Abstract
The recessive mouse mutant Mpv17 is characterized by the development of early-onset glomerulosclerosis, concomitant hypertension, and structural alterations of the inner ear. The primary cause of the disease is the loss of function of the Mpv17 protein, a peroxisomal gene product involved in reactive oxygen metabolism. In our search of a common mediator exerting effects on several aspects of the phenotype, we discovered that the absence of the Mpv17 gene product causes a strong increase in matrix metalloproteinase 2 (MMP-2) expression. This was seen in the kidney and cochlea of Mpv17-negative mice as well as in tissue culture cells derived from these animals. When these cells were transfected with the human Mpv17 homolog, an inverse causal relationship between Mpv17 and MMP-2 expression was established. These results indicate that the Mpv17 protein plays a crucial role in the regulation of MMP-2 and suggest that enhanced MMP-2 expression might mediate the mechanisms leading to glomerulosclerosis, inner ear disease, and hypertension in this model.
INTRODUCTION
The mouse mutant Mpv17 carries a retroviral insert in the Mpv17 gene. Failure to express this gene causes a phenotype of glomerulosclerosis and nephrotic syndrome (Weiher et al., 1990; Weiher, 1993) in such animals. In addition, hypertension occurs in this model (Clozel, submitted for publication) as well as characteristic alterations in the inner ear closely resembling Alport syndrome (Meyer zum Gottesberge et al., 1996). The Mpv17 gene product appears to be a peroxisomal protein involved in the metabolism of reactive oxygen (Zwacka et al., 1994). There is a human homolog of the Mpv17 gene localized on chromosome 2 which can, if introduced into the mutant mouse as a transgene, complement the kidney phenotype (Schenkel et al., 1995). Thus, this gene in humans is a candidate gene for kidney disorders or deafness.
In mice, the Mpv17 gene is expressed in a nearly ubiquitous manner, posing the question of how the loss of function of this gene in Mpv17-negative mice causes such diverse but defined phenotypes. Thus, Mpv17 expression may directly or indirectly affect several effector functions responsible for particular aspects of the phenotype. Molecular changes seen in both major locations of pathology, the kidney and the inner ear, might thereby be upstream in the chain of events. Candidates for such changes might be enzymes involved in basement membrane metabolism, because the basement membrane shows morphologic changes in both organs in Mpv17-negative mice (Weiher et al., 1990; Weiher, 1993; Meyer zum Gottesberge et al., 1996). In the present study we therefore explored the matrix metalloproteinase 2 (MMP-2) as a mediating function in the development of the phenotype in the kidney as well as in the inner ear, as it has been described as critically involved in the basement turnover within the glomerulus (Johnson et al., 1992; Carome et al., 1994; Nakamura et al., 1994). We indeed find an enhancement of MMP-2 expression in the absence of Mpv17 function in the kidney and the inner ear of Mpv17- deficient mice as well as in fibroblasts derived from these animals. Moreover, when the human Mpv17 gene was introduced into Mpv17-negative cells, MMP-2 expression was repressed. We therefore conclude that the phenotype caused by Mpv17 deficiency is mediated directly or indirectly by overexpression of MMP-2.
MATERIALS AND METHODS
Imunohistochemistry of the Inner Ear
Paraffin sections (10 μm) were incubated in 1% H2O2 for 30 min, washed twice for 5 min, blocked with 10% FCS in PBS for 1 h, and then incubated with the primary antibody (mouse anti-MMP-2, 1:100 dilution in 1% BSA in PBS) overnight at 4°C in a humid chamber. Sections were then washed, incubated with the secondary antibody (peroxidase-conjugated rabbit anti-mouse IgG, 1:2000 dilution), washed in PBS, and incubated in chromogen (3-amino-9-ethylcarbazide, Sigma Chemical, St. Louis, MO) for 2–5 min and counterstained with hematoxylin. Samples incubated without primary antibody served as negative controls.
Immunohistochemistry of the Kidney
Preparation of the kidneys, blocking, and incubation of the secondary antibody was performed the same way as described above for the inner ear. Detection was performed using an alkaline phosphatase-conjugated secondary antibody.
Western Blot Analysis
The membraneous labyrinth and kidneys were collected. Samples were homogenized with a Branson Sonifier (Branson, Plainview, NY) and boiled for 5 min in 2% SDS, 10% glycerol, 7.5 mM Tris-HCl, pH 6.8, 5% 2-mercaptoethanol, and 0.005% bromophenol blue. Total protein (100 μg) was electrophoresed on a 12% SDS-PAGE and transferred to an Immobilon-P membrane (Millipore, Bedford, MA) using a semidry electroblotting apparatus (Bio-Rad, Richmond, CA). The filter was blocked in 5% nonfat dry milk in PBS containing 0.1% Tween 20 for 1 h and incubated with the mouse anti-MMP-2 antibody (dilution 1:500). The filter was then washed and treated with secondary antibody (peroxidase-conjugated rabbit anti-mouse IgG, 1:4000), and the signals were detected using the ECL system (Amersham, Arlington Heights, IL).
Northern Analysis
Northern blots were performed as described by Maniatis et al. (1982). The cells were lysed with SDS, followed by proteinase K digestion. Poly(A)+ RNA was selected by binding to oligo(dT) cellulose (Biolabs, Beverly, MA) according to the manufacturers recommendations. Samples of RNA were denatured at 65°C for 10 min in a solution containing 50% (vol/vol) formamide, 2.2 M formaldehyde, and RNA running buffer (20 mM MOPS, pH 7.0, 5 mM sodium acetate, and 1 mM EDTA). Electrophoresis was carried out in 1% agarose gels containing RNA running buffer and 2.2 M formaldehyde.
Substrate Gel Analysis/Zymogram
Cells (5 × 105) were plated in a 3-cm culture dish and grown in 2.5% FCS in DMEM. After 48 h the proteins were pelleted by adding 100 μl 100% trichloroacetic acid to 875 μl of the medium and incubated on ice for 1 h. The proteins were pelleted by centrifugation and dissolved in 100 μl of 8% SDS, 4% sucrose, 250 mM Tris, pH 6.8, 0.01% bromophenol blue. The samples were subjected to electrophoresis in a 12% SDS-polyacrylamide gel containing 0.1% gelatin, and the gel was incubated twice in 2.5% Triton X-100 for 15 min. After a short rinse with distilled water, the gel was incubated in 50 mM Tris, pH 7.5, 10 mM CaCl2 overnight, stained with 0.25% Coomassie brilliant blue, and destained with 40% methanol, 10% acetic acid.
In Situ Hybridization
In situ hybridization was performed on sections of heads from mice of different genotypes essentially as described by Gack et al. (1995). As a probe for the Mpv17 gene, an in vitro 35S-labeled transcript from a 383-base pair (bp) SacI–EcoRI fragment of the human cDNA was used; for the MMP-2 gene, a 340-bp PvuII–SacI fragment was used (Gack et al., 1995).
RESULTS
Mpv17 and MMP-2 Are Expressed in the Cochlea
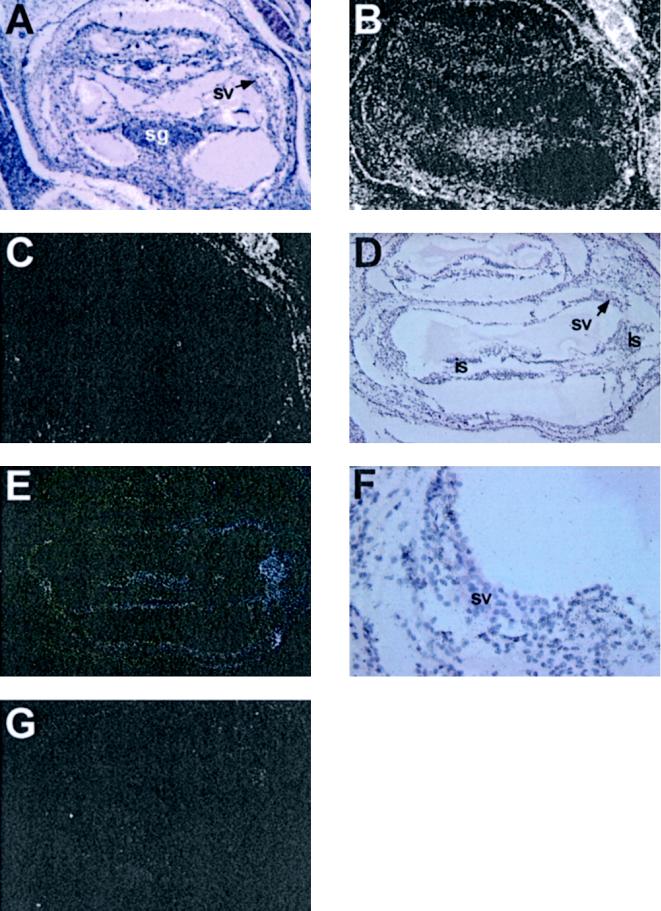
The phenotypical changes seen in both major locations of pathology of the Mpv17-negative mouse, the kidney and the inner ear, consist mainly of basement membrane alterations (Weiher et al., 1990; Weiher, 1993; Meyer zum Gottesberge et al., 1996). We therefore reasoned that failure to express the Mpv17 gene may cause a deregulation of matrix-degrading enzymes, which, in turn, may lead to changes in the basement membrane composition. As a prerequisite of this idea, Mpv17 and such candidate genes should be coexpressed in the respective tissues. For the kidney, expression of the matrix metalloproteinase II (MMP-2) has been shown (Harendza et al., 1995; Knowlden et al., 1995), and Mpv17 has been demonstrated to be expressed overlapping with this pattern (Zwacka, 1995). We therefore first explored the expression pattern of Mpv17 and MMP-2 in the other location of pathology, the inner ear. In situ hybridization on inner ears of 4-d-old mice was performed and the result is depicted in Figure 1. As expected from the nearly ubiquitous expression pattern in the other tissues (Weiher et al., 1990), Mpv17-specific signals were detected almost everywhere in the inner ear (Figure 1, A and B), being particularly pronounced in the spiral ganglion and the stria vascularis. The MMP-2 expression was also present, although in a a less ubiquitous manner (Figure 1, D–F). Here particularly strong expression was seen in the region of the outer sulcus with the type II fibroblasts of the spiral ligament and the spiral prominence, and the limbus spiralis region adjacent to the inner sulcus. Remarkably, there was only very weak expression detectable in the stria vascularis (Figure 1F). Thus, as previously shown for the kidney, the expression sites of Mpv17 and MMP-2, although not completely identical, overlap in the inner ear as well.
Figure 1.
In situ hybridization analysis of Mpv17 and MMP-2 expression in murine cochleae at 4 d of age. (A–C) Mpv17 expression in wild types: (A) bright field picture; (B) dark field picture of a Mpv17 antisense probe hybridization; (C) Mpv17 sense probe hybridization. Exposure time (A–C), 16 d; magnification 50×. (D–G) MMP-2 expression: (D) bright field picture; (E) dark field picture of a MMP-2 antisense probe hybridization. Exposure time (D-G), 12 d; magnification 50×. (F) Bright field picture of the stria vascularis of panel E. Magnification, 200x. (G) MMP-2 sense probe hybridization. Magnification 50×. sv, stria vascularis; ls, ligamentum spirale; is, inner sulcus; sg, spiral ganglion. The pictures were scanned with a Scan Maker Designer Pro and assembled using Adobe Photoshop 4.0.
Enhanced Expression of MMP-2 in the Kidney and Inner Ear of Mpv17- Negative Mice
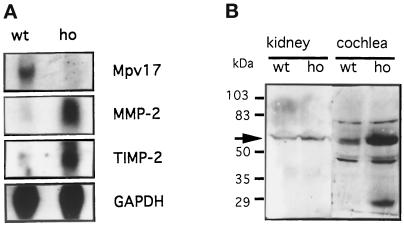
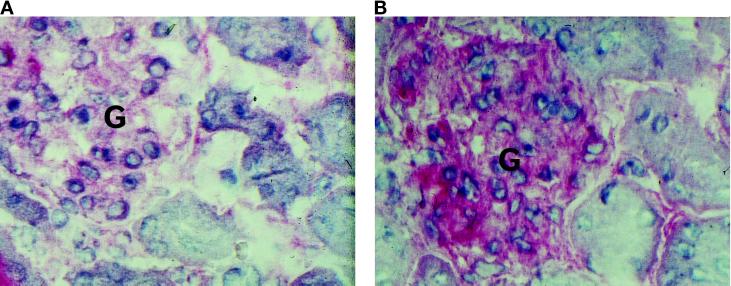
To test for the potential role of MMP-2 in the pathomechanism of the Mpv17 mutation, we analyzed whether the expression of the MMP-2 gene was influenced by the lack of Mpv17 expression in Mpv17-negative mice. We therefore studied the MMP-2 expression in Mpv17-negative mice at the mRNA and protein level. As depicted in Figure 2A, in the kidney of Mpv17- deficient mice, strongly elevated MMP-2 expression was detected as compared with control mice. In addition, stronger expression of tissue-specific inhibitor of metalloproteinase 2 (TIMP-2), a modulator of MMP-2 activity that is involved in membrane binding of the TIMP-2/proMMP-2 complex before the activation of the proenzyme to the active enzyme (Emmert-Buck et al., 1995; Sato et al., 1996) could also be seen. The increase in MMP-2 was also seen on the protein level. In particular, immunohistochemistry on glomeruli revealed an enhancement of MMP-2 expression in Mpv17-deficient mice (Figure 3). A Western blot analysis depicted in Figure 2 corroborates this notion. In the kidney, despite some unspecific reactivity in the high molecular mass range, a slight enhancement of specific reactivity at 62 kDa, the size of the active enzyme is observed. Since in this analysis whole kidneys were investigated, sites of MMP-2 expression other than glomeruli are also analyzed, blurring the enhancement observed in the glomeruli in immunohistochemistry. In contrast, in isolated cochleae of the inner ear it appeared that in the Western blot analysis the 62-kDa band was increased by fivefold in Mpv17- negative mice as seen in comparison with the bands of unspecific reactivity detected in this tissue. The 28-kDa band also elevated in Mpv17-negative mice represents most likely a degradation product of MMP-2, as such products have been observed earlier (Bergmann et al., 1995).
Figure 2.
MMP-2 gene expression in Mpv17- negative (ho) and wild-type (wt) mice. (A) Northern blots of polyA+-RNA from kidney. As a loading control the blot was probed with a cDNA from GAPDH. (B) Western blot analysis of proteins from kidney and cochlea. The arrow denotes the MMP-2 signal at 62 kDa. For specification of the antibody and detection, see MATERIALS AND METHODS.
Figure 3.
MMP-2 expression in glomeruli of Mpv17-negative (B) and wild-type (A) animals. Paraffin sections were stained with a monoclonal mouse anti-human MMP-2 antibody. For detection an alkaline phosphatase-coupled secondary anti-mouse antibody was used (APAP). Magnification 200×. G, Glomerulus
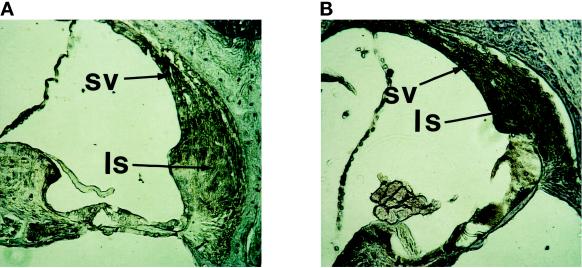
Accordingly, the immunohistochemical analysis of the cochleae showed a strong overexpression of MMP-2 in both ligamentum spirale and stria vascularis of Mpv17-negative animals (Figure 4). Therefore, the sites of detection do not differ between mice of different genotype. Taken together, both locations of pathology show increased MMP-2 expression in the absence of the Mpv17 gene product.
Figure 4.
MMP-2 expression in the cochleae of Mpv17-negative (B) and wild-type (A) mice. The primary antibody was the same as in Figure 2. For detection a peroxidase-coupled secondary antibody was used. Magnification 200×. ls, ligamentum spirale; sv, stria vascularis
Thus, it seems conceivable that MMP-2 mediates the molecular mechanisms involved in the pathology of both organs.
Expression of Mpv17 and MMP-2 Is Negatively Correlated in Fibroblast Tissue Culture
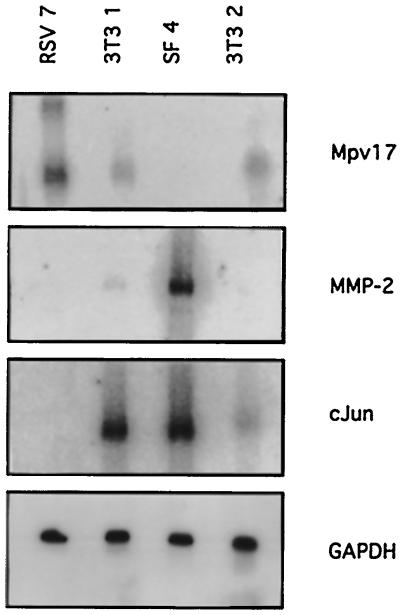
Primary cells were derived from the skin of newborn Mpv17-negative animals. These cells (SF4), like all tissue tested from these animals (Weiher et al. 1990), do not express Mpv17 mRNA (Figure 5 lane 3). When standard 3T3 cells were investigated, they showed intermediate and, dependent upon the particular preparation, somewhat variable levels of Mpv17 mRNA expression (lanes 2 and 4). To obtain a constitutively expressing cell line, 3T3 cells were transfected with a Mpv17 expression construct. These RSV7 cells show high, constitutive Mpv17 expression (Zwacka et al. 1994). Lane 1 shows two Mpv17-specific bands in these cells. The smaller one represents a mRNA species of approximately 1.4 kb, which is initiated and terminated on the transfected construct. The larger band is consistent with being a readthrough product into the neighboring sequences (Reuter, unpublished). The endogenous Mpv17 band of 1.7 kilobases (kb) (compare lanes 2 and 4) is below the limits of detection in these cells. Therefore it is possible that, in the presence of exogenous Mpv17 expression, the endogenous mRNA expression is inhibited. When looking at the respective MMP-2 stable mRNA expression levels, we found that these negatively correlate with Mpv17 mRNA levels (Figure 5, second panel). Thus, Mpv17-negative SF4 cells show high expression, 3T3 cells show intermediate, and RSV7 cells show no detectable MMP-2 expression. Therefore, it appears that the MMP-2 levels in tissue culture fibroblasts tested reflect the in vivo situation in tissue (see above). This negative correlation is not a nonspecific effect of general mRNA levels, since the expression of control genes like GAPDH is not affected by Mpv17 expression (Figure 5, lowest panel). However, this effect is not restricted to MMP-2 expression as other genes, such as the immediate early gene c-jun, is expressed in a similar pattern as MMP-2 (Figure 5, third panel). These data suggest that if Mpv17 expression influences the expression of MMP-2, the effect, may, however, not be direct and could possibly be mediated by other regulatory genes.
Figure 5.
MMP-2 and cJun expression in fibroblasts of different genotype. Northern blots of polyA+-RNA from different cell lines were analyzed. SF4, Primary skin fibroblasts from Mpv17-negative animals; 3T3 1 and 3T3 2, two different isolates of NIH 3T3 cells; RSV7, 3T3 cells, stably transfected with a RSV promotor-driven Mpv17 expression construct (Zwacka et al., 1994). As a loading control, the filter was hybridized with a GAPDH probe (see Figure 2).
Mpv17 Expression Represses MMP-2 Expression and Activity
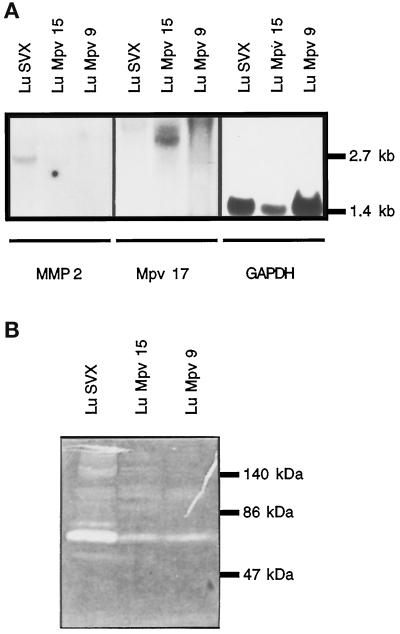
To test for a causal relationship between Mpv17 and MMP-2 expression, we transfected a construct constitutively expressing the human Mpv17 mRNA in Mpv17-negative cells. We have shown earlier that the human gene can complement the missing function in Mpv17-negative mice after transgenesis (Schenkel et al., 1995). We first isolated lung fibroblasts from Mpv17-negative mice immortalized with a SV40 T-antigen–containing retrovirus (Jat et al., 1986) and derived several cell clones. These LUSVX cells, like the primary skin fibroblasts SF4, show no Mpv17 mRNA signal but high MMP-2 expression upon Northern analysis (Figure 6). These cells were transfected with the expression vectors pBabePuroMpv17 and pBabePuroMpv17His containing the human Mpv17 coding region cloned into the vector pBabePuro (Morgenstern and Land, 1990) as well as an His tag in the pBabePuroMpv17His. Several transfectants were derived with either this construct or the empty vector. As depicted in Figure 6A, it revealed that Mpv17-expressing clones repressed MMP-2 expression at the level of mRNA. By contrast, the expression had no effect on GAPDH stable mRNA levels. Furthermore, we tested whether these expression changes were also evident at the level of MMP-2 enzyme activity. Therefore, we monitored gelatinase activity in supernatants from the different transfectants and LuSVX cells using an in-gel gelatinase assay (Vallon et al. 1997). Figure 6B shows that a prominent band at 72 kDa disappeared in the transfectants, indicating that, indeed, overall MMP-2 enzyme activity was also influenced by the expression of the Mpv17 gene. In summary, a strong decrease of MMP-2 mRNA and enzymatic activity is seen dependent upon the presence of Mpv17 expression, thereby establishing an inverse causal relationship between Mpv17 gene expression and MMP-2 expression and activity.
Figure 6.
MMP-2 expression and activity in Mpv17 transfectants. (A) Northern analysis of Mpv17-negative LUSVX cells and two different clones of LUSVX cells, stably transfected with different Mpv17 expression constructs (LuMpv9, LuMpv15). The two Mpv17 transcripts can be detected at 3.5 kb and 4.5 kb, respectively; 2.7 kb is the size of the MMP-2 transcript and 1.4 kb is the size of the GAPDH transcript. (B) Substrate gel analysis (Zymogram) of LUSVX, LU-Mpv9, and LU-Mpv15 cells.
DISCUSSION
A strong relationship between the kidney and the inner ear is established by clinical data. Numerous drugs have nephrotoxic as well as ototoxic effects (Begg and Barclay, 1995), while congenital abnormalies exist that cause lesions in both organs (Schuknecht, 1973, Arnold and Friedmann, 1992). A paradigm for the latter, Alport syndrome, is characterized by mutations in type IV collagen (Barker et al., 1990; Tryggvason et al., 1993; Mochizuki et al., 1994), a major component of the basement membranes in the glomerulus and the cochlea (Tokahashi and Hokunan, 1992; Cosgrove et al., 1996). The recessive mouse mutant Mpv17, which is characterized by a failure to express the Mpv17 gene, shows a phenotype of glomerulosclerosis in the kidney (Weiher et al., 1990) that is similar to Alport syndrome in the inner ear (Meyer zum Gottesberge et al., 1996). It displays characteristic changes in the basement membranes in both locations. Since the Mpv17 gene product is not a structural component of the basement membrane but rather a peroxisomal protein involved in the metabolism of reactive oxygen species (Zwacka et al., 1994), we hypothesize that the failure to express this gene might cause regulatory changes finally leading to defects in the basement membrane. In this paper we establish a causal relationship between Mpv17 expression and regulation of MMP-2, a protein known to be involved in basement membrane metabolism (Johnson et al., 1992; Carome et al., 1994; Nakamura et al., 1994; ). On the one hand, MMP-2 is overexpressed in tissues and cultured cells derived from Mpv17-negative mice. On the other hand, constitutive overexpression of the Mpv17 gene in such Mpv17-negative fibroblasts turns off the expression of MMP-2. These data are in accord with our initial hypothesis and suggest that MMP-2 is indeed a common mediator of both disease phenotypes. The expression of the TIMP-2, which controls the activity of MMP-2 and other metalloproteinases (Emmert-Buck et al., 1995; Sato et al., 1996) not only by binding the metalloproteinase in a stoichiometric complex but also in playing an essential role in activation of the proteinase, is induced in the kidney of Mpv17-negative mice as well. However, it is not clear whether this reflects a common regulatory pathway for both MMP-2 and TIMP-2 or whether it might constitute a secondary event in the complicated systemic reaction to the primary defect.
The molecular mechanism by which Mpv17 gene expression controls MMP-2 expression is yet unknown. We have established earlier a role of the Mpv17 gene in the metabolism of reactive oxygen species (Zwacka et al., 1994), and it has been observed that expression of MMP-2 and TIMP-2 is redox-dependent (Kawaguchi et al., 1996, Tyagi et al., 1996). Moreover, recent experiments have shown that the glomerulosclerosis in Mpv17 deficient mice is therapeutically responsive to treatment with compounds scavenging reactive oxygen in the kidney (Kerjaschki et al., unpublished data). However, whether this regulation involves the transcription factors c-jun or NF[κ]B, which have been identified as players in the redox-dependent regulation of several genes (Abate et al., 1990; Meyer et al., 1993) remains to be tested in future experiments. Of note, the c-jun expression parallels the MMP-2 expression pattern in tissue culture cells analyzed in our experiments (Figure 5).
Mpv17-negative mice display a characteristic thickening of basement membranes in both the kidney and the cochlea (Meyer zum Gottesberge et al., 1996) as well as a longitudinal splitting of the basement membrane of the strial vasculature. Immunohistochemical analyses at the resolution of light microscopy revealed so far no loss of particular type IV collagen or laminin components in these animals, although an overexpression of collagen IV α1/α2 and laminin (laminin β1 in the kidney, laminin β2 in the cochlea) could be detected (Reuter, unpublished results). These data are remarkably similar to the phenotype seen in the recently generated collagen IV α3 knockout mouse (Miner and Sanes, 1996). Comparable to those α3(IV) knockout mice, Mpv17- negative mice show an increased glomerular permeability resulting in proteinuria, focal and segmental glomerulosclerosis, and inner ear defects such as degeneration of the stria vascularis, thickened and multilaminated basal laminae of the stial vasculature, degeneration of the organ of Corti, and atrophy of the spiral ganglion. Although the characteristic “basket-weave” appearance of the glomerular basement membrane seen in α3(IV)-negative mice could not be detected in Mpv17-negative mice, the overall similarity between the phenotypes further points toward a basement membrane defect as the primary defect in the Mpv17-negative mouse. This suggests that, although the molecular cause is different, both mouse models may share pathological mechanisms finally leading to similar phenotypes. More detailed immuno-electronmicroscopic analyses are necessary to detect structural defects on the level of the macromolecular scaffold, on which the basement membrane is formed. Finally, these mice may represent a model for those cases of Alport syndrome in which none of the structural components of type IV collagen are mutated.
ACKNOWLEDGMENTS
The generous support of Professor Heinz Schaller, University of Heidelberg, is gratefully acknowledged. This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (to H.W.). A.R. was awarded a stipend from the Forschungszentrum Karlsruhe GmbH.
REFERENCES
- Abate C, Patel L, Rauscher FJ, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Arnold W, Friedmann I. Pathology of the Inner Ear. London: Churchill Livingstone; 1992. [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Begg EJ, Barclay ML. Aminoglycosids—50 years on. Br J Clin Pharmacol. 1995;39:597–603. [PMC free article] [PubMed] [Google Scholar]
- Bergmann U, Tuuttila A, Stetler-Stevenson WG, Tryggvason K. Autolytic activation of recombinant human 72 kilodalton type IV collagenase. Biochemistry. 1995;34:2819–2825. doi: 10.1021/bi00009a011. [DOI] [PubMed] [Google Scholar]
- Carome MA, Striker LJ, Peten EP, Elliot SJ, Yang C-W, Stetler-Stevenson WG, Reponen P, Tryggvason K. Assessment of 72-kilodalton gelatinase and TIMP-1 gene expression in normal and sclerotic murine glomeruli. J Am Soc Nephrol. 1994;5:1391–1399. doi: 10.1681/ASN.V561391. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Samuelson D, Pinnt J. Immunohistochemical localization of basement membrane collagens and associated proteins in the murine cochlea. Hearing Res. 1996;97:54–65. [PubMed] [Google Scholar]
- Emmert-Buck M, Emonard HP, Corcoran ML, Krutzsch HC, Foidart JM, Stetler-Stevenson WG. Cell surface binding of TIMP-2 and pro-MMP-2/TIMP-2 complex. FEBS Lett. 1995;364:28–32. doi: 10.1016/0014-5793(95)00345-a. [DOI] [PubMed] [Google Scholar]
- Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, Weiher H, Wagner EF, Angel P. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995;6:759–767. [PubMed] [Google Scholar]
- Harendza S, Pollock AS, Mertens PR, Lovett DH. Tissue-specific enhancer-promoter interactions regulate high level constitutive expression of matrix metalloproteinase 2 by glomerular mesangial cells. J Biol Chem. 1995;270:18786–18796. doi: 10.1074/jbc.270.32.18786. [DOI] [PubMed] [Google Scholar]
- Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Yamabe H, Chen YP, Campbell C, Gordon K, Baker D, Lovett D, Couser WG. Glomerular epithelial cells secrete a glomerular basement membrane-degrading metalloproteinase. J Am Soc Nephrol. 1992;2:1388–1397. doi: 10.1681/ASN.V291388. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Tanaka H, Okada T, Konishi H, Takahashi M, Ito M, Asai J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and ist tissue inhibitor in cultured human dermal fibroblasts. Arch Dermatol Res. 1996;288:39–44. doi: 10.1007/BF02505041. [DOI] [PubMed] [Google Scholar]
- Knowlden J, Martin J, Davies M, Williams JD. Metalloproteinase generation by human glomerular epithelial cells. Kidney Int. 1995;47:1682–1689. doi: 10.1038/ki.1995.233. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Reuter A, Weiher H. Inner ear defect similar to Alport’s syndrome in the glomerulosclerosis mouse model Mpv17. Eur Arch Otorhinolaryngol. 1996;253:470–474. doi: 10.1007/BF00179952. [DOI] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Molecular and functional defects in kindeys of mice lacking collagen alpha 3 (IV): implications for Alport syndrome. J Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, et al. Identification of mutations in the a3(IV) and a4(IV) collagen genes in autosomal recessive Alport syndrome. Nature Genet. 1994;8:77–82. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Fukui M, Ebihara I, Tomio Y, Koide H. Low protein diet blunts the rise in glomerular gene expression in focal glomerulosclerosis. Kidney Int. 1994;45:1593–1605. doi: 10.1038/ki.1994.210. [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler-Stevenson WG, Seiki M. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Lett. 1996;385:238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- Schenkel J, Zwacka RM, Rutenberg C, Reuter A, Waldherr R, Weiher H. Functional rescue of the glomerulosclerosis phenotype in Mpv17 mice by transgenesis with the human Mpv17 homologue. Kidney Int. 1995;48:80–84. doi: 10.1038/ki.1995.270. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Pathology of the Ear. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- Tokahashi M, Hokunan K. Localization of type IV collagen and Laminin in the guinea pig inner ear. Ann Otol Rhinol Laryngol. 1992;191:58–62. doi: 10.1177/0003489492101s1012. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Zhou J, Hostikka SL, Shows TB. Molecular genetics of Alport syndrome. Kidney Int. 1993;43:38–44. doi: 10.1038/ki.1993.8. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Kumar SG, Borders S. Reduction-oxidation (redox) state regulation of extracellular matrix metalloproteinases and tissue inhibitors in cardiac normal and transformed fibroblast cells. J Cell Biochem. 1996;61:139–151. doi: 10.1002/(sici)1097-4644(19960401)61:1<139::aid-jcb15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Vallon R, Müller R, Moosmayer D, Gerlach E, Angel P. The catalytic domain of activated collagenase I (MMP-1) is absolutely required for interaction with its specific inhibitor, tissue specific inhibitor of metalloproteinases I (TIMP-1) Eur J Biochem. 1997;244:81–88. doi: 10.1111/j.1432-1033.1997.00081.x. [DOI] [PubMed] [Google Scholar]
- Weiher H. Glomerular sclerosis in transgenic mice: the Mpv17 gene and ist human homologue. In: Grünfeld J-P, Bach JF, Kreis H, Maxwell MH, editors. Advances in Nephrology. St. Louis, MO: Mosby Year Books; 1993. pp. 37–42. [PubMed] [Google Scholar]
- Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Zwacka RM, Reuter A, Pfaff E, Moll J, Gorgas K, Karasawa M, Weiher H. The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J. 1994;12:5129–5134. doi: 10.1002/j.1460-2075.1994.tb06842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwacka RM. The human homologue of the murine glomerulosclerosis gene Mpv17. Wissenschaftliche Berichte FZKA 5621 B. Karlsruhe, Germany: Forschungszentrum Karlsruhe GmbH; 1995. [Google Scholar]