Abstract
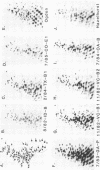
Vesicular stomatitis virus (VSV) has been shown previously to be capable of undergoing rapid mutational change during sequential experimental infections in various tissue culture cell systems (J. Holland, K. Spindler, F. Horodyski, E. Grabau, S. Nichol, and S. Vandepol, Science 215:1577-1585, 1982). The present study was undertaken to determine the degree of genetic diversity and evolution of the virus under natural infection conditions and to gain insight into the epizootiology of the disease. Between 1982 and 1985, numerous outbreaks of VSV of the New Jersey serotype were reported throughout regions of the United States and Mexico. A T1 RNase fingerprint analysis was performed on the RNA genomes of 43 virus isolates from areas of epizootic and enzootic virus activity. This indicates that virus populations were genetically relatively homogeneous within successive U.S. virus epizootics. The data included virus isolates from different epizootic stages, geographical locations, host animals, and host lesion sites. In contrast, only distant genome RNA T1 fingerprint similarities were observed among viruses of the different U.S. epizootics. However, Mexican viruses isolated before or concurrent with U.S. epizootics had very similar RNA genome fingerprints, suggesting that Mexico may have been the possible origin of virus initiating recent U.S. VSV New Jersey outbreaks. Comparison of T1 fingerprints of viruses with enzootic disease areas revealed a greater extent of virus genetic diversity in these areas relative to that observed in epizootic areas. The evolutionary significance of these findings and their relationship to experimental data on VSV evolution are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRIS D. H., HANSON R. P., DICKE R. J., ROBERTS R. H. Experimental transmission of vesicular stomatitis virus by Diptera. J Infect Dis. 1955 Mar-Apr;96(2):184–192. doi: 10.1093/infdis/96.2.184. [DOI] [PubMed] [Google Scholar]
- Fletcher W. O., Stallknecht D. E., Jenney E. W. Serologic surveillance for vesicular stomatitis virus on Ossabaw Island, Georgia. J Wildl Dis. 1985 Apr;21(2):100–104. doi: 10.7589/0090-3558-21.2.100. [DOI] [PubMed] [Google Scholar]
- HANSON R. P. The natural history of vesicular stomatitis. Bacteriol Rev. 1952 Sep;16(3):179–204. doi: 10.1128/br.16.3.179-204.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., O'Hara P. J., Holland J. J., Perrault J. Structure and origin of a novel class of defective interfering particle of vesicular stomatitis virus. Nucleic Acids Res. 1984 Mar 26;12(6):2775–2790. doi: 10.1093/nar/12.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P. J., Horodyski F. M., Nichol S. T., Holland J. J. Vesicular stomatitis virus mutants resistant to defective-interfering particles accumulate stable 5'-terminal and fewer 3'-terminal mutations in a stepwise manner. J Virol. 1984 Mar;49(3):793–798. doi: 10.1128/jvi.49.3.793-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Schnitzlein W. M., Bishop D. H., Lazzerini R. A., Beatrice S. T., Wagner R. R. Classification of the New Jersey serotype of vesicular stomatitis virus into two subtypes. J Virol. 1978 Jan;25(1):446–449. doi: 10.1128/jvi.25.1.446-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. Characterization of New Jersey vesicular stomatitis virus isolates from horses and black flies during the 1982 outbreak in Colorado. Virology. 1985 Apr 30;142(2):426–431. doi: 10.1016/0042-6822(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Shelokov A., Peralta P. H. Vesicular stomatitis virus, Indiana type: an arbovirus infection of tropical sandflies and humans? Am J Epidemiol. 1967 Jul;86(1):149–157. doi: 10.1093/oxfordjournals.aje.a120720. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Stallknecht D. E., Nettles V. F., Erickson G. A., Jessup D. A. Antibodies to vesicular stomatitis virus in populations of feral swine in the United States. J Wildl Dis. 1986 Jul;22(3):320–325. doi: 10.7589/0090-3558-22.3.320. [DOI] [PubMed] [Google Scholar]
- Stallknecht D. E., Nettles V. F., Fletcher W. O., Erickson G. A. Enzootic vesicular stomatitis New Jersey type in an insular feral swine population. Am J Epidemiol. 1985 Nov;122(5):876–883. doi: 10.1093/oxfordjournals.aje.a114170. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Direct method for quantitation of extreme polymerase error frequencies at selected single base sites in viral RNA. J Virol. 1986 Jan;57(1):219–228. doi: 10.1128/jvi.57.1.219-228.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudia W. D., Fields B. N., Calisher C. H. The isolation of vesiculay stomatitis virus (Indiana strain) and other viruses from mosquitoes in New Mexico, 1965. Am J Epidemiol. 1967 Nov;86(3):598–602. doi: 10.1093/oxfordjournals.aje.a120769. [DOI] [PubMed] [Google Scholar]
- Tesh R. B., Chaniotis B. N., Johnson K. M. Vesicular stomatitis virus (Indiana serotype): transovarial transmission by phlebotomine sandlies. Science. 1972 Mar 31;175(4029):1477–1479. doi: 10.1126/science.175.4029.1477. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Jones E. V., Kelly M., Frielle D. W. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981 Jan 15;108(1):87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]