Abstract
Secretory carrier membrane proteins (SCAMPs) are ubiquitously expressed proteins of post-Golgi vesicles. In the presence of the tyrosine phosphatase inhibitor vanadate, or after overexpression in Chinese hamster ovary (CHO) cells, SCAMP1 and SCAMP3 are phosphorylated selectively on tyrosine residue(s). Phosphorylation is reversible after vanadate washout in situ or when isolated SCAMP3 is incubated with the recombinant tyrosine phosphatase PTP1B. Vanadate also causes the partial accumulation of SCAMP3, but not SCAMP1, in “patches” at or near the cell surface. A search for SCAMP kinase activities has shown that SCAMPs 1 and 3, but not SCAMP2, are tyrosine phosphorylated in EGF-stimulated murine fibroblasts overexpressing the EGF receptor (EGFR). EGF catalyzes the progressive phosphorylation of the SCAMPs up to 1 h poststimulation and may enhance colocalization of the EGFR and SCAMP3 within the cell interior. EGF also induces SCAMP–EGFR association, as detected by coimmunoprecipitation, and phosphorylation of SCAMP3 is stimulated by the EGFR in vitro. These results suggest that phosphorylation of SCAMPs, either directly or indirectly, may be functionally linked to the internalization/down-regulation of the EGFR.
INTRODUCTION
An emerging theme in the regulation of vesicular trafficking is the involvement of multiple phosphorylation and dephosphorylation events (Greengard et al., 1993; McClure and Robinson, 1996; Stanley, 1996). While phosphorylation of serine and threonine has been studied for some time, the important role of tyrosine phosphorylation in influencing protein activity, conformation, and interactions with other proteins has been realized more recently (Hu et al., 1992; Lamaze and Schmid, 1995; Ferrier-Montial et al., 1996; McClure and Robinson, 1996; Umemori et al., 1997). Indeed, while the early focus of tyrosine phosphorylation was on events related to control of cell growth and differentiation, more recent insight suggests a role in regulating molecular and vesicular trafficking (Futter et al., 1993; Sorkin and Carpenter, 1993; Lamaze and Schmid, 1995; Austin and Shields, 1996; McClure and Robinson, 1996; Okabayashi et al., 1996; van Delft et al., 1997; Wang and Moran, 1996). Many growth factor receptors possess tyrosine kinase activity (Baass et al., 1995; Seaman et al., 1996). This kinase activity is essential for signaling, internalization, and receptor down-regulation (Lamaze and Schmid, 1995; Seaman et al., 1996; van Delft et al., 1997). The human EGF receptor (EGFR), HER1, exemplifies this.
The intrinsic tyrosine kinase activity of the EGFR is activated upon EGF binding (Ushiro and Cohen, 1980). Subsequent to dimerization and activation, the receptors are internalized into the endocytic pathway, by a process thought to involve the proteins Eps15, Grb2, Shc, dynamin, and the AP-2 complex (McClure and Robinson, 1996; Okabayashi et al., 1996; Seaman et al., 1996; Wang and Moran, 1996; van Delft et al., 1997). Internalized EGFR kinase remains active, thus extending the period of signal transduction (Baass et al., 1995; Bevan et al., 1996). Within the endosomal system, EGFR enters invaginated vesicles, a process that may reflect sorting for lysosomal degradation and receptor down-regulation (Dunn et al., 1986; Futter et al., 1993). Notably, tyrosine phosphorylation of annexin I may be involved in vesicle invagination (Futter et al., 1993). However, whether other putative substrates for EGFR kinase might participate in its trafficking remains largely unresolved.
Secretory carrier membrane proteins (SCAMPs) are integral membrane proteins of apparent Mr 37–40 kDa found in membranes that function as vesicular carriers in the cell surface recycling system. While SCAMPs have been identified in vesicles that function in regulated trafficking, including synaptic vesicles, secretory granules, and the GluT4 glucose transporter compartment (Brand et al., 1991; Brand and Castle, 1993; Laurie et al., 1993), they also inhabit endosomal pathways, colocalizing with endocytosed transferrin and mannose-6-phosphate receptor (Brand and Castle, 1993; Wu and Castle, 1997). Indeed, SCAMPs have been found in recycling carriers in all cell and tissue types thus far examined, and they are highly conserved across mammalian species and phylogenetically (Singleton et al., 1997). Three mammalian SCAMPs that are products of distinct, but related, genes have been characterized to date (Singleton et al., 1997). SCAMPs 1 and 2 substantially codistribute within cells, and they appear to be part of a larger SCAMP-containing protein complex (Wu and Castle, 1997). While SCAMP3 extensively colocalizes with SCAMPs 1 and 2, it is partially concentrated in peripheral sites at or near the cell surface that are distinct from those where most of SCAMPs 1 and 2 are concentrated (Singleton et al., 1997).
In this paper, we report the discovery that selected SCAMPs are phosphorylated on tyrosine residues. Using vanadate, a tyrosine phosphatase inhibitor, we show that SCAMP1 and SCAMP3 are phosphorylated with little or no detectable phosphorylation of SCAMP2. Vanadate treatment is also correlated with a progressive and accentuated accumulation of SCAMP3, but not SCAMP1, at the cell periphery. We also show that overexpressed epitope-tagged SCAMP3 as well as endogenous SCAMP3 are tyrosine phosphorylated in the absence of vanadate treatment. Among cellular tyrosine kinases that were tested for the ability to phosphorylate SCAMPs, we find that EGF/EGFR, but not other growth factor/receptor pairs, stimulates phosphorylation of SCAMPs 1 and 3 in vivo. Phosphorylation appears to reflect the direct or indirect association of EGFR with the SCAMPs in the presence of EGF, and both the time course of phosphorylation and intracellular colocalization of SCAMP3 and EGFR suggest that phosphorylated SCAMPs may function in EGFR trafficking after internalization.
MATERIALS AND METHODS
Cell Culture
Chinese hamster ovary (CHO-K1) cells, normal rat kidney (NRK) cells, and Vero cells were grown in DMEM (Life Technologies, Grand Island, NY) containing 10% FCS, penicillin (100 U/ml), and streptomycin (100 U/ml) (normal growth media). Mouse Neo (expressing the Neo resistance gene), NeoR (expressing human EGFR), 5H (expressing c-Src), and 5HR cells (expressing c-Src and EGFR) were a kind gift from Richard Roof and Dr. Sarah J. Parsons. The generation and characterization of these cell lines from parent mouse fibroblasts, 10T1/2, are described elsewhere (Luttrell et al., 1988; Wilson et al., 1989; Maa et al., 1995). Neo, NeoR, 5H, and 5HR cells were grown in normal growth media supplemented with 0.4 mg/ml Geneticin (G418, Life Technologies, Gaithersburg, MD). 293T human embryonic kidney cells were a kind gift from Drs. Lorraine Hernandez and Judy White. 293T cells were maintained in 10% calf serum, DMEM, 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 U/ml). A431 cells were obtained from American Type Tissue Culture Collection (ATCC, Bethesda, MD) and were maintained in DMEM containing 10% FCS.
Incubations
Pervanadate solution was prepared by adding hydrogen peroxide to 10 mM sodium orthovanadate to a final concentration of 0.8% (vol/vol). This solution was added to Chinese hamster ovary (CHO) cells (80% confluent) as a 1:2000 dilution (5 μM final) for 20 min. Cells designated as vanadate-untreated were incubated in a 1:2000 dilution of 0.8% (vol/vol) hydrogen peroxide. For vanadate washout experiments, after 20 min pervanadate treatment, cells were washed twice in DMEM, and then incubated for 1 h in vanadate-free media (10% FCS in DMEM).
EGF (Sigma Chemical, St. Louis, MO.) was prepared as a 100 μg/ml stock solution in water and was used at 100 ng/ml for the specified stimulation times. Fluorescent EGF-Oregon Green (Molecular Probes Inc., Eugene, OR) was used at 500 ng/ml. NeoR cells were serum-starved overnight in 0.1% BSA in DMEM before EGF stimulation.
IgA protease was obtained from Boehringer Mannheim (Indianapolis, IN). SCAMP3 immunoprecipitates were incubated with 400 nM IgA protease in PBS for 1 h at 37°C. Control samples were incubated in PBS alone. The proteolysis was terminated by boiling all samples in Laemmli sample buffer plus 100 mM DTT.
Antibodies
mAb 7C12 was generated and characterized as previously described (Brand et al., 1991; Brand and Castle, 1993). Anti-SCAMP1 polyclonal antibodies 1α and 1ς were prepared against SCAMP1 peptides NH2-SDFDSNPFADPDLNN-NorLeu-C-COOH for 1α and NH2-KKVHGLYRTTGASFEK-COOH for 1ς. The preparation of anti-SCAMP polyclonal antibodies 1Ω and 3γ is described elsewhere (Singleton et al., 1997; Wu and Castle, 1997). Monoclonal anti-EGFR clone F4 (Sigma Chemical) was used to immunoprecipitate EGFR. Monoclonal anti-EGFR clone 13 (Transduction Laboratories, Lexington, KY) was used for immunostaining of A431 cells. Recombinant RC20-HRP anti-phosphotyrosine antibody (Transduction Laboratories) was used for Western blotting of phosphotyrosine (P-Tyr) proteins.
The 9E10 hybridoma cell line was a kind gift from Dr. Jeremy Thorner. mAb 9E10 (Evan et al., 1985) directed against the human c-myc epitope (EQKLISEEDL) was purified by ammonium sulfate precipitation, dialyzed into 20 mM potassium phosphate, 10 mM EDTA, pH 7.0, and frozen at −20°C. The anti-transferrin receptor antibody H68.4 was a kind gift from Dr. Ian Trowbridge.
Construction of SCAMP cDNAs and Expression in 293T Cells
Construction of SCAMP3-myc was performed using PCR. Oligonucleotide site-directed mutagenesis was performed using a human SCAMP3 cDNA single-stranded template. The following oligonucleotide was used: 5′-CGGGCAGTTGCAACAGATCTATGGAACAAAAGCTTATTTCTGAAGAAGACTTGGGAGGTGGAATGGCTCAGAGCAGA-3′. It includes a myc epitope tag (EQKLISEEDL [Evan et al., 1985]) upstream of the SCAMP3 coding sequence. The mutation (insertion) was verified by sequencing, and the SCAMP3-myc cDNA was subcloned into the NheI/XhoI site of pBK/CMV (Stratagene, La Jolla, CA). The construct was expressed in 293T cells by the calcium phosphate method (Wigler et al., 1977).
Immunofluorescent Labeling and Confocal Microscopy
CHO and Vero cells grown on uncoated glass coverslips were permeabilized in 0.05% saponin, fixed in 3% formaldehyde, and immunostained according to methods developed by Zerial et al. (1992) and as described elsewhere (Wu and Castle, 1997). Secondary antibody staining was achieved using fluorescein-conjugated donkey anti-mouse (Jackson Immunoresearch Laboratories, West Grove, PA) and Texas Red-conjugated goat anti-rabbit (Jackson Immunoresearch Laboratories). Cells were mounted on slides with Gel-Mount (Biomeda, Foster City, CA) and slides were examined using a Laser Scan Confocal Microscope 410 (Carl Zeiss, Thornwood, NY).
For estimating the incidence of patched immunostaining of SCAMP3, patches in CHO cells were defined as regions of immunostaining at the cell periphery at least 4 × 4 pixels in area. Cells were examined in multiple sections to ensure that all patches for a particular cell were included in the quantitation.
Immunoprecipitations and Western blotting
A 15-cm dish of CHO or NeoR cell clones were grown to 70–90% confluence, rinsed once with ice-cold PBS, and lysed in 1 ml CTEG buffer (1% CHAPS, 10 mM Tris-HCl, 1 mM EDTA, 150 mM sodium glutamate, pH 7.5) plus protease and phosphatase inhibitors (1 mM 4-(2-aminoethyl)benzenesulfonylfluoride (AEBSF), 0.1 trypsin inhibitor unit/ml aprotinin, 2 mM sodium orthovanadate) for 20 min on ice. Cells were spun (11,000 × gav, 10 min), and the supernatants were precleared for 15 min at 25°C with Pansorbin (Calbiochem, La Jolla, CA). Staphylococcus aureus cells were pelleted (11,000 × gav, 10 min), and the supernatants were incubated with either 1.0 mg Protein A Sepharose (for immunoprecipitation with anti-SCAMP3γ) or 1.0 mg Protein A-Sepharose with prebound rabbit anti-mouse Fcγ (Jackson Immunoresearch Laboratories) (for immunoprecipitation with 7C12 and H68.4) for 1 h at 25°C with primary antibody.
Where reimmunoprecipitation was performed, samples were boiled for 5 min in 1% SDS, 10 mM Tris, 1 mM EDTA, pH 7.5, and then diluted 1:20 in CTEG. After mixing for 15 min at 25°C, samples were reimmunoprecipitated with the specified antibody for 1 h at 25°C.
Samples were washed three times in ice-cold CTEG buffer and once in ice-cold PBS, and then boiled 5 min in Laemmli sample buffer plus 100 mM DTT and resolved by 10% SDS-PAGE (Laemmli, 1970). Western blotting was performed as described (Brand and Castle, 1993) using ECL for semiquantitative visualization.
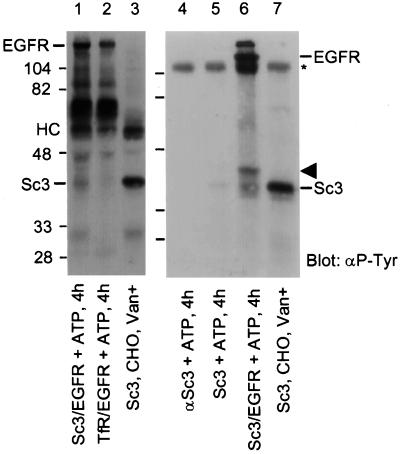
The in vitro kinase assay was performed as follows. NeoR cells (15 cm dishes, 70–90% confluent) were stimulated with 100 ng/ml EGF for 5 min, and then lysed in CTEG buffer plus protease inhibitors (see above). Untreated and vanadate-treated CHO cells (10-cm dishes, 70–90% confluent) were also lysed in CTEG buffer plus protease inhibitors. EGFR was immunoprecipitated from NeoR lysates (3.0 mg protein) with 5 μg anti-EGFR antibody (F4), while SCAMP3 was immunoprecipitated from CHO cell lysates (1.0 mg protein) with 5.0 μg anti-SCAMP3 antibody (3γ). In each case, the lysates were incubated with primary antibody for 2 h at 4°C. To each anti-EGFR–containing sample was added 1.0 mg Protein A Sepharose that had been precoated with 5 μg rabbit anti-mouse Fcγ (bridge antibody). To each anti-SCAMP3–containing sample was added 1.0 mg Protein A Sepharose. All samples were adsorbed for an additional hour at 4°C and then washed three times in CTEG buffer. SCAMP3 immunoprecipitation samples were washed for 10 min in 2 M KCl, 60 mM Tris, 2 mM EDTA, 2 mM EGTA, pH 7.5, to remove associated proteins. All samples were then washed once in PBS and twice in 20 mM Tris, 10 mM MgCl2, 10 mM MnCl2, pH 7.2 (TMM). For the kinase assay, the following controls and experimental samples were prepared: 1) a negative control of anti-SCAMP3 antibody alone incubated with ATP for 4 h; 2) a negative control of SCAMP3 alone incubated with ATP for 4 h; 3) a negative control of EGFR mixed with TFR, incubated with ATP for 4 h; 4) an experimental sample consisting of EGFR mixed with SCAMP3, incubated with ATP for 4 h; 5) and a positive control consisting of SCAMP3 immunoprecipitated from vanadate-treated (20 min, 5 μM pervanadate) CHO cells. Reactions 1–4 were performed in 1 ml TMM buffer at 30°C with continuous mixing. At the end of the incubation, reactions were terminated by boiling in Laemmli sample buffer plus 10 mM DTT (Figure 8, lanes 1–3) or by incubating in 8 M urea, 2.5% SDS, 10% glycerol, 125 mM Tris, pH 6.8, for 5 min at 25°C (Figure 8, lanes 4–7). Samples were resolved by SDS-PAGE, transferred to nitrocellulose, Western blotted for phosphotyrosine using Rc20-HRP (Transduction Laboratories), and visualized using ECL.
Figure 8.
In vitro phosphorylation of SCAMP3 by EGFR. EGFR was immunoprecipitated from NeoR cells stimulated with 100 ng/ml EGF for 5 min. SCAMP3 and transferrin receptor were immunoprecipitated from CHO cells. Lanes 1 and 6, EGFR and SCAMP3 incubated with cold ATP for 4 h; lane 2, EGFR and transferrin receptor incubated with cold ATP for 4 h. Lanes 3 and 7, SCAMP3 immunoprecipitated from vanadate-treated CHO cells; lane 4, anti-SCAMP3 alone incubated with ATP for 4 h; lane 5, SCAMP3 incubated with ATP for 4 h. HC indicates the position of the IgG heavy chain; * indicates the position of nonreduced IgG. The arrowhead indicates the position of an unknown phosphotyrosine-containing protein. Note that the samples in lanes 1–3 were reduced with DTT before electrophoresis, whereas those in lanes 4–7 were not. Many of the nonspecific bands observed in the vicinity of the IgG heavy chain (HC, lanes 1–3) redistribute to higher apparent Mr (lanes 4–7). All samples were Western blotted for phosphotyrosine and visualized using ECL.
RESULTS
SCAMP1 and 3 Are Tyrosine Phosphorylated in Vanadate-treated CHO Cells
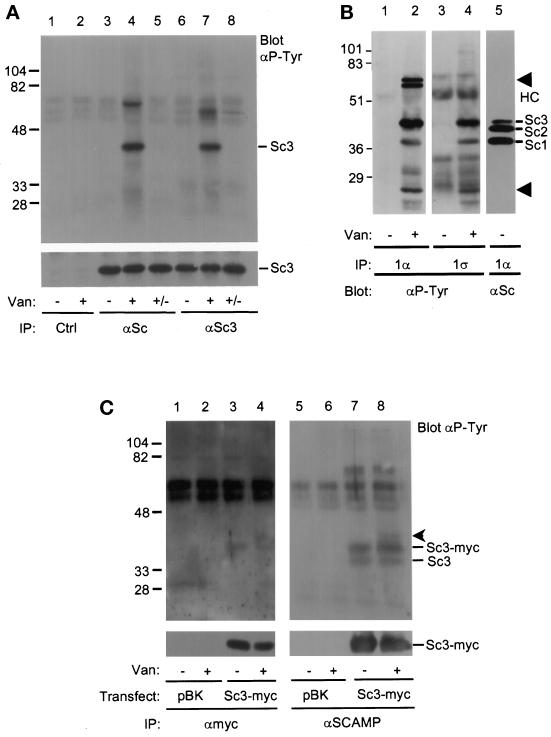
CHO cells were treated with vanadate, a phosphotyrosine phosphatase inhibitor, and immunoprecipitated with 3γ, a polyclonal antibody specific for SCAMP3, or antiSCAMP (7C12), a mAb that recognizes SCAMPs 1, 2, and 3. The immunoprecipitates were Western blotted for phosphotyrosine and for SCAMPs (Figure 1A). The phosphotyrosine blot indicated the appearance of a prominent ∼40-kDa band, consistent with the molecular weight of SCAMP3 (lanes 4 and 7), in vanadate-treated samples immunoprecipitated with both anti-SCAMP3 and anti-SCAMP antibodies. In contrast, the ∼40-kDa band was not seen in vanadate-untreated cells (lanes 3 and 6) or in cell lysates immunoprecipitated with a control antibody (lanes 1 and 2). The ∼40-kDa band disappeared after vanadate washout and a 1-h incubation in vanadate-free tissue culture media (lanes 5 and 8), indicating that the vanadate effect was fully reversible. Blotting for SCAMP3 served to normalize the samples (Figure 1A, lower panel). We also observed the variable appearance of three unknown phosphotyrosine-containing bands: a faint ∼37-kDa band in lane 4, a prominent ∼65-kDa band in lane 4, and a prominent ∼60-kDa band in lane 7. The ∼65-kDa band does not Western blot with anti-Shc, and the ∼60-kDa band does not blot with anti-c-Src (our unpublished observations).
Figure 1.
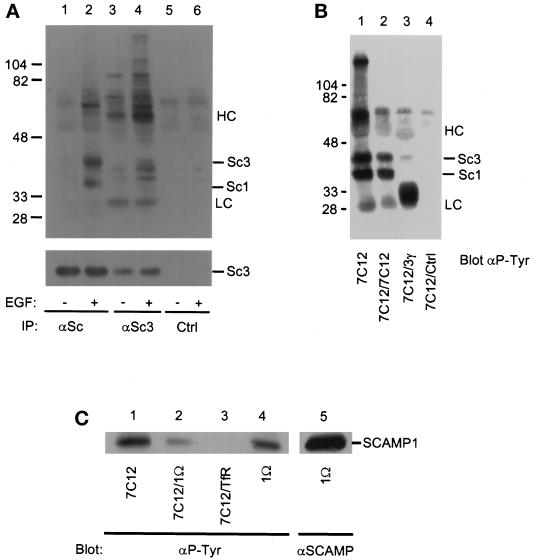
SCAMP3 is tyrosine phosphorylated in vanadate-treated CHO cells. (A) CHO cells were either untreated (lanes 1, 3, and 6) or treated (lanes 2, 4, and 7) with 5 μM pervanadate for 20 min and immunoprecipitated with anti-SCAMP (7C12, lanes 3–5), anti-SCAMP3 (3γ, lanes 6–8), or a control antibody (9E10, lanes 1 and 2). Two samples (lanes 5 and 8, +/−) were washed in vanadate-free media and further incubated for 1 h. Immunoprecipitates were Western blotted with anti-phosphotyrosine (αP-Tyr, upper panel) or anti-SCAMP3 (3γ, lower panel) and visualized using ECL. The corresponding 7C12 blot demonstrated that bands with the mobility of SCAMP1 and SCAMP3, but not SCAMP2, were phosphorylated on tyrosine (our unpublished observations). (B) Untreated (lanes 1, 3, and 5) or vanadate-treated (lanes 2 and 4) CHO cells were lysed in CHAPS buffer and immunoprecipitated with anti-SCAMP1 antibodies 1α (lanes 1, 2, and 5) or 1ς (lanes 3 and 4). Samples were resolved by SDS-PAGE, transferred to nitrocellulose, and Western blotted with anti-phosphotyrosine antibody (αP-Tyr, lanes 1–4) or with anti-SCAMP (7C12, lane 5). The positions of SCAMPs 1, 2, and 3 are indicated (Sc1, Sc2, Sc3), as well as the position of several unidentified phosphotyrosine proteins (lanes 2 and 4, arrowheads) and the IgG heavy chain (HC). (C) Tyrosine phosphorylation of endogenous SCAMP3 and transiently overexpressed SCAMP3-myc. 293T cells were transfected with expression vector pBK alone (lanes 1 and 2) or with human SCAMP3-myc/pBK (lanes 3 and 4), lysed in CHAPS buffer 48 h post-transfection, and immunoprecipitated with anti-myc antibody (9E10, lanes 1–4). The supernatants from samples 1–4 were then immunoprecipitated with anti-SCAMP antibody (7C12, lanes 5–8). Immunoprecipitates were Western blotted with anti-phosphotyrosine antibody (upper panels) or anti-myc (lower panel) and visualized by ECL. An unknown protein of ∼40 kDa coimmunoprecipitates with SCAMP3-myc (lanes 4 and 8, arrowhead).
The faint ∼37-kDa band migrated at a gel position consistent with that of SCAMP1. Thus, we immunoprecipitated SCAMP1 from vanadate-treated CHO cells using two anti-SCAMP1 antibodies, 1α and 1ς, each with an epitope distinct from the other and from that of anti-SCAMP antibody, 7C12. Both 1α and 1ς specifically immunoprecipitated the ∼37-kDa phosphotyrosine band (lanes 2 and 4) from vanadate-treated CHO cells, but not from untreated cells (lanes 1 and 3). We have also immunoprecipitated the ∼37-kDa protein using yet another anti-SCAMP1 antibody with a unique epitope, 1Ω, but have been unable to immunoprecipitate this protein with two different negative control antibodies (our unpublished observations). Taken together, these results indicate that SCAMP1 is tyrosine phosphorylated in vanadate-treated CHO cells.
To confirm the presence of phosphotyrosine in the ∼40-kDa protein, an immunoprecipitate from vanadate-treated CHO cells was incubated with the recombinant tyrosine phosphatase, GST-PTP1B fusion protein. The 40-kDa protein was completely dephosphorylated and no longer detectable by anti-phosphotyrosine (our unpublished observations).
To establish that the ∼40-kDa tyrosine-phosphorylated polypeptide was indeed SCAMP3 and not simply a coimmunoprecipitating protein of the same electrophoretic mobility, we engineered a myc epitope tag onto the N terminus of SCAMP3 using the human SCAMP3 cDNA, transiently expressed the construct in 293T cells, and conducted immunoprecipitations with or without prior vanadate treatment. Since human SCAMP3 migrates as a 38-kDa polypeptide (Singleton et al., 1997), we expected that the endogenous SCAMP3 in 293T cells (a human cell line) would migrate at 38 kDa while SCAMP3-myc would migrate slightly slower. Also because immunoprecipitation with anti-myc antibody is inefficient (Wu and Castle, 1997), we conducted successive immunoprecipitations with anti-myc and anti-SCAMP. As shown in Figure 1C, both antibodies immunoprecipitate a ∼39-kDa tyrosine-phosphorylated polypeptide (SCAMP3-myc) while anti-SCAMP also immunoprecipitates an additional 38-kDa polypeptide (endogenous SCAMP3 as determined by Western blotting with 3γ [our unpublished observations]). Notably, overexpression of SCAMP3-myc causes tyrosine phosphorylation of both endogenous and epitope-tagged SCAMP3 even in the absence of vanadate treatment. This observation suggests that phosphorylation of SCAMP3 is regulated by enzyme:substrate ratio, and, along with the vanadate washout shown in Figure 1A, it argues that phosphorylation is transient. We also note the coimmunoprecipitation of a minor unknown phosphotyrosine-containing protein of ∼40 kDa that cannot be blotted by any of our SCAMP antibodies (our unpublished observations).
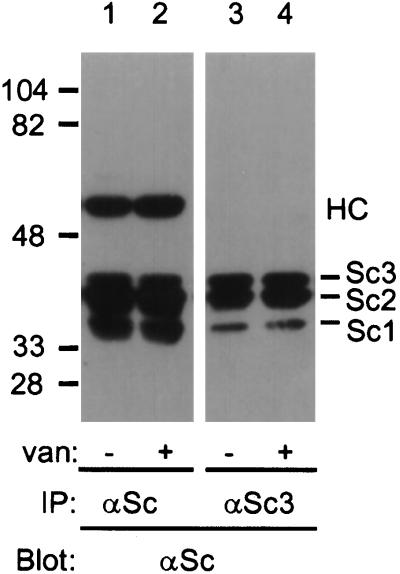
We have previously presented evidence for the existence of a protein complex involving SCAMP1 and SCAMP2 (Wu and Castle, 1997). Apparently, some portion of the same complex includes SCAMP3, since it can be coimmunoprecipitated with the SCAMP1-specific polyclonal antibody 1Ω (Wu and Castle, 1997). To determine whether the SCAMP complex could be immunoprecipitated with the SCAMP3-specific antibody, 3γ, we prepared lysates from mock or vanadate-treated CHO cells, immunoprecipitated with 3γ, and blotted with 7C12. As shown in Figure 2, SCAMPs 1, 2, and 3 were coimmunoprecipitated with 3γ in both mock and vanadate-treated cells (lanes 3 and 4). Note that only a fraction of the total SCAMP1 and SCAMP2 coimmunoprecipitated with SCAMP3 (compare lanes 1 and 3 and 2 and 4), suggesting that some SCAMP1 and SCAMP2 may be present in monomeric form, or that these SCAMPs may be part of other protein complexes. Vanadate treatment did not increase the amount of SCAMP1 or SCAMP2 coimmunoprecipitating with SCAMP3 (compare lanes 3 and 4).
Figure 2.
Vanadate does not alter coimmunoprecipitation of SCAMPs 1, 2, and 3. Vanadate-untreated (lanes 1 and 3) or vanadate-treated (lanes 2 and 4) CHO cells were lysed in CHAPS buffer and immunoprecipitated with anti-SCAMP (7C12, lanes 1 and 2) or anti-SCAMP3 (3γ, lanes 3 and 4), and then Western blotted with anti-SCAMP and visualized by ECL. HC indicates the position of the IgG heavy chain.
Distribution of SCAMP3 in Vanadate-treated CHO cells
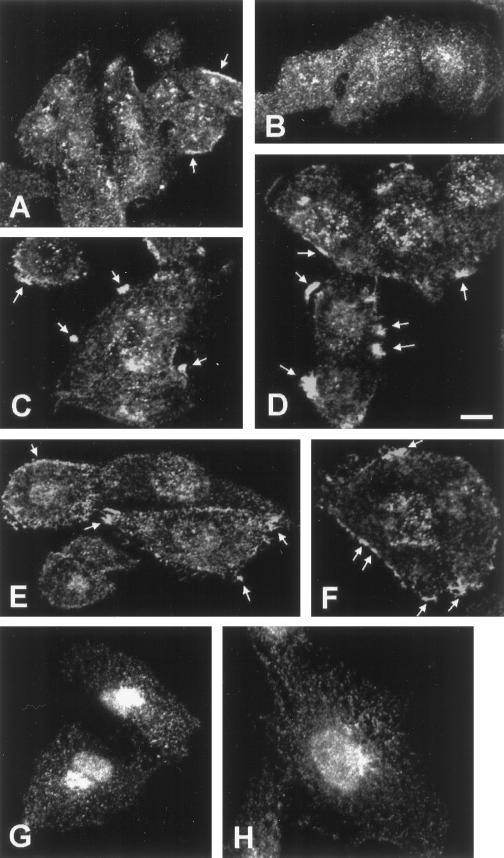
Previously we have found that SCAMPs 1 and 2 codistribute extensively (Wu and Castle, 1997) but that SCAMP3 distribution, while similar, shows some degree of concentration at distinct sites (Singleton et al., 1997; Wu and Castle, 1997). We were curious whether vanadate treatment visibly affected SCAMP1 or SCAMP3 distribution, possibly reflecting altered trafficking of tyrosine-phosphorylated forms. Therefore, we examined this possibility by indirect immunofluorescence and confocal microscopy. As shown in Figure 3, A and B, SCAMP3 normally has a punctate cytoplasmic and perinuclear distribution reminiscent of previous SCAMP immunostaining, but in many cells also shows “patches” of peripheral antigen staining that are concentrated in the vicinity of the plasma membrane (Brand and Castle, 1993; Singleton et al., 1997; Wu and Castle, 1997). The incidence of such patches increased with vanadate treatment, although the patches were not observed in all cells, regardless of the optical section examined. In 32% of vanadate-untreated cells (n = 221), we observed SCAMP3 patches (Figure 3, arrowheads, panel A). After 5 min of vanadate treatment, 65% of CHO cells (n = 208) displayed these patches (panels C and D, arrowheads). Compared with untreated cells, the patches appeared rounder and less elongate, and most cells displayed more than one patch. After 20 min of vanadate treatment, 65% of CHO cells (n = 228) still displayed patches (panels E and F, arrowheads), but many of the patches appeared elongated and less continuous compared with those at 5 min of vanadate treatment. A similar time course of SCAMP1 immunostaining revealed no change in SCAMP1 distribution with vanadate treatment (Figure 3, compare panels G and H). SCAMP1 staining was restricted to post-Golgi vesicular compartments, consistent with earlier findings (Singleton et al., 1997; Wu and Castle, 1997), and there was no appreciable concentration of this SCAMP isoform in the vicinity of the plasma membrane.
Figure 3.
Distribution of SCAMP1 and SCAMP3 in vanadate-treated CHO cells. CHO cells were vanadate treated for 0 min (A, B, and G), 5 min (C and D), or 20 min (E, F, and H) and immunolabeled with anti-SCAMP3 (3γ, A–F) or anti-SCAMP1 (1Ω, G and H) for visualization by confocal microscopy. The presence of peripheral patches of SCAMP3 immunostaining is indicated by the arrows. Bar, 10 μm.
SCAMP1 and SCAMP3 Are Tyrosine Phosphorylated in EGF-stimulated NeoR Cells
While SCAMPs 1 and 3 are detectably tyrosine phosphorylated when treated pharmacologically with vanadate to inhibit tyrosine phosphatase activity, we were curious whether we could identify prospective kinases that might catalyze their phosphorylation. Thus, we checked for phosphorylation of SCAMPs 1 and 3 by cellular tyrosine kinases using a variety of cell lines and agonists.
We first examined 10T1/2 murine fibroblasts overexpressing EGFR (NeoR) that had been stimulated with 100 ng/ml EGF. Upon immunoprecipitation with anti-SCAMP antibodies and Western blotting for phosphotyrosine, 37- and 40-kDa bands consistent with the molecular weights of SCAMPs 1 and 3 were revealed when lysates from EGF-stimulated cells were immunoprecipitated with anti-SCAMP (7C12, Figure 4, lane 2). The 40-kDa band was also observed in anti-SCAMP3 (3γ) immunoprecipitates from EGF-stimulated cells (lane 4), as were two other slightly lower Mr bands that do not appear to be related to SCAMPs. Neither of the prospective SCAMPs was observed in control immunoprecipitates (lanes 5 and 6).
Figure 4.
(A) SCAMP3 is tyrosine phosphorylated in EGF-stimulated NeoR cells. Serum-starved NeoR cells were either unstimulated (lanes 1, 3, and 5) or stimulated (lanes 2, 4, and 6) with 100 ng/ml EGF for 30 min, lysed in CHAPS buffer, and immunoprecipitated with either anti-SCAMP (7C12, lanes 1 and 2), anti-SCAMP3 (3γ, lanes 3 and 4), or a control antibody (9E10, lanes 5 and 6). The immunoprecipitates were blotted for phosphotyrosine (αP-Tyr, upper panel) or for SCAMP3 (3γ, lower panel). (B) Reimmunoprecipitation of SCAMP3. NeoR cells were EGF stimulated for 30 min, lysed in CHAPS buffer, and immunoprecipitated with anti-SCAMP (7C12). Samples 2, 3, and 4 were boiled in 1% SDS and reconstituted in CHAPS buffer, and then reimmunoprecipitated with anti-SCAMP (7C12, lane 2), anti-SCAMP3 (3γ, lane 3), or a control antibody (9E10, lane 4). Samples were Western blotted for phosphotyrosine and visualized using ECL. (C) Reimmunoprecipitation of SCAMP1. NeoR cells were stimulated with EGF for 1 h, lysed in CHAPS buffer, and immunoprecipitated with anti-SCAMP (7C12, lanes 1–3) or anti-SCAMP1 (1Ω, lanes 4 and 5) in the first round. Samples 2 and 3 were boiled in SDS and reconstituted in buffer containing 1% CHAPS, and then subjected to a second round of immunoprecipitation with either anti-SCAMP1 (1Ω, lane 2) or a control antibody (anti-transferrin receptor, lane 3). Samples were resolved by SDS-PAGE, transferred to nitrocellulose, Western blotted with either anti-phosphotyrosine (lanes 1–4) or anti-SCAMP (lane 5), and visualized by ECL.
We have also used 7C12 to immunoprecipitate a ∼40-kDa tyrosine- phosphorylated protein from NRK cells overexpressing EGFR after EGF stimulation (our unpublished observations), suggesting that this effect is not cell type specific. However, we could not detect this band in SCAMP3 immunoprecipitates from EGF-stimulated mock-transfected 10T1/2 cells (Neo), suggesting a requirement for overexpressed EGFR.
We sought to confirm the identities of the 37- and 40-kDa phosphorylated polypeptides as SCAMPs by reimmunoprecipitation. EGF-stimulated NeoR cells were immunoprecipitated with 7C12; after boiling in SDS to disrupt protein-protein interactions, the samples were diluted in immunoprecipitation buffer containing 1% CHAPS and then reimmunoprecipitated with a control antibody or with anti-SCAMP (7C12), anti-SCAMP3 (3γ), or anti-SCAMP1 (1Ω). Anti-phosphotyrosine blotting (Figure 4B) showed that the 40,000-Da band was immunoprecipitated with anti-SCAMP (lane 2) and anti-SCAMP3 (lane 3), but not with the control antibody (lane 4), confirming its identity as SCAMP3. Similarly, the 37-kDa phosphotyrosine protein that was reimmunoprecipitated by anti-SCAMP (lane 2) strongly resembled SCAMP1. As shown in Figure 4C, the identity of SCAMP1 was confirmed by specific reimmunoprecipitation with anti-SCAMP1 (1Ω, lane 2).
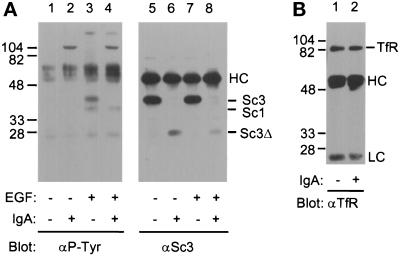
We used IgA protease digestion to gain insight regarding which tyrosine residues in SCAMPs 1 and 3 might be phosphorylated. IgA protease from Neisseria gonorrhea has been shown to preferentially cleave the motif PPXP, where X is A, S, or T, and it has been previously demonstrated to cleave one of the SCAMPs (SCAMPs 2 and 3 were not distinguished but SCAMP1 was not cleaved) from rat adipocytes (Cheatham et al., 1996). Comparison of SCAMP sequences (Singleton et al., 1997) shows that SCAMP3 alone has an appropriate PPXP site (PPAP, residues 55–58). This site lies upstream of the epitope for the anti-SCAMP3 antibody (residues 76–89). SCAMP3 from vanadate-untreated and vanadate-treated CHO cells is completely cleaved by IgA protease (our unpublished observations). SCAMP3 from unstimulated (Figure 5A, lane 6) and EGF-stimulated (lane 8) NeoR cells was cleaved to a polypeptide of ∼28 kDa mobility (Sc3Δ) on SDS-PAGE, consistent with the predicted formation of a ∼30-kDa cleavage product. Cleavage abrogated the phosphotyrosine signal on SCAMP3, while the signal on SCAMP1 was unaffected (lanes 3 and 4). Note that the anti-phosphotyrosine blot shows a band in all lanes (light chain of IgG) that has a slightly lower Mr and is unrelated to SCAMP. Transferrin receptor, a control for nonspecific proteolysis by IgA protease, was unaffected (Figure 5B). These data argue that tyrosine phosphorylation of SCAMP3 is proximal to residue 55.
Figure 5.
The 40-kDa tyrosine-phosphorylated polypeptide is SCAMP3. (A) IgA protease cleaves SCAMP3. NeoR cells were either unstimulated (lanes 1, 2, 5, and 6) or stimulated (lanes 3, 4, 7, and 8) with 100 ng/ml EGF for 30 min, lysed in CHAPS buffer, and immunoprecipitated with 7C12. Samples were either incubated in PBS alone (lanes 1, 3, 5, and 7) or in PBS plus 400 nM IgA protease (lanes 2, 4, 6, and 8) for 1 h. Samples were Western blotted for phosphotyrosine (αP-Tyr, left panel) or for SCAMP3 (3γ, right panel). “Sc3Δ” indicates the presence of the putative SCAMP3 cleavage fragment. (B) Transferrin receptor is not cleaved by IgA protease. NeoR cells were stimulated with EGF for 30 min, lysed in CHAPS buffer, and immunoprecipitated with anti-transferrin receptor antibody (H68.4). Samples were incubated in PBS alone (lane 1) or in PBS plus 400 nM IgA protease (lane 2) for 1 h. Immunoprecipitates were Western blotted for transferrin receptor and visualized using ECL.
In addition to examining EGFR as a prospective kinase for SCAMP1 and SCAMP3, we also considered other tyrosine kinases. Given the abundance of SCAMPs in the insulin-regulated GLUT4 compartment (Laurie et al., 1993), we examined insulin-stimulated rat adipocytes and insulin-like growth factor 1 (IGF-1)–stimulated Rat1 fibroblasts overexpressing IGF-1 receptor (IGF-1R) for SCAMP phosphorylation. Neither SCAMP1 nor SCAMP3 was detectably tyrosine phosphorylated in rat adipocytes that were stimulated with 200 nM insulin over a 30-min time course (Turner and Castle, unpublished observations), nor was SCAMP1 or SCAMP3 tyrosine phosphorylated in Rat1/IGF-1R cells stimulated with IGF-1 up to 60 min (our unpublished observations). We also examined the effect of platelet-derived growth factor (PDGF) on NeoR cells, since PDGF receptor and EGFR share common substrates, such as phospholipase-Cγ, GTPase-activating protein, and phosphatidylinositol 3-kinase (Fedi et al., 1994; Baass et al., 1995). PDGF stimulation of NeoR cells over a 60-min time course did not result in detectable tyrosine phosphorylation of either SCAMP (our unpublished observations). Finally, we examined a possible role for the nonreceptor tyrosine kinase, c-Src, in SCAMP phosphorylation, because both SCAMPs and c-Src are abundant in endosomes and because c-Src is known to enhance the mitogenic effects of overexpressed EGFR (Kaplan et al., 1992; Maa et al., 1995). For this experiment, we compared tyrosine phosphorylation of SCAMPs 1 and 3 in three cell types derived from 10T1/2 murine fibroblasts: NeoR cells, 5H cells (c-Src overexpressors), and 5HR cells (c-Src and EGFR double overexpressors). Both SCAMPs are tyrosine phosphorylated in EGF-stimulated NeoR and 5HR cells, but not in 5H cells, suggesting that overexpression of c-Src alone does not result in enhanced SCAMP tyrosine phosphorylation. Furthermore, tyrosine phosphorylation was not enhanced in 5HR cells above the level achieved in EGF-stimulated NeoR cells, suggesting that overexpression of c-Src in combination with EGFR does not lead to synergistic phosphorylation (our unpublished observations).
Time Course of SCAMP3 Tyrosine Phosphorylation in EGF-stimulated NeoR Cells
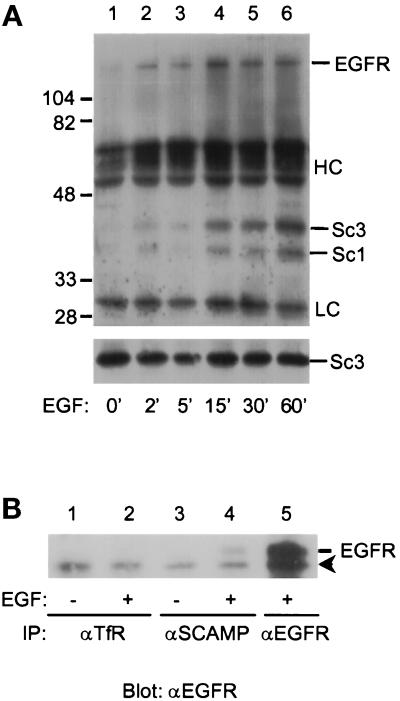
In an attempt to place the phosphorylation of SCAMPs 1 and 3 in relation to events following the binding of EGF to its receptor, we examined the time course of EGF-stimulated tyrosine phosphorylation over the first hour of EGF treatment. For this experiment and subsequent experiments involving SCAMP3, we used anti-SCAMP antibody 7C12 to follow both SCAMPs. As shown in Figure 6, tyrosine phosphorylation of both SCAMPs was detected at 2 min and progressively increased over the time course. No increase in SCAMP1 or SCAMP3 tyrosine phosphorylation beyond the 1 h level was observed after 5 h (our unpublished observations). Also, both SCAMP phosphotyrosine signals remained stable, after 1 h EGF stimulation, for an additional 4 h incubation in serum-free media lacking EGF. For reference, activated EGFR is internalized into endosomes with a t1/2 of 1 min, with maximal tyrosine phosphorylation of EGFR and the EGFR substrate, Shc, occurring at 15 min poststimulation, but persisting beyond 60 min poststimulation (Baass et al., 1995).
Figure 6.
Time course of SCAMP3 phosphorylation. (A) NeoR cells were stimulated with 100 ng/ml EGF for 0, 2, 5, 15, 30, or 60 min, lysed in CHAPS buffer, and immunoprecipitated with anti-SCAMP (7C12). Samples were Western blotted for phosphotyrosine (αP-Tyr, upper panel) or for SCAMP3 (3γ, lower panel). (B) EGF-inducible association of SCAMPs with EGFR. NeoR cells were either unstimulated (lanes 1 and 3) or EGF stimulated (lanes 2, 4, and 5), and then immunoprecipitated with a control antibody (anti-TfR, lanes 1 and 2), anti-SCAMP (7C12, lanes 3 and 4), or anti-EGFR (lane 5). Samples were resolved by 7.5% SDS-PAGE, transferred to nitrocellulose, Western blotted for EGFR, and visualized by ECL. The arrowhead indicates the position of a nonspecific band of ∼150 kDa.
An unknown phosphotyrosine protein of ∼170 kDa coimmunoprecipitated with SCAMPs beginning at 2 min EGF stimulation (Figure 6A, lanes 2–6). This band comigrated precisely with EGFR on a 7.5% SDS-PAGE gel (our unpublished observations). To determine whether the ∼170-kDa band might represent EGFR itself, we immunoprecipitated SCAMPs from EGF-stimulated NeoR cells and blotted with anti-EGFR. As shown in Figure 6B, EGFR did not coimmunoprecipitate with the control antibody (lanes 1 and 2) or with anti-SCAMP from unstimulated NeoR cells (lane 3). However, anti-SCAMP coimmunoprecipitated EGFR from EGF-stimulated NeoR cells (lane 4), suggesting that EGFR associates with the SCAMP immune complex in an EGF-inducible manner.
We have also examined the effect of EGF stimulation on SCAMP–SCAMP interactions. NeoR cells were stimulated up to 60 min, and then immunoprecipitated with anti-SCAMP3 to immunoprecipitate SCAMP3 and coimmunoprecipitate SCAMPs 1 and 2. EGF stimulation did not affect the ability of SCAMP1 or SCAMP2 to coimmunoprecipitate with SCAMP3 at any of the time points examined (our unpublished observations).
Distribution of SCAMP3 in EGF-stimulated NeoR Cells
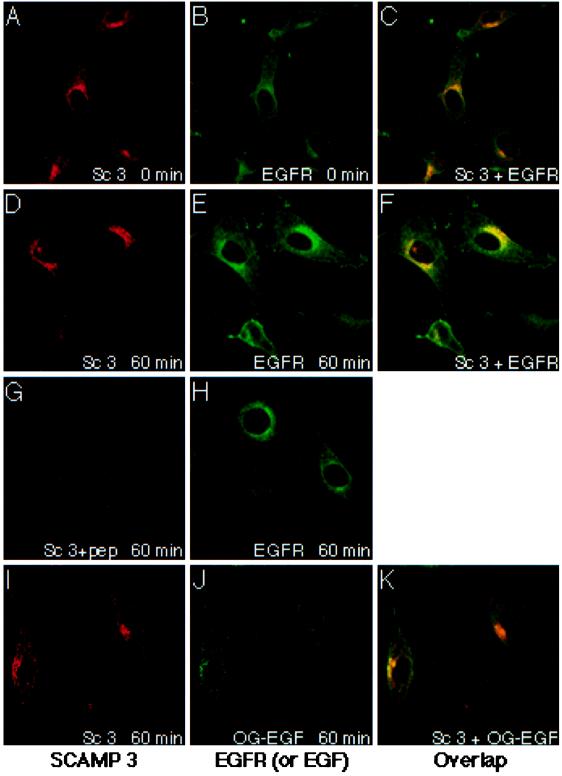
In view of the parallel time course of SCAMP and EGFR phosphorylation and the well-known effect of EGF on EGFR internalization, we examined whether EGF stimulation of NeoR cells altered the distribution of EGFR relative to the SCAMPs. The distributions of SCAMP3 and EGFR were compared at 0 and 60 min after EGF addition using double-label immunofluorescence and confocal microscopy. As shown in Figure 7, SCAMP3 displays a punctate cytoplasmic and perinuclear staining consistent with earlier findings (Singleton et al., 1997). It does not appear to redistribute in response to EGF stimulation of NeoR cells (compare panels A, D, and I). Unlike in CHO cells, we do not observe peripheral patches of SCAMP3 staining in NeoR cells. The specificity of the SCAMP3 signal was demonstrated by blocking staining with excess epitope peptide (Figure 7G).
Figure 7.
Distribution of SCAMP3, EGFR, and internalized EGF in NeoR cells. Serum-starved NeoR cells were stimulated with 100 ng/ml EGF for 0 min (panels A–C) or 60 min (panels D–H) or with 500 ng/ml EGF-Oregon Green (panels I–K). Subsequently, samples were fixed in 3% formaldehyde, permeabilized in 0.05% saponin, immunolabeled with anti-SCAMP3 (3γ; panels A, D, G, and I) or anti-EGFR (clone 13; panels B, E, and H) and analyzed by confocal microscopy. SCAMP3 immunostaining in panel G was conducted in the presence of 20-fold excess epitope peptide. Representative single optical sections are shown, and coconcentration is indicated by mixed staining in overlapped images (panels C, F, and K).
While SCAMP3 staining is not noticeably changed, EGFR immunostaining appears to partially redistribute from the cell surface to perinuclear endosomes in the presence of EGF (Figure 7, E, H, and I), consistent with the time course of receptor internalization/down-regulation (Dunn et al., 1986; Wiley et al., 1991; Masui et al., 1993; Herbst et al., 1994). Internalization was confirmed by uptake of Oregon Green-labeled EGF (Figure 7J). Pairwise comparison of SCAMP3 and EGFR immunostaining on overlapped images demonstrates coconcentration in the perinuclear region that appears moderately increased at 60 min (compare Figure 7, C and F). Notably, the overlapped images of SCAMP3 and internalized EGF show the same perinuclear concentration, but part of the EGF appears in greener-staining structures, signifying decreased SCAMP (Figure 7K). This appearance is consistent with accumulation of a portion of the EGF in lysosomes that are known to be SCAMP-poor (Brand and Castle, 1993). Thus, only a portion of EGF and its receptor seems to codistribute with SCAMP3 after EGF stimulation and internalization.
SCAMP3 Is Tyrosine Phosphorylated by EGFR in Vitro
In light of the apparent progressive codistribution of SCAMP3 and EGFR, the parallel time courses of phosphorylation of SCAMPs 1 and 3 and EGFR, and the observation of ligand-dependent coimmunoprecipitation, we wished to determine whether SCAMP3 could be directly phosphorylated by EGFR. Therefore, we examined phosphorylation in vitro. We immunoprecipitated SCAMP3 from CHO cells and EGFR from EGF-stimulated NeoR cells, combined the immunoprecipitates, and incubated the samples in the presence of ATP, magnesium, and manganese. As shown in Figure 8, phosphorylation of SCAMP3 was detected in two separate experiments. In the first experiment, coincubation of SCAMP3 and EGFR (lane 1) resulted in SCAMP phosphorylation, whereas coincubation of transferrin receptor and EGFR did not (lane 2). In the second experiment, the presence of EGFR enhanced phosphorylation above a low level observed when SCAMP3 was incubated in the presence of ATP alone (compare lane 6 vs. lane 5). The low level of phosphorylation of SCAMP3 in the presence of ATP was observed despite the inclusion of a 2 M KCl wash after immunoprecipitation to remove associated proteins. Thus, while phosphorylation of SCAMP is stimulated by EGFR in vitro, the low level of phosphorylation observed with the SCAMP3 immunoprecipitate alone does not allow us to distinguish whether EGFR directly or indirectly phosphorylates this SCAMP isoform. Note that analysis of the primary sequence of SCAMPs 1 and 3 does not indicate the presence of the 11 conserved subdomains common to protein kinases; therefore, we do not believe that SCAMPs themselves are kinases (Hanks et al., 1988).
DISCUSSION
The tyrosine phosphorylation of SCAMP1 and SCAMP3 is the first post-translational modification of SCAMPs to be described for this relatively new family of membrane proteins. As for numerous other phosphorylation events, our findings suggest that phosphorylation of SCAMPs may be a constitutive cycle, as we were able to affect the level of phosphorylation either by blocking phosphatase activity (Figure 1A) or by increasing the substrate relative to the amount of phosphatase (by overexpressing epitope-tagged SCAMP3, Figure 1C). While phosphorylation observed in the presence of vanadate gives the impression that SCAMP3 may be favored over SCAMP1 as a substrate (Figure 1), these two SCAMPs are probably phosphorylated to about the same extent, since the actual amount of SCAMP3 is underrepresented relative to SCAMP1 in Western blots using 7C12 (Figure 2 and Singleton et al., 1997). Consequently, the kinase that phosphorylates SCAMPs 1 and 3 may codistribute with both SCAMPs even though the steady-state distributions of the two SCAMPs are somewhat different (Figure 3 and Singleton et al., 1997).
While we don’t yet know the functional consequences of tyrosine phosphorylation of SCAMPs 1 and 3, we have noted that phosphorylation in the presence of vanadate in CHO cells is correlated with a shift in the steady-state distribution of SCAMP3 but not SCAMP1. Specifically, the accumulation of SCAMP3 at the cell periphery is exacerbated by the treatment (Figure 3). We have not detected rearrangement of other proteins that function in recycling (e.g., α-adaptin, clathrin). We presume that the peripheral accumulation of SCAMP3 is a specific consequence of inhibiting its dephosphorylation and thus identifies a site in SCAMP3 trafficking that is normally regulated by this process. There are already many examples of proteins that either undergo trafficking to the cell surface or function in this type of trafficking where phosphorylation regulates subcellular distribution, e.g., furin, EGFR, dynamin, synaptojanin (McPherson et al., 1994; Jones et al., 1995; Lamaze and Schmid, 1995; McClure and Robinson, 1996). Further study is required to identify the pathways through which the various SCAMPs circulate and to pinpoint the site where SCAMP3 accumulates. Likely possibilities include the plasma membrane and peripheral endosomes, in which case phosphorylation/dephosphorylation of SCAMP3 may regulate entry and exit at the cell surface. By analogy, it is possible that phosphorylation of SCAMP1 may regulate its distribution within internal compartments and that changes in distribution in the presence of vanadate have gone undetected in our observations using immunofluorescence microscopy. Notably, phosphorylation of SCAMPs 1 and 3 does not appear to affect the association between SCAMPs as detected by coimmunoprecipitation (Figure 2), suggesting that phosphorylation/dephosphorylation on tyrosine residues does not regulate interactions between SCAMPs at sites where their trafficking pathways overlap.
In our efforts to identify cellular tyrosine kinases that phosphorylate SCAMPs, we are quite intrigued by the observation that among a number of kinases, only the EGFR exhibits activity toward SCAMPs. Further, we find it noteworthy that phosphorylation requires EGF and also results in phosphorylation of both SCAMPs 1 and 3 as observed in the presence of vanadate. The time course of phosphorylation of both SCAMPs in EGF-stimulated cells (Figure 6) parallels the process of EGFR internalization and progression toward down-regulation (Baass et al., 1995). Evidently, phosphorylation is occurring at cellular sites inhabited by both SCAMPs, and the ligand-induced association of EGFR with SCAMPs (Figure 6B) supports the hypothesis that phosphorylation of one or both SCAMPs regulates an interaction with the receptor on its internalization pathway. It has been shown previously that EGF-catalyzed autophosphorylation of the EGFR promotes its association with Shc and Grb2 (Baass et al., 1995) and with other proteins, Eps15, annexin I, and the AP-2 complex, that are involved in receptor internalization and trafficking within endosomes (Futter et al., 1993; Okabayashi et al., 1996; Wang and Moran, 1996; van Delft et al., 1997). Perhaps SCAMPs are components of the endosomal sorting machinery such that their phosphorylation and association with the EGFR govern the fate of the latter on its route to lysosomes. Such a possibility is consistent with the partial localization of SCAMP3 and the EGFR at perinuclear sites that we have observed by immunofluorescence (Figure 7). Further, the sites of apparent colocalization may place the interaction of SCAMPs with the EGFR later than AP-2 in the intracellular itinerary of the receptor (van Delft et al., 1997), possibly at the level of annexin I (Futter et al., 1993) or sorting nexin I (Kurten et al., 1996).
Potentially, very interesting insight into the function of phosphorylation of SCAMPs derives from considering the prospective structural site(s) of phosphorylation. By comparing the primary structures of the mammalian SCAMPs characterized to date (Singleton et al., 1997), we have observed that there are two tyrosines conserved in SCAMP1 and SCAMP3, which are not found in SCAMP2. Of these two tyrosines (Tyr37 and Tyr73 in SCAMP1; Tyr 41 and Tyr83 in SCAMP3), we consider Tyr37/41 to be a more likely site for tyrosine phosphorylation, because it lies upstream of the IgA protease cleavage site (Figure 5) and because it loosely resembles the consensus site for EGFR kinase phosphorylation (Songyang et al., 1995). These deductions strongly suggest to us that SCAMPs 1 and 3 are each tyrosine phosphorylated at a single site. Further, because the selectivity of phosphorylation among SCAMP paralogs is the same for vanadate treatment and EGF stimulation, we believe that the same phosphorylation site is involved in each case. Future studies with appropriate site-specific mutant SCAMPs are needed to confirm that this is so. Notably, Tyr 37/41 is found in close proximity to one of three asparagine-proline-phenylalanine (NPF) repeats that are conserved elements near the N terminus of SCAMPs 1–3. NPF repeats have recently been identified as in vivo targets of the Eps15 homology (EH) domain (Salcini et al. 1997), a domain of Eps15 that functions in protein–protein interaction with other proteins that are involved in vesicular trafficking, particularly during endocytosis (van Delft et al., 1997). In view of these new insights, we suggest the possibilities that SCAMPs may interact with Eps15 or other EH-containing proteins during their intracellular trafficking in vivo and that phosphorylation near one of the NPF repeats may serve to regulate this interaction. The potential interrelationship between SCAMPs1 and 3 and Eps15 is interesting from another standpoint in that the tyrosine kinase specificities of the two proteins are the same (our studies and van Delft et al. [1997]). The potential interaction and functional relationship of SCAMPs and Eps15 is being considered in ongoing studies.
Finally, we note that while we have obtained evidence for EGF-stimulated phosphorylation and EGFR association with SCAMPs1 and 3, we presently are unable to distinguish whether both the phosphorylation and association are direct or indirect. Indeed, our earlier studies pointed to the presence of SCAMPs in macromolecular complexes (Wu and Castle, 1997), and both the low-level phosphorylation of SCAMP3 observed in the absence of EGFR in vitro (Figure 8) and the possible presence of a more broadly distributed kinase inferred from the studies with vanadate suggest that kinases other than the EGFR may be involved. Consequently, we feel that it is relevant to consider whether a constitutively active kinase and ligand-activated EGFR might function synergistically as has been observed previously for src family kinases and the EGFR (Parsons and Parsons, 1997).
ACKNOWLEDGMENTS
We would like to thank members of the Castle laboratory, Dr. Sarah J. Parsons, Dr. Carl Creutz, and Dr. Michael Cox, for valuable discussion and helpful suggestions, and the University of Virginia Biomolecular Research Facility (with support from the University of Virginia Comprehensive Cancer Center) for oligonucleotide synthesis and synthetic peptides. Special thanks to Dr. David Singleton for preparation of the SCAMP3-myc construct by PCR and to Amy Huang for help in obtaining data and preparing Figure 7. These studies were supported by grant DE09655 and Medical Scientist Training Program grant T32 GM07267–20 from the National Institutes of Health.
REFERENCES
- Austin C, Shields D. Formation of nascent secretory vesicles from the trans-Golgi network of endocrine cells is inhibited by tyrosine kinase and phosphatase inhibitors. J Cell Biol. 1996;135:1471–1483. doi: 10.1083/jcb.135.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baass P, Guglielmo G, Authier F, Posner B, Bergeron J. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- Bevan A, Drake P, Bergeron J, Posner B. Intracellular signal transduction: the role of endosomes. Trends Endocrinol Metab. 1996;7:13–21. doi: 10.1016/1043-2760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- Brand S, Castle JD. SCAMP 37, a new marker within the general cell surface recycling system. EMBO J. 1993;12:3753–3761. doi: 10.1002/j.1460-2075.1993.tb06053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Laurie S, Mixon M, Castle JD. Secretory carrier membrane proteins 31–35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991;266:18949–18957. [PubMed] [Google Scholar]
- Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W, Connolly T, Hubbard A. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway. J Cell Biol. 1986;102:24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G, Lewis G, Ramsay G, Bishop M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi P, Pierce J, DiFiore P, Kraus M. Efficient coupling with phosphtidylinositol 3-kinase, but not phospholipase Cγ or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier-Montial A, Canaves J, DasGupta B, Wilson M, Montal M. Tyrosine phosphorylation modulates the activity of clostridial neurotoxins. J Biol Chem. 1996;271:18322–18325. doi: 10.1074/jbc.271.31.18322. [DOI] [PubMed] [Google Scholar]
- Futter C, Felder S, Schlessinger J, Ullrich A, Hopkins C. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik A, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Herbst J, Oprekso L, Walsh B, Lauffenburger D, Wiley H. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J Biol Chem. 1994;269:12865–12873. [PubMed] [Google Scholar]
- Hu P, Margolis B, Skolnik E, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Thomas L, Molloy S, Thulin C, Fry M, Walsh K, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K, Swedlow J, Varmus H, Morgan D. Association of p60c-Src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten Richard C, Cadena Deborah L, Gill Gordon N. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid S. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie S, Cain C, Lienhard G, Castle J. The glucose transporter GluT4 and secretory carrier membrane proteins (SCAMPs) colocalize in rat adipocytes and partially segregate during insulin stimulation. J Biol Chem. 1993;268:19110–19117. [PubMed] [Google Scholar]
- Luttrell D, Luttrell L, Parsons SJ. Augmented mitogenic responsiveness to epidermal growth factor in murine fibroblasts that overexpress Pp 60c-Src. Mol Cell Biol. 1988;8:497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa M, Leu T, McCarley D, Schatzman R, Parsons S. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H, Castro L, Mendelsohn J. Consumption of EGF by A431 cells: evidence for receptor recycling. J Cell Biol. 1993;120:85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S, Robinson P. Dynamin, endocytosis, and intracellular signalling (Review) Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- McPherson P, Takei K, Schmid S, DeCamilli P. P145, a major Grb2-binding protein in brain, is colocalized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. J Biol Chem. 1994;269:30132–30139. [PubMed] [Google Scholar]
- Okabayashi Y, Sugimoto Y, Totty N, Hsuan J, Kido Y, Sakaguchi K, Gout I, Waterfield M, Kasuga M. Interaction of Shc with adaptor protein adaptins. J Biol Chem. 1996;271:5265–5269. doi: 10.1074/jbc.271.9.5265. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signalling pathways (Review) Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, DiFiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M, Burd C, Emr S. Receptor signalling and the regulation of endocytic membrane transport. Curr Opin Cell Biol. 1996;8:549–556. doi: 10.1016/s0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- Singleton D, Wu T, Castle JD. Three mammalian SCAMPs (secretory carrier membrane proteins) are highly related products of distinct genes having similar subcellular distributions. J Cell Sci. 1997;110:2099–2107. doi: 10.1242/jcs.110.17.2099. [DOI] [PubMed] [Google Scholar]
- Songyang Z, et al. Catalytic specificity of protein tyrosine kinases is critical for selective signalling. Nature. 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Stanley KK. Regulation of targetting signals in membrane proteins (review) Mol Membr Biol. 1996;13:19–27. doi: 10.3109/09687689609160570. [DOI] [PubMed] [Google Scholar]
- Umemori H, Inoue T, Kume S, Sekiyamu N, Nagao M, Itoh H, Nakanishi S, Mikoshiba K, Yamamoto T. Activation of the G protein Gq/11 through tyrosine phosphorylation of the α subunit. Science. 1997;276:1878–1881. doi: 10.1126/science.276.5320.1878. [DOI] [PubMed] [Google Scholar]
- Ushiro H, Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A431 cell membranes. J Biol Chem. 1980;255:8363–8365. [PubMed] [Google Scholar]
- Van Delft S, Schumacher C, Hage W, Verkleij A, Van Bergen en Henegouwen P. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J Cell Biol. 1997;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moran M. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Wigler M, Silverstein S, Lee L, Pellicer A, Cheng Y, Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11:223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wiley H, Herbst J, Walsh B, Lauffenburger D, Rosenfeld M, Gill G. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Wilson L, Luttrell D, Parsons J, Parsons S. Pp 60c-Src tyrosine kinase, myristylation, and modulatory domains are required for enhanced mitogenic responsiveness to epidermal growth factor seen in cells overexpressing c-Src. Mol Cell Biol. 1989;9:1536–1544. doi: 10.1128/mcb.9.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TT, Castle JD. Evidence for colocalization and interaction between 37 and 39 kDa isoforms of secretory carrier membrane proteins. J Cell Sci. 1997;110:1533–1541. doi: 10.1242/jcs.110.13.1533. [DOI] [PubMed] [Google Scholar]
- Zerial M, Parton R, Chavrier P, Frank R. Localization of Rab family members in animal cells. Methods Enzymol. 1992;219:398–407. doi: 10.1016/0076-6879(92)19039-9. [DOI] [PubMed] [Google Scholar]