Abstract
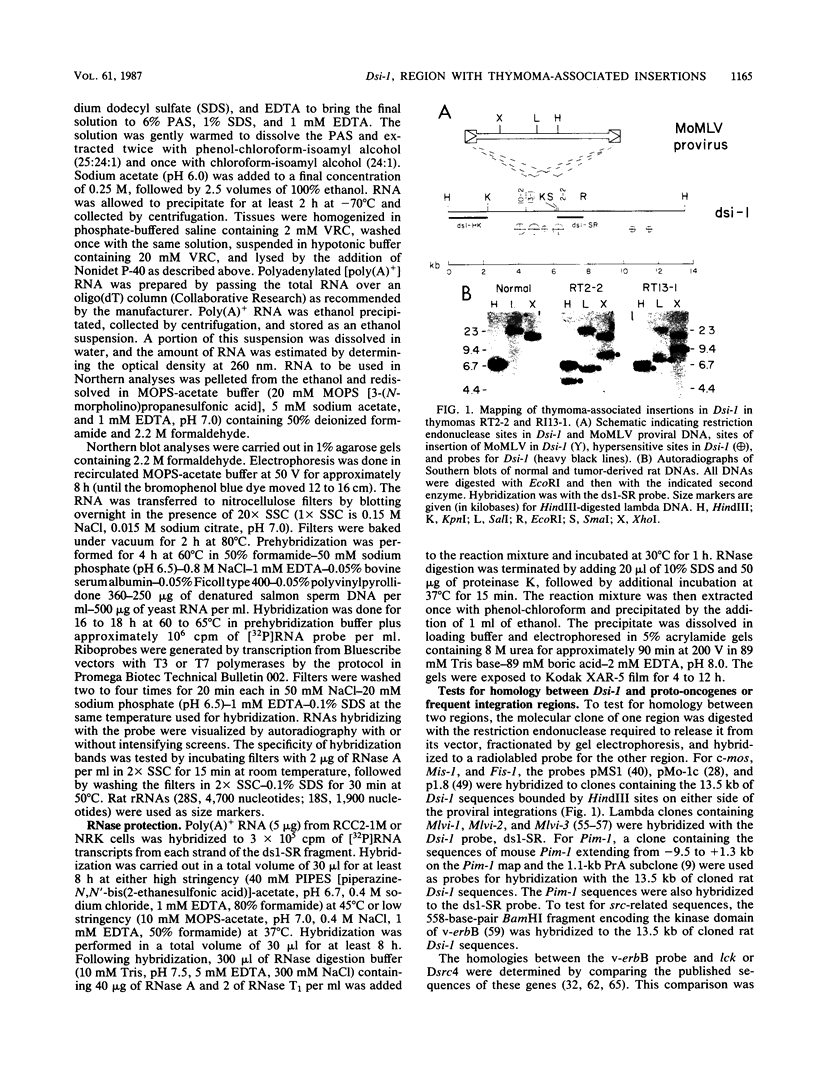
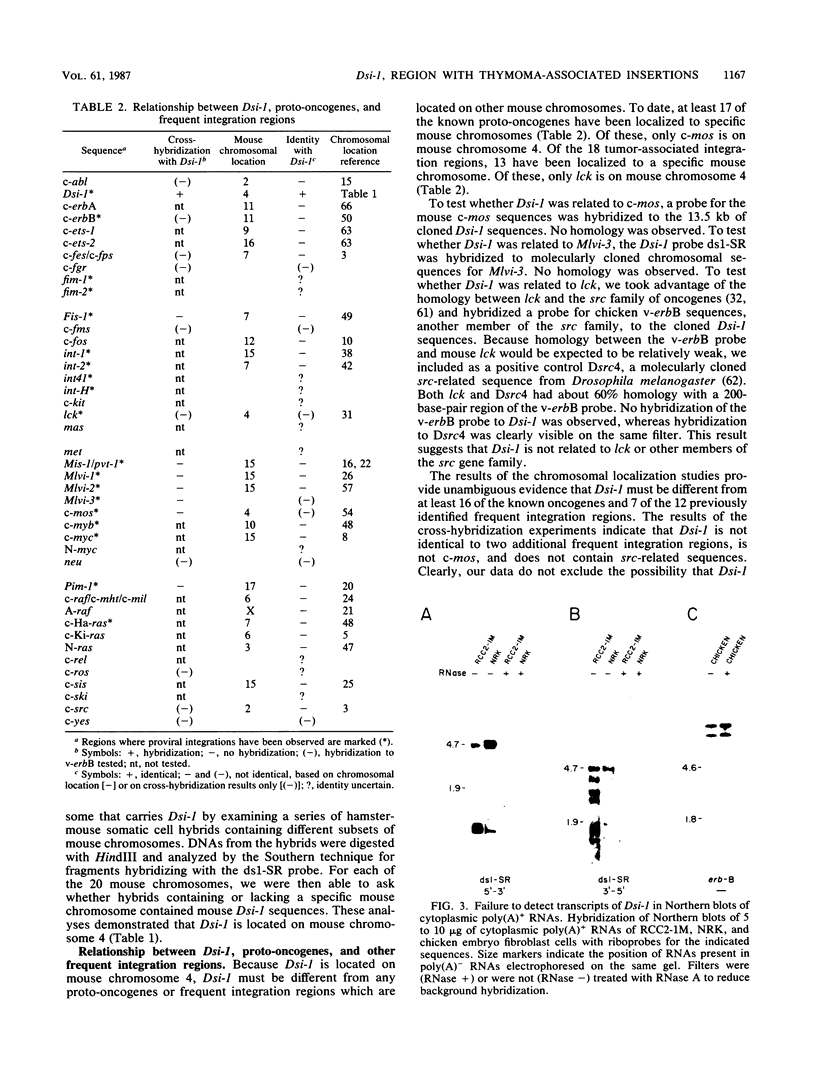
Dsi-1 is a region of chromosomal DNA that underwent proviral insertion in 3 of 24 Moloney murine leukemia virus-induced rat thymomas. In one of these tumors, a provirus is also integrated adjacent to the proto-oncogene c-myc. The proviruses in Dsi-1 have been characterized and appear to be complete. The proviruses were located within a 2-kilobase region that contained four prominent DNase I-hypersensitive sites. These hypersensitive sites were observed in Moloney murine leukemia virus-induced thymomas but not in NRK cells. The region of Dsi-1 immediately 3' to the insertions cross-hybridized with human and chicken DNA, indicating that it contains highly conserved sequences. No evidence could be found for the expression of this highly conserved region. Dsi-1 was mapped to mouse chromosome 4. This location demonstrates that Dsi-1 is different from 16 of the known proto-oncogenes (c-abl, c-erbA c-erbB, c-ets-1, c-ets-2, c-fes, c-fos, c-myb, c-myc, c-raf, A-raf, c-Ha-ras, c-Ki-ras, N-ras, c-sis, and c-src) and 12 cellular regions of tumor-associated integrations in retrovirus-induced tumors (c-erbB, Fis-1, int-1, int-2, Mis-1/pvt-1, Mlvi-1, Mlvi-2, c-mos, c-myb, c-myc, Pim-1, and c-Ha-ras). Hybridization experiments indicated that Dsi-1 is probably different from five additional proto-oncogenes (c-fgr, c-fms, c-mos, neu, and c-yes) and from two additional frequent integration regions (lck and Mlvi-3).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacheler L. T., Fan H. Multiple integration sites for Moloney murine leukemia virus in productively infected mouse fibroblasts. J Virol. 1979 Jun;30(3):657–667. doi: 10.1128/jvi.30.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Blatt C., Harper M. E., Franchini G., Nesbitt M. N., Simon M. I. Chromosomal mapping of murine c-fes and c-src genes. Mol Cell Biol. 1984 May;4(5):978–981. doi: 10.1128/mcb.4.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Wildin R. S., Prendergast T. J., Varmus H. E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986 Sep 26;46(7):1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- Cahilly L. A., George D. L. Regional mapping of cKi-ras proto-oncogene on mouse chromosome 6 by in situ hybridization. Cytogenet Cell Genet. 1985;39(2):140–144. doi: 10.1159/000132123. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Crews S., Barth R., Hood L., Prehn J., Calame K. Mouse c-myc oncogene is located on chromosome 15 and translocated to chromosome 12 in plasmacytomas. Science. 1982 Dec 24;218(4579):1319–1321. doi: 10.1126/science.7146913. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P. A genetic map of mouse chromosome 12 composed of polymorphic DNA fragments. J Exp Med. 1984 Sep 1;160(3):827–838. doi: 10.1084/jem.160.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L., Kuehl M., Weir L., Leder P., Max E. E. A conserved sequence in the immunoglobulin J kappa-C kappa intron: possible enhancer element. Nature. 1983 Aug 4;304(5925):447–449. doi: 10.1038/304447a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Garcia M., Wellinger R., Vessaz A., Diggelmann H. A new site of integration for mouse mammary tumor virus proviral DNA common to BALB/cf(C3H) mammary and kidney adenocarcinomas. EMBO J. 1986 Jan;5(1):127–134. doi: 10.1002/j.1460-2075.1986.tb04186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Goff S. P., D'Eustachio P., Ruddle F. H., Baltimore D. Chromosomal assignment of the endogenous proto-oncogene C-abl. Science. 1982 Dec 24;218(4579):1317–1319. doi: 10.1126/science.6293057. [DOI] [PubMed] [Google Scholar]
- Graham M., Adams J. M., Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. 1985 Apr 25-May 1Nature. 314(6013):740–743. doi: 10.1038/314740a0. [DOI] [PubMed] [Google Scholar]
- Gray D. A., McGrath C. M., Jones R. F., Morris V. L. A common mouse mammary tumor virus integration site in chemically induced precancerous mammary hyperplasias. Virology. 1986 Jan 30;148(2):360–368. doi: 10.1016/0042-6822(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hilkens J., Cuypers H. T., Selten G., Kroezen V., Hilgers J., Berns A. Genetic mapping of Pim-1 putative oncogene to mouse chromosome 17. Somat Cell Mol Genet. 1986 Jan;12(1):81–88. doi: 10.1007/BF01560730. [DOI] [PubMed] [Google Scholar]
- Huebner K., ar-Rushdi A., Griffin C. A., Isobe M., Kozak C., Emanuel B. S., Nagarajan L., Cleveland J. L., Bonner T. I., Goldsborough M. D. Actively transcribed genes in the raf oncogene group, located on the X chromosome in mouse and human. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3934–3938. doi: 10.1073/pnas.83.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Villeneuve L., Rassart E., Kozak C. Mouse chromosomal mapping of a murine leukemia virus integration region (Mis-1) first identified in rat thymic leukemia. J Virol. 1985 Dec;56(3):1045–1048. doi: 10.1128/jvi.56.3.1045-1048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A. Genetic mapping of a mouse chromosomal locus required for mink cell focus-forming virus replication. J Virol. 1983 Oct;48(1):300–303. doi: 10.1128/jvi.48.1.300-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Sears J. F., Hoggan M. D. Genetic mapping of the mouse proto-oncogene c-sis to chromosome 15. Science. 1983 Aug 26;221(4613):867–869. doi: 10.1126/science.6308764. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Strauss P. G., Tsichlis P. N. Genetic mapping of a cellular DNA region involved in induction of thymic lymphomas (Mlvi-1) to mouse chromosome 15. Mol Cell Biol. 1985 Apr;5(4):894–897. doi: 10.1128/mcb.5.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C., Gunnell M. A., Rapp U. R. A new oncogene, c-raf, is located on mouse chromosome 6. J Virol. 1984 Jan;49(1):297–299. doi: 10.1128/jvi.49.1.297-299.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski J. F., Potter M., Bauer S. R., Reddy E. P. DNA rearrangement and altered RNA expression of the c-myb oncogene in mouse plasmacytoid lymphosarcomas. Science. 1983 May 20;220(4599):795–798. doi: 10.1126/science.6687762. [DOI] [PubMed] [Google Scholar]
- Nagarajan L., Louie E., Tsujimoto Y., ar-Rushdi A., Huebner K., Croce C. M. Localization of the human pim oncogene (PIM) to a region of chromosome 6 involved in translocations in acute leukemias. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2556–2560. doi: 10.1073/pnas.83.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Kozak C., Dickson C. Mouse mammary tumor virus integration regions int-1 and int-2 map on different mouse chromosomes. Mol Cell Biol. 1984 Feb;4(2):375–378. doi: 10.1128/mcb.4.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Gagnon G. C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986 Jan;57(1):28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Ryan J., Hart C. P., Ruddle F. H. Molecular cloning and chromosome assignment of murine N-ras. Nucleic Acids Res. 1984 Aug 10;12(15):6063–6072. doi: 10.1093/nar/12.15.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi A. Y., Lalley P. A., Zabel B. U., Ellis R. W., Scolnick E. M., Naylor S. L. Chromosome assignments of four mouse cellular homologs of sarcoma and leukemia virus oncogenes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):525–529. doi: 10.1073/pnas.81.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Kozak C. Common proviral integration region on mouse chromosome 7 in lymphomas and myelogenous leukemias induced by Friend murine leukemia virus. J Virol. 1986 Feb;57(2):526–533. doi: 10.1128/jvi.57.2.526-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Whitney J. B., 3rd, Kozak C., Hollis G., Kirsch I. Erbb is linked to the alpha-globin locus on mouse chromosome 11. Mol Cell Biol. 1985 Jul;5(7):1784–1786. doi: 10.1128/mcb.5.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola B., Fichelson S., Bordereaux D., Tambourin P. E., Gisselbrecht S. fim-1 and fim-2: two new integration regions of Friend murine leukemia virus in myeloblastic leukemias. J Virol. 1986 Nov;60(2):718–725. doi: 10.1128/jvi.60.2.718-725.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Swan D., Oskarsson M., Keithley D., Ruddle F. H., D'Eustachio P., Vande Woude G. F. Chromosomal localization of the Moloney sarcoma virus mouse cellular (c-mos) sequence. J Virol. 1982 Nov;44(2):752–754. doi: 10.1128/jvi.44.2.752-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Lohse M. A., Szpirer C., Szpirer J., Levan G. Cellular DNA regions involved in the induction of rat thymic lymphomas (Mlvi-1, Mlvi-2, Mlvi-3, and c-myc) represent independent loci as determined by their chromosomal map location in the rat. J Virol. 1985 Dec;56(3):938–942. doi: 10.1128/jvi.56.3.938-942.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Kozak C. A. Cellular DNA region involved in induction of thymic lymphomas (Mlvi-2) maps to mouse chromosome 15. Mol Cell Biol. 1984 May;4(5):997–1000. doi: 10.1128/mcb.4.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Robinson H. L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J Virol. 1986 Nov;60(2):683–692. doi: 10.1128/jvi.60.2.683-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wadsworth S. C., Madhavan K., Bilodeau-Wentworth D. Maternal inheritance of transcripts from three Drosophila src-related genes. Nucleic Acids Res. 1985 Mar 25;13(6):2153–2170. doi: 10.1093/nar/13.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Kozak C., Reeves R., Gearhart J., Nunn M. F., Nash W., Fowle J. R., 3rd, Duesberg P., Papas T. S. Conserved chromosomal positions of dual domains of the ets protooncogene in cats, mice, and humans. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1792–1796. doi: 10.1073/pnas.83.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Papkoff J., Moscovici C., Varmus H. E. Identification of a provirally activated c-Ha-ras oncogene in an avian nephroblastoma via a novel procedure: cDNA cloning of a chimaeric viral-host transcript. EMBO J. 1986 Feb;5(2):301–309. doi: 10.1002/j.1460-2075.1986.tb04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Zabel B. U., Fournier R. E., Lalley P. A., Naylor S. L., Sakaguchi A. Y. Cellular homologs of the avian erythroblastosis virus erb-A and erb-B genes are syntenic in mouse but asyntenic in man. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4874–4878. doi: 10.1073/pnas.81.15.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]