Abstract
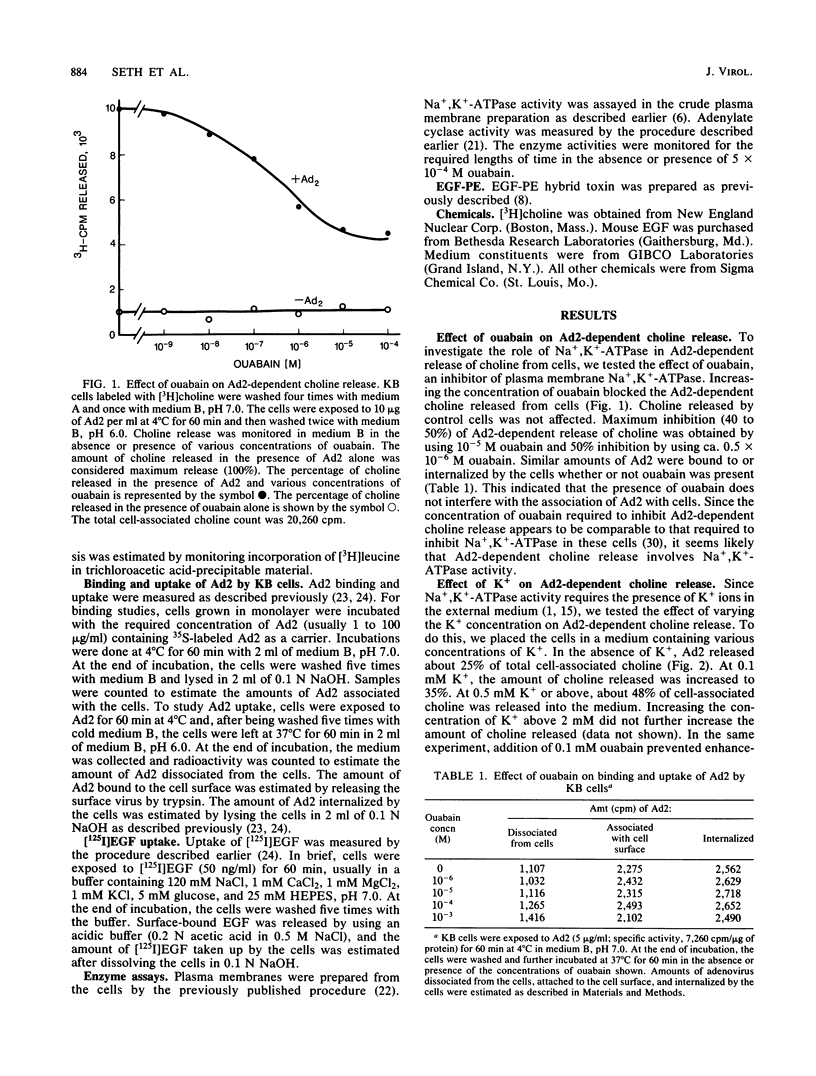
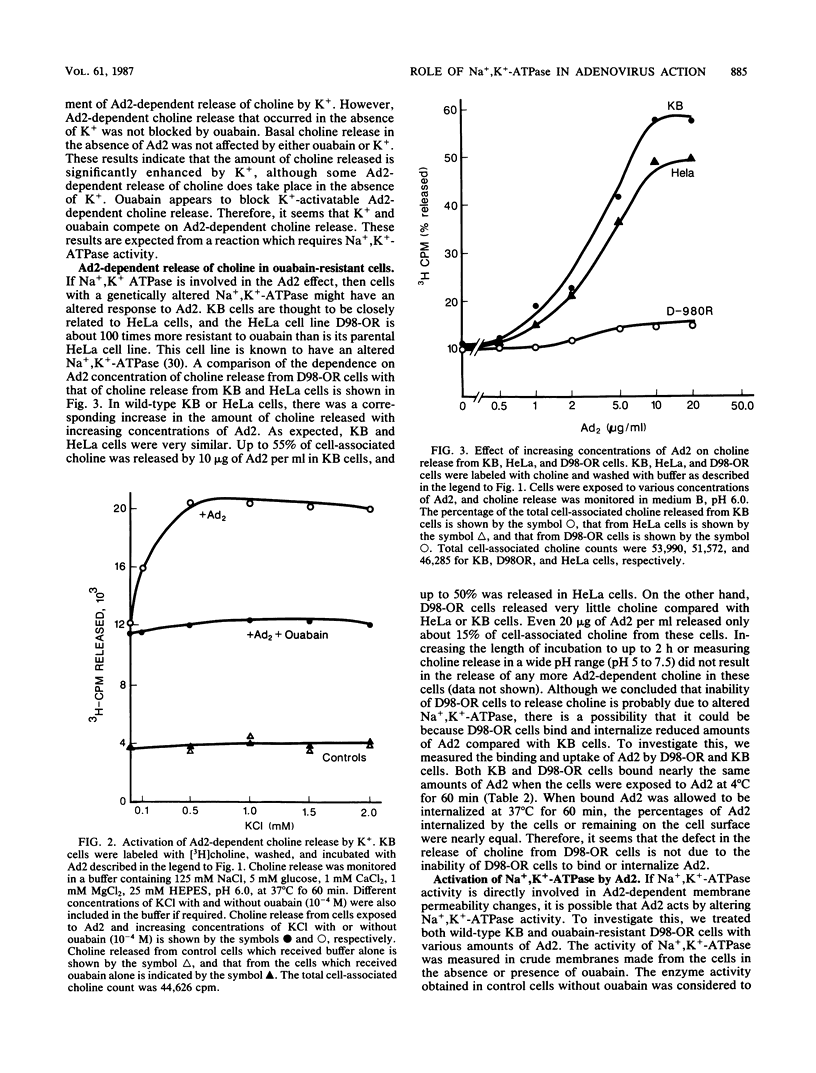
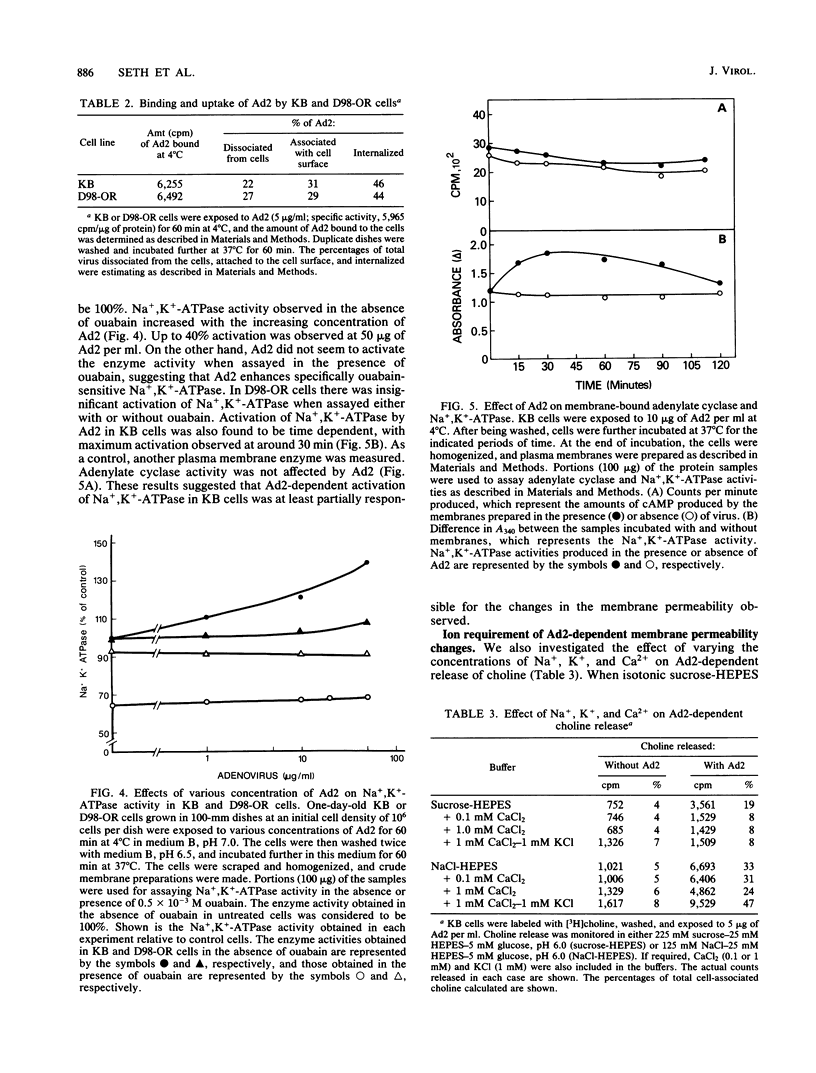
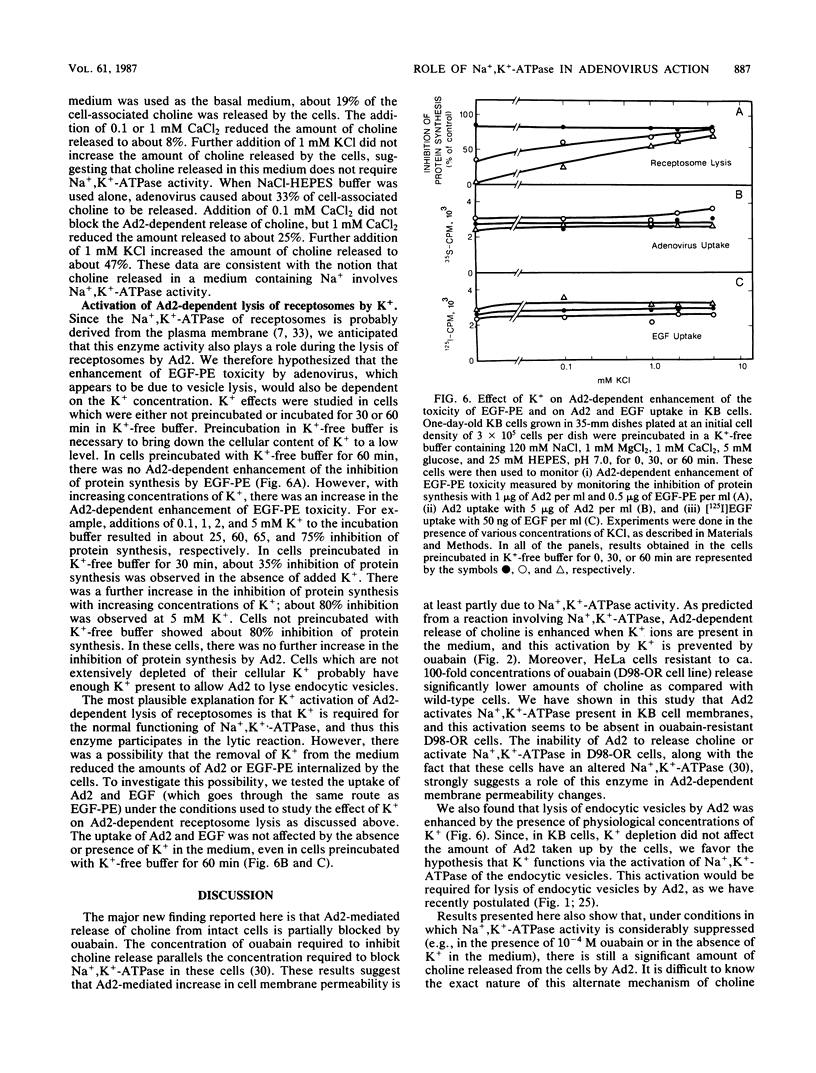
Adenovirus-dependent release of choline phosphate from KB cells at pH 6.0 was partially blocked by ouabain. In K+-containing medium, maximum inhibition of release was obtained by 10(-5) M ouabain and half-maximal inhibition was achieved by about 0.5 X 10(-6)M ouabain. Ouabain did not block either the binding or the uptake of adenovirus by KB cells. Without K+, about 25% of cell-associated choline phosphate was released by adenovirus, whereas with 1 mM K+ about 50% was released. This activation by K+ was blocked by 0.1 mM ouabain. HeLa cells behaved like KB cells, but a mutant of HeLa cells resistant to ouabain (D98-OR) released much lower amounts of choline phosphate in response to human adenovirus type 2 (Ad2). Wild-type D98-OR cells bound nearly the same amount of adenovirus as did normal HeLa cells. Ad2 also increased the activity of Na+,K+-ATPase in KB cells, with maximum activation at 50 micrograms of Ad2 per ml. In D98-OR cells, Ad2 failed to activate Na+,K+-ATPase activity. Ad2-dependent lysis of endocytic vesicles (receptosomes) was assayed by measuring Ad2-dependent enhancement of epidermal growth factor-Pseudomonas exotoxin toxicity. This action of adenovirus was increased when K+ was present in the medium. Under the conditions used, K+ had no effect on the amount of Ad2 or epidermal growth factor taken up by the cells. On the basis of these results, it is suggested that Ad2-dependent cellular efflux of choline phosphate and adenovirus-dependent lysis of receptosomes may require Na+,K+-ATPase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner B. M. The receptor function of the Na+, K+-activated adenosine triphosphatase system. Biochem J. 1985 Apr 1;227(1):1–11. doi: 10.1042/bj2270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Gray M. A., Micklem K. J., Taylor C. C., Turek P. J., Pasternak C. A. Oxonol dyes as monitors of membrane potential: the effect of viruses and toxins on the plasma membrane potential of animal cells in monolayer culture and in suspension. J Cell Physiol. 1985 Jun;123(3):326–336. doi: 10.1002/jcp.1041230306. [DOI] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Modulation of junctional permeability. Fed Proc. 1984 Sep;43(12):2692–2696. [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. H. Receptor-mediated endocytosis of alpha 2-macroglobulin: inhibition by ionophores and stimulation by Na+ and HCO3(-). Ann N Y Acad Sci. 1982;401:38–49. doi: 10.1111/j.1749-6632.1982.tb25705.x. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Phillips M. C., Levine B. A., Williams R. J. Ion-binding to phospholipids. Interaction of calcium and lanthanide ions with phosphatidylcholine (lecithin). Eur J Biochem. 1975 Oct 1;58(1):133–144. doi: 10.1111/j.1432-1033.1975.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Foster K. A., Micklem K. J., Pasternak C. A. Nature of virally mediated changes in membrane permeability to small molecules. Biochem J. 1980 Mar 15;186(3):847–860. doi: 10.1042/bj1860847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Levanon A., Kohn A., Inbar M. Increase in lipid fluidity of cellular membranes induced by adsorption of RNA and DNA virions. J Virol. 1977 May;22(2):353–360. doi: 10.1128/jvi.22.2.353-360.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Kühne J., Zibirre R., Koch G. Poliovirus-induced alterations in HeLa cell membrane functions. J Virol. 1982 Nov;44(2):445–449. doi: 10.1128/jvi.44.2.445-449.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Pastan I., Willingham M. C. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985 Aug 15;260(17):9598–9602. [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984 Dec 10;259(23):14350–14353. [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Binding of adenovirus and its external proteins to Triton X-114. Dependence on pH. J Biol Chem. 1985 Nov 25;260(27):14431–14434. [PubMed] [Google Scholar]
- Spiegelstein P. F., Haimsohn M., Gitelman J., Kohn A. Early changes in the membrane of HeLa cells adsorbing Sendai virus under conditions of fusion. J Cell Physiol. 1978 May;95(2):223–233. doi: 10.1002/jcp.1040950212. [DOI] [PubMed] [Google Scholar]
- Svensson U., Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984 Sep;51(3):687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman B., Stanbridge E. J. Characterization of ouabain resistant, hypoxanthine phosphoribosyl transferase deficient human cells and their usefulness as a general method for the production of human cell hybrids. Cytogenet Cell Genet. 1980;28(4):227–239. doi: 10.1159/000131536. [DOI] [PubMed] [Google Scholar]
- Wenner C., Hackney J. Inhibition of 86Rb+ and 22Na+ efflux of Ehrlich lettré tumor cells by Ca2+. Arch Biochem Biophys. 1976 Sep;176(1):37–42. doi: 10.1016/0003-9861(76)90138-7. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Formation of receptosomes from plasma membrane coated pits during endocytosis: analysis by serial sections with improved membrane labeling and preservation techniques. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5617–5621. doi: 10.1073/pnas.80.18.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]



