Abstract
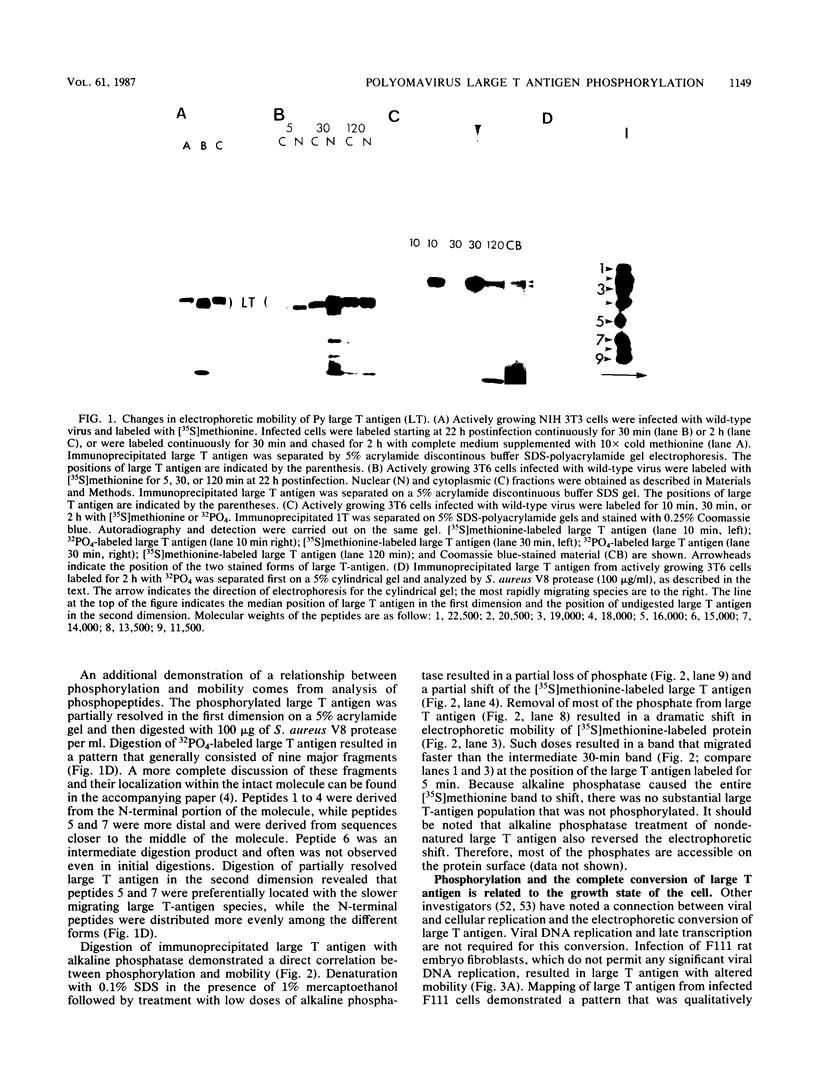
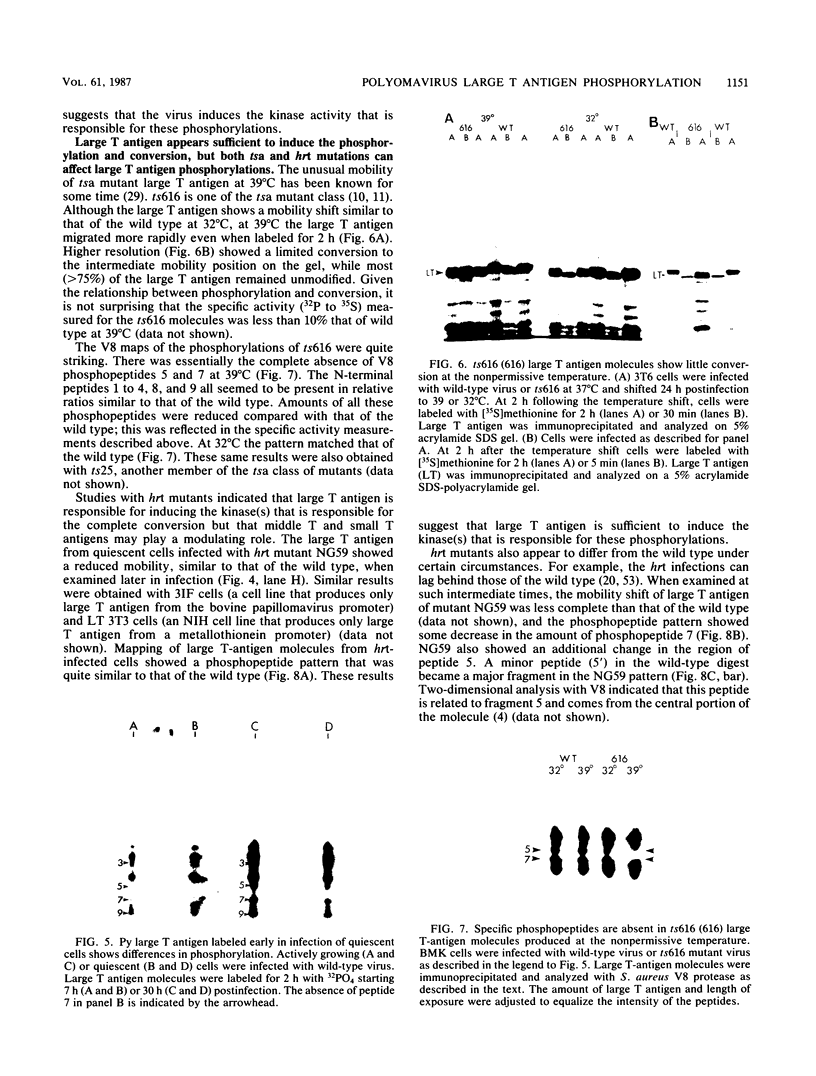
Phosphorylation is responsible for the shift in electrophoretic mobility of polyomavirus large T antigen observed in pulse-chase or continuous-labeling experiments. Phosphorylated forms migrated more slowly than newly synthesized [35S]methionine large T antigen, and alkaline phosphatase treatment reversed the mobility shift. Analysis of phosphopeptides with Staphylococcus aureus V8 protease showed that large T antigen forms of intermediate mobility were enriched in peptides 1 to 4, 8, and 9, while the slower migrating species had all nine phosphopeptides, including peptides 5 and 7. The phosphorylations represented by phosphopeptides 5 and 7 were of particular interest. These phosphopeptides were entirely lacking in large T antigen from tsa mutants such as ts616 labeled at the nonpermissive temperature. Also, the phosphorylation of peptides 5 and 7 depends on the growth state of the cell. Early in infection of quiescent cells intermediate mobility forms of large T antigen with little or no phosphorylation, particularly of peptides 5 and 7, were seen, whereas peptides 5 and 7 were well represented at the same time in patterns from growing cells. Later in infection of growth-arrested cells, these phosphorylations were observed, suggesting that infection stimulates the relevant kinase. Because large T antigen of hrt mutants, which lack middle and small T antigens, showed phosphorylation of peptides 5 and 7, large T antigen was apparently responsible for the stimulation. Because some differences in the distribution of phosphopeptides were noted between hrt mutants and the wild type, middle T antigen, small T antigen, or both may play a modulating role in large T antigen phosphorylation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselin C., Bastin M. Sequences from polyomavirus and simian virus 40 large T genes capable of immortalizing primary rat embryo fibroblasts. J Virol. 1985 Dec;56(3):958–968. doi: 10.1128/jvi.56.3.958-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Zouzias D., Della-Valle G., Gattoni S., Colantuoni V., Fenton R., Dailey L. Integration and excision of polyoma virus genomes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):611–620. doi: 10.1101/sqb.1980.044.01.064. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockus B. J., Schaffhausen B. Localization of the phosphorylations of polyomavirus large T antigen. J Virol. 1987 Apr;61(4):1155–1163. doi: 10.1128/jvi.61.4.1155-1163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. K., Hudson J., Villanueva M. S., Livingston D. M. Specific in vitro adenylylation of the simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6574–6578. doi: 10.1073/pnas.81.21.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherington V., Morgan B., Spiegelman B. M., Roberts T. M. Recombinant retroviruses that transduce individual polyoma tumor antigens: effects on growth and differentiation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4307–4311. doi: 10.1073/pnas.83.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen B. Virus-specific early RNA in 3T6 cells infected by a tsA mutant of polyoma virus. Virology. 1978 Mar;85(1):222–230. doi: 10.1016/0042-6822(78)90426-9. [DOI] [PubMed] [Google Scholar]
- Cowie A., Kamen R. Multiple binding sites for polyomavirus large T antigen within regulatory sequences of polyomavirus DNA. J Virol. 1984 Dec;52(3):750–760. doi: 10.1128/jvi.52.3.750-760.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmerie W. G., Folk W. R. Regulation of polyomavirus transcription by large tumor antigen. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6919–6923. doi: 10.1073/pnas.81.22.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R. G., Basilico C. Changes in the topography of early region transcription during polyoma virus lytic infection. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7142–7146. doi: 10.1073/pnas.79.23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Sompayrac L., Fluck M., Benjamin T. Localization of gene functions in polyoma virus DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4169–4173. doi: 10.1073/pnas.73.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Staneloni R. J., Benjamin T. L. Hr-t and ts-a: two early gene functions of polyoma virus. Virology. 1977 Apr;77(2):610–624. doi: 10.1016/0042-6822(77)90486-x. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Gilden R. V., Vernon M. L., Wolford R. G., Hugunin P. E., Huebner R. J. 5-Bromo-2'-deoxyuridine potentiation of transformation of rat-embryo cells induced in vitro by 3-methylcholanthrene: induction of rat leukemia virus gs antigen in transformed cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2415–2419. doi: 10.1073/pnas.70.8.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R. L., Ballmer-Hofer K., Benjamin T. L. Virion assembly defect of polyomavirus hr-t mutants: underphosphorylation of major capsid protein VP1 before viral DNA encapsidation. J Virol. 1985 May;54(2):311–316. doi: 10.1128/jvi.54.2.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R. L., Benjamin T. L. Host range transforming gene of polyoma virus plays a role in virus assembly. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3613–3617. doi: 10.1073/pnas.80.12.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Sequential phsophorylation of histone subfractions in the Chinese hamster cell cycle. J Biol Chem. 1975 May 25;250(10):3936–3944. [PubMed] [Google Scholar]
- Hancock R., Weil R. Biochemical evidence for induction by polyoma virus of replication of the chromosomes of mouse kidney cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1144–1150. doi: 10.1073/pnas.63.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassauer M., Scheidtmann K. H., Walter G. Mapping of phosphorylation sites in polyomavirus large T antigen. J Virol. 1986 Jun;58(3):805–816. doi: 10.1128/jvi.58.3.805-816.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Chaudry F., Fried M. Loss of polyoma virus infectivity as a result of a single amino acid change in a region of polyoma virus large T-antigen which has extensive amino acid homology with simian virus 40 large T-antigen. J Virol. 1983 Feb;45(2):693–699. doi: 10.1128/jvi.45.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Translation of polyoma virus T antigens in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5917–5921. doi: 10.1073/pnas.75.12.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Iida H., Oda K. Stimulation of non-histone chromosomal protein synthesis in simian virus 40-infected simian cells. J Virol. 1975 Mar;15(3):471–478. doi: 10.1128/jvi.15.3.471-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Dulbecco R. Characterization of polyoma virus T antigen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1259–1263. doi: 10.1073/pnas.74.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Butel J. S. Modification of simian virus 40 large tumor antigen by glycosylation. Virology. 1985 Mar;141(2):173–189. doi: 10.1016/0042-6822(85)90250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylation: a new post-translational modification specific for plasma membrane-associated simian virus 40 large T-antigen. FEBS Lett. 1983 Jan 24;151(2):257–259. doi: 10.1016/0014-5793(83)80081-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Carmichael G. G., Benjamin T. L. A polyoma mutant that encodes small T antigen but not middle T antigen demonstrates uncoupling of cell surface and cytoskeletal changes associated with cell transformation. Mol Cell Biol. 1984 Dec;4(12):2774–2783. doi: 10.1128/mcb.4.12.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Nilsson S. V., Magnusson G. Activities of polyomavirus large-T-antigen proteins expressed by mutant genes. J Virol. 1984 Sep;51(3):768–775. doi: 10.1128/jvi.51.3.768-775.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. V., Magnusson G. T-antigen expression by polyoma mutants with modified RNA splicing. EMBO J. 1983;2(12):2095–2101. doi: 10.1002/j.1460-2075.1983.tb01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Schley C., Mahoney M., Harlow E., Schaffhausen B. S., Roberts T. M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986 Dec;60(3):1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Gaudray P., Canning M., Trejo-Avila L., Cuzin F. Two polyoma virus gene functions involved in the expression of the transformed phenotype in FR 3T3 rat cells. I. Localization of a transformation maintenance function in the proximal half of the large T coding region. Virology. 1981 Oct 30;114(2):489–500. doi: 10.1016/0042-6822(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. Transforming genes and gene products of polyoma and SV40. CRC Crit Rev Biochem. 1982;13(3):215–286. doi: 10.3109/10409238209114230. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Silver J., Schaffhausen B., Benjamin T. Tumor antigens induced by nontransforming mutants of polyoma virus. Cell. 1978 Oct;15(2):485–496. doi: 10.1016/0092-8674(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Song M. K., Adolph K. W. Phosphorylation of nonhistone proteins during the HeLa cell cycle. Relationship to DNA synthesis and mitotic chromosome condensation. J Biol Chem. 1983 Mar 10;258(5):3309–3318. [PubMed] [Google Scholar]
- Stahl H., Dröge P., Zentgraf H., Knippers R. A large-tumor-antigen-specific monoclonal antibody inhibits DNA replication of simian virus 40 minichromosomes in an in vitro elongation system. J Virol. 1985 May;54(2):473–482. doi: 10.1128/jvi.54.2.473-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H., Salomon C. Small and middle T antigens contribute to lytic and abortive polyomavirus infection. J Virol. 1985 Feb;53(2):579–586. doi: 10.1128/jvi.53.2.579-586.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. The tumor antigens and the early functions of polyoma virus. Mol Cell Biochem. 1980 Sep 15;32(2):63–93. doi: 10.1007/BF00227801. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]