar 1;149(3):702–712. doi: 10.1084/jem.149.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge M. D., Green A. R., Heathcliffe G. R., Meacock P. A., Schuch W., Scanlon D. B., Atkinson T. C., Newton C. R., Markham A. F. Total synthesis of a human leukocyte interferon gene. Nature. 1981 Aug 20;292(5825):756–762. doi: 10.1038/292756a0. [DOI] [PubMed] [Google Scholar]
- Govindan M. V., Spiess E., Majors J. Purified glucocorticoid receptor-hormone complex from rat liver cytosol binds specifically to cloned mouse mammary tumor virus long terminal repeats in vitro. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5157–5161. doi: 10.1073/pnas.79.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Repaske R., Garon C. F., Chan H. W., Rowe W. P., Martin M. A. Characterization of proviruses cloned from mink cell focus-forming virus-infected cellular DNA. J Virol. 1982 Feb;41(2):435–448. doi: 10.1128/jvi.41.2.435-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of ecotropic murine leukemia virus-inducing loci in six inbred strains. J Exp Med. 1982 Feb 1;155(2):524–534. doi: 10.1084/jem.155.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of xenotropic murine leukemia virus-inducing loci in five mouse strains. J Exp Med. 1980 Jul 1;152(1):219–228. doi: 10.1084/jem.152.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. A genetic locus regulates the expression of tissue-specific mRNAs from multiple transcription units. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5823–5827. doi: 10.1073/pnas.79.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hering C., Hartley J. W., Rowe W. P., Hopkins N. Analysis of the genomes of mink cell focus-inducing murine type-C viruses: a progress report. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1269–1274. doi: 10.1101/sqb.1980.044.01.138. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E., Obata Y., Old L. J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981 Jul 30;112(2):548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Hamagishi Y., Steele P. E., Tykocinski M., Martin M. A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985 Oct;56(1):176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Young J. M., Mural R. J., Bell T. E., Ihle J. N. Molecular cloning and characterization of murine leukemia virus-related DNA sequences from C3H/HeN mouse DNA. J Virol. 1982 Jul;43(1):113–126. doi: 10.1128/jvi.43.1.113-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]