Abstract
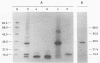
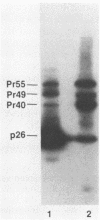
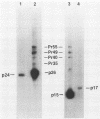
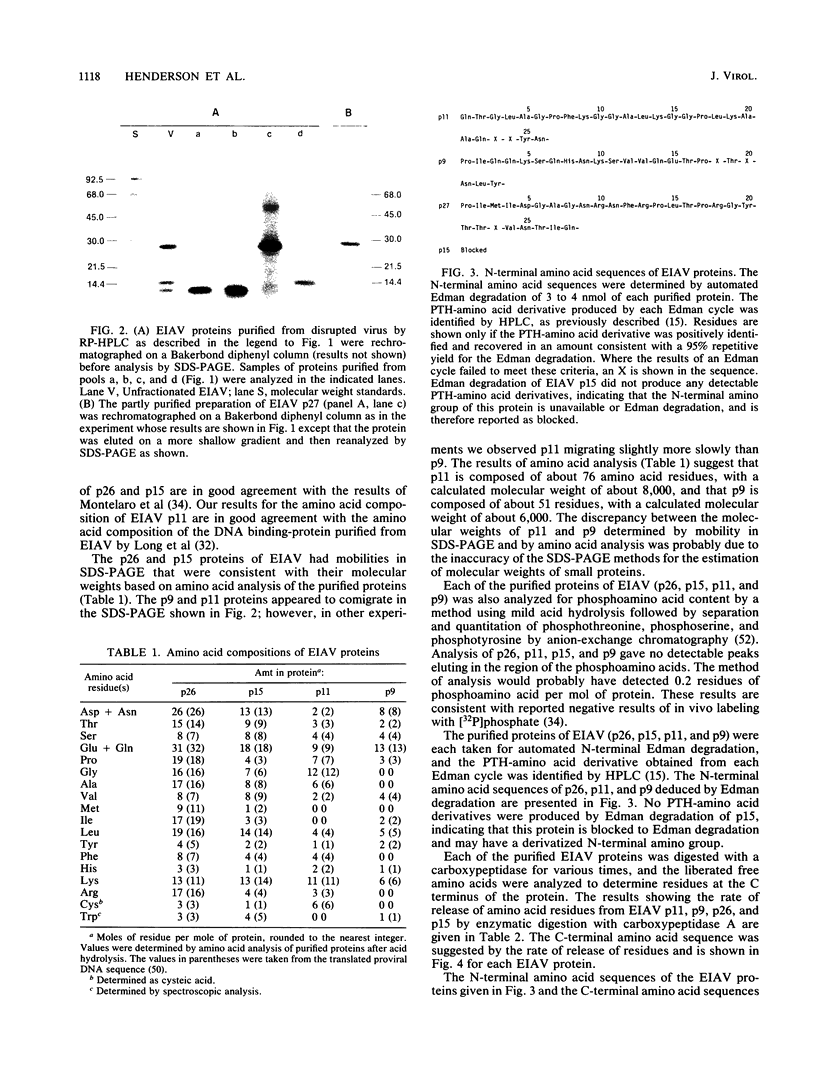
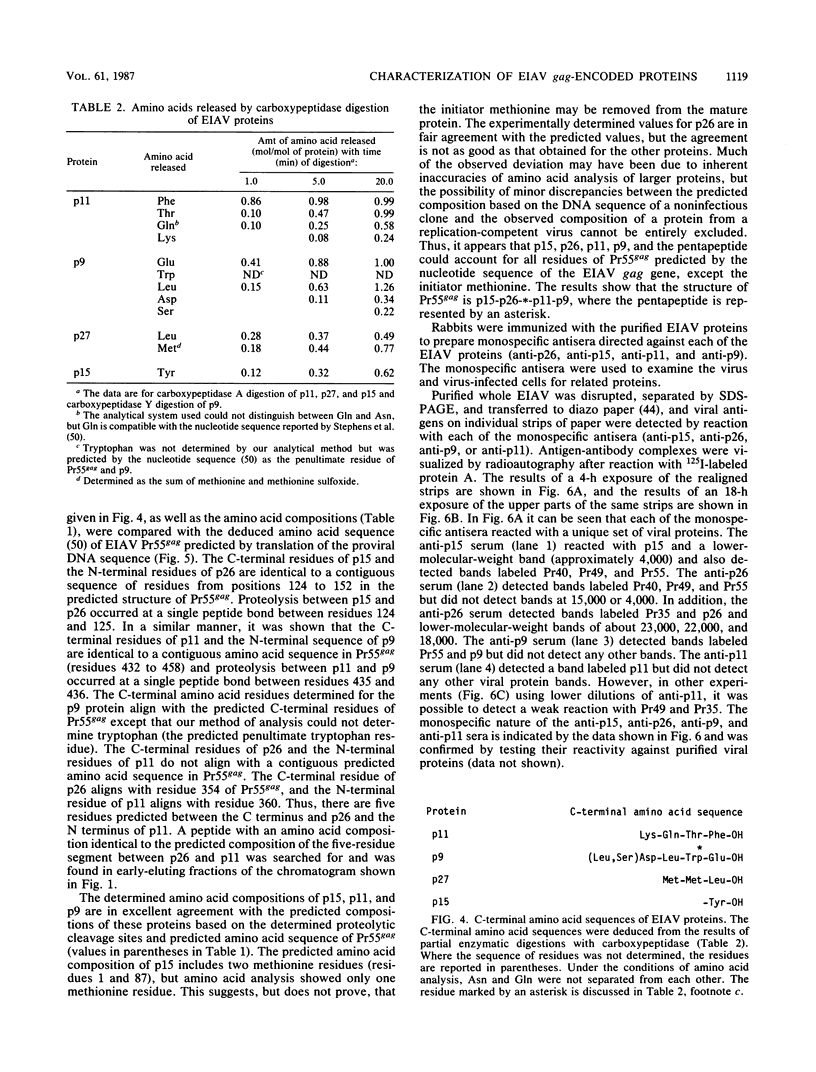
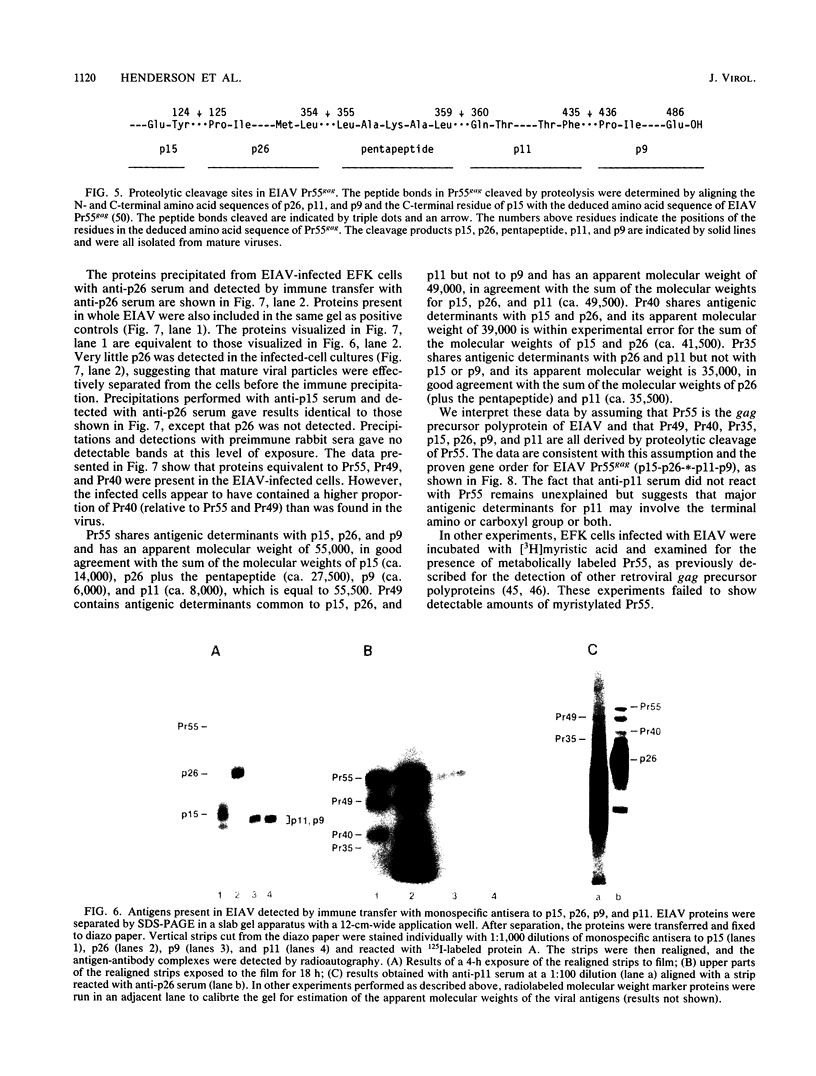
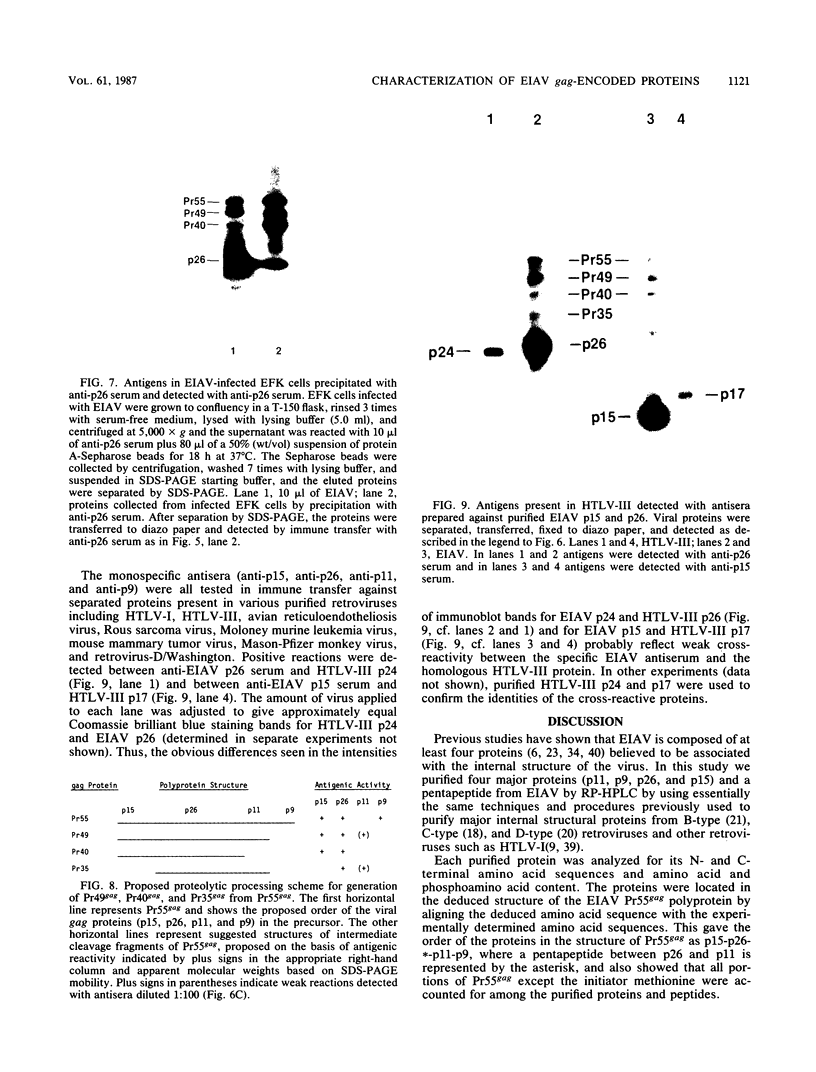
The viral core proteins (p15, p26, p11, and p9) of equine infectious anemia virus (EIAV) (Wyoming strain) were purified by reverse-phase high-pressure liquid chromatography. Each purified protein was analyzed for amino acid content, N-terminal amino acid sequence, C-terminal amino acid sequence, and phosphoamino acid content. The results of N- and C-terminal amino acid sequence analysis of each gag protein, taken together with the nucleotide sequence of the EIAV gag gene (R. M. Stephens, J. W. Casey, and N. R. Rice, Science 231:589-594, 1986), show that the order of the proteins in the precursor is p15-p26-*-p11-p9, where a pentapeptide also found in the virus is represented by the asterisk. The data are in complete agreement with the predicted structure of the gag polyprotein and show the peptide bonds cleaved during proteolytic processing. The N-terminus of p15 is blocked to Edman degradation. The p11 protein is identical to the nucleic acid-binding protein of EIAV previously isolated (C. W. Long, L. E. Henderson, and S. Oroszlan, Virology 104:491-496, 1980). High-titer rabbit antiserum was prepared against each purified protein. These antisera were used to detect the putative gag precursor (Pr55gag) and intermediate cleavage products designated Pr49 (p15-p26-*-p11), Pr40 (p15-p26), and Pr35 (p26-*-p11) in the virus and in virus-infected cells. High-titer antisera to EIAV p15 and p26 showed cross-reactivity with the homologous protein of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer B. G., Crawford T. B., McGuire T. C., Frazier M. E. RNA-dependent DNA polymerase associated with equine infectious anemia virus. J Virol. 1977 Apr;22(1):16–22. doi: 10.1128/jvi.22.1.16-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. M., Kim Y., Andersen P. R., Watson K. F., Fox J. L., Devare S. G. Human T-cell lymphotropic virus type III: immunologic characterization and primary structure analysis of the major internal protein, p24. J Virol. 1985 Aug;55(2):417–423. doi: 10.1128/jvi.55.2.417-423.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman H. P., Bladen S., Gilden R. V., Coggins L. Equine infectious anemia virus: evidence favoring classification as a retravirus. J Virol. 1976 Sep;19(3):1073–1079. doi: 10.1128/jvi.19.3.1073-1079.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Ackley C. M., Crawford T. B. Structural proteins of equine infectious anemia virus. J Virol. 1978 Dec;28(3):997–1001. doi: 10.1128/jvi.28.3.997-1001.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Archer B. G., Crawford T. B. Characterization of RNA from equine infectious anemia virus. J Virol. 1977 Nov;24(2):489–497. doi: 10.1128/jvi.24.2.489-497.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland T. D., Morgan M. A., Oroszlan S. Complete amino acid sequence of the basic nucleic acid binding protein of feline leukemia virus. Virology. 1984 Feb;133(1):137–145. doi: 10.1016/0042-6822(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Oroszlan S., Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Complete amino acid sequence of human T-cell leukemia virus structural protein p15. FEBS Lett. 1983 Oct 17;162(2):390–395. doi: 10.1016/0014-5793(83)80793-5. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Oroszlan S. Separation of amino acid phenylthiohydantoins by high-performance liquid chromatography on phenylalkyl support. Anal Biochem. 1980 Feb;102(1):1–7. doi: 10.1016/0003-2697(80)90307-3. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Copeland T. D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984 Nov;52(2):492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Benveniste R. E., Oroszlan S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J Virol. 1985 Sep;55(3):778–787. doi: 10.1128/jvi.55.3.778-787.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Oroszlan S. Terminal amino acid sequences and proteolytic cleavage sites of mouse mammary tumor virus env gene products. J Virol. 1983 Oct;48(1):314–319. doi: 10.1128/jvi.48.1.314-319.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R., Green R. W., Bolognesi D. P. The structural polypeptides of equine infections anemia virus. Intervirology. 1978;9(5):286–294. doi: 10.1159/000148946. [DOI] [PubMed] [Google Scholar]
- Issel C. J., Coggins L. Equine infectious anemia: current knowledge. J Am Vet Med Assoc. 1979 Apr 1;174(7):727–733. [PubMed] [Google Scholar]
- Kanki P. J., Alroy J., Essex M. Isolation of T-lymphotropic retrovirus related to HTLV-III/LAV from wild-caught African green monkeys. Science. 1985 Nov 22;230(4728):951–954. doi: 10.1126/science.2997923. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Barin F., M'Boup S., Allan J. S., Romet-Lemonne J. L., Marlink R., McLane M. F., Lee T. H., Arbeille B., Denis F. New human T-lymphotropic retrovirus related to simian T-lymphotropic virus type III (STLV-IIIAGM). Science. 1986 Apr 11;232(4747):238–243. doi: 10.1126/science.3006256. [DOI] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Long C. W., Henderson L. E., Oroszlan S. Isolation and characterization of low-molecular-weight DNA-binding proteins from retroviruses. Virology. 1980 Jul 30;104(2):491–496. doi: 10.1016/0042-6822(80)90352-9. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Lohrey N., Parekh B., Blakeney E. W., Issel C. J. Isolation and comparative biochemical properties of the major internal polypeptides of equine infectious anemia virus. J Virol. 1982 Jun;42(3):1029–1038. doi: 10.1128/jvi.42.3.1029-1038.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Nakajima H., Tanaka S., Ushimi C. Physicochemical studies of equine infectious anemia virus. IV. Determination of the nucleic acid type in the virus. Arch Gesamte Virusforsch. 1970;31(3):273–280. doi: 10.1007/BF01253762. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Strandberg J. D., Cork L. C., Griffin D. E. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Sep;50(1):69–79. doi: 10.1099/0022-1317-50-1-69. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B., Issel C. J., Montelaro R. C. Equine infectious anemia virus, a putative lentivirus, contains polypeptides analogous to prototype-C oncornaviruses. Virology. 1980 Dec;107(2):520–525. doi: 10.1016/0042-6822(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Perini F., Sadow J. B., Hixson C. V. Fluorometric analysis of polyamines, histamine, and 1-methylhistamine. Anal Biochem. 1979 Apr 15;94(2):431–439. doi: 10.1016/0003-2697(79)90386-5. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Tajima M., Nakajima H., Ito Y. Electron microscopy of equine infectious anemia virus. J Virol. 1969 Oct;4(4):521–527. doi: 10.1128/jvi.4.4.521-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. C., Fujitaki J. M., Smith R. A. Separation of phosphohydroxyamino acids by high-performance liquid chromatography. Anal Biochem. 1982 May 15;122(2):360–363. doi: 10.1016/0003-2697(82)90295-0. [DOI] [PubMed] [Google Scholar]